Sodium Butyrate Supplementation Modulates Neuroinflammatory Response Aggravated by Antibiotic Treatment in a Mouse Model of Binge-like Ethanol Drinking
Abstract
:1. Introduction
2. Results
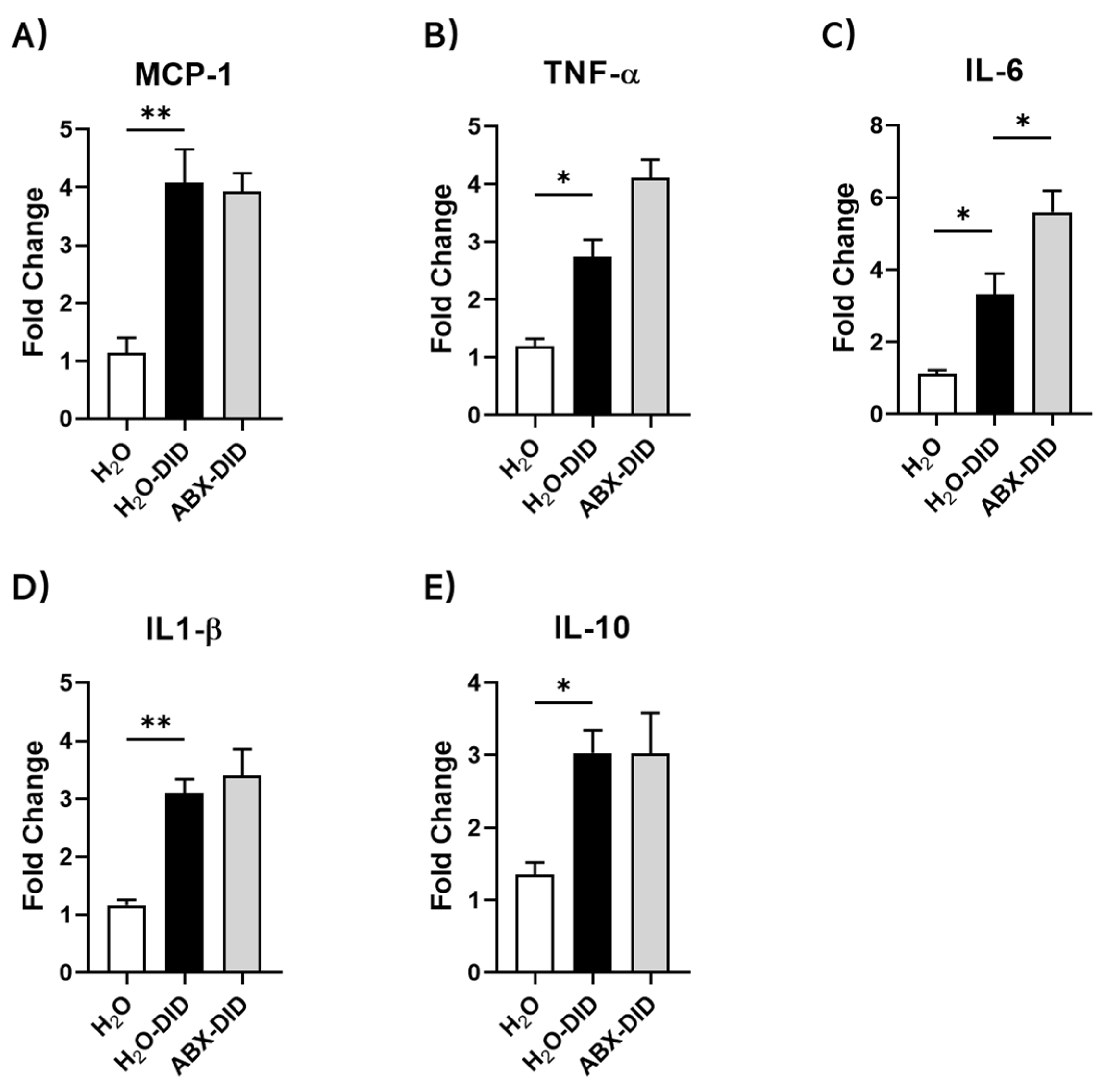
2.1. ABX Treatment Enhanced Ethanol-Induced Increases in IL-6 and TNF-α mRNA Levels in Mouse Brain
2.2. Sodium Butyrate Supplementation Reduced Ethanol-Induced and ABX-Enhanced Increases in mRNA Levels of Key Inflammatory Cytokines
2.3. Sodium Butyrate Supplementation Prevented Ethanol-Induced and ABX-Enhanced Activation of Hippocampal Microglial Cells
2.4. Sodium Butyrate Supplementation Prevented Ethanol-Induced and ABX-Enhanced Reduction in the Activity of Hippocampal Astrocytes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibiotic Cocktail and Sodium Butyrate (SB) Treatment
4.3. Drinking in the Dark (DID) Ethanol Self-Administration Procedure
4.4. RT-qPCR
4.5. Immunohistochemistry (IHC)
4.6. Image Analysis
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erickson, E.K.; Grantham, E.K.; Warden, A.S.; Harris, R.A. Neuroimmune Signaling in Alcohol Use Disorder. Pharmacol. Biochem. Behav. 2019, 177, 34–60. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.C.; Jones, J.L.; Jarnecke, A.M.; Back, S.E. Behavioral Treatments for Alcohol Use Disorder and Post-Traumatic Stress Disorder. Alcohol Res. 2018, 39, 181–192. [Google Scholar] [PubMed]
- Huynh, N.; Arabian, N.; Naito, A.; Louie, S.; Jakowec, M.W.; Asatryan, L.; Davies, D.L. Preclinical Development of Moxidectin as a Novel Therapeutic for Alcohol Use Disorder. Neuropharmacology 2017, 113, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swift, R.M.; Aston, E.R. Pharmacotherapy for Alcohol Use Disorder: Current and Emerging Therapies. Harv. Rev. Psychiatry 2015, 23, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Witkiewitz, K.; Litten, R.Z.; Leggio, L. Advances in the Science and Treatment of Alcohol Use Disorder. Sci. Adv. 2019, 5, eaax4043. [Google Scholar] [CrossRef] [Green Version]
- Kauer, J.A. Learning Mechanisms in Addiction: Synaptic Plasticity in the Ventral Tegmental Area as a Result of Exposure to Drugs of Abuse. Annu. Rev. Physiol. 2004, 66, 447–475. [Google Scholar] [CrossRef]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar]
- Leclercq, S.; de Timary, P.; Delzenne, N.M.; Stärkel, P. The Link between Inflammation, Bugs, the Intestine and the Brain in Alcohol Dependence. Transl. Psychiatry 2017, 7, e1048. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Stärkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric Dysbiosis Associated with a Mouse Model of Alcoholic Liver Disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Singhal, R.; Donde, H.; Ghare, S.; Stocke, K.; Zhang, J.; Vadhanam, M.; Reddy, S.; Gobejishvili, L.; Chilton, P.; Joshi-Barve, S.; et al. Decrease in Acetyl-CoA Pathway Utilizing Butyrate-Producing Bacteria Is a Key Pathogenic Feature of Alcohol-Induced Functional Gut Microbial Dysbiosis and Development of Liver Disease in Mice. Gut Microbes 2021, 13, 1946367. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Jang, Y.A.; Kim, Y.M.; Kim, J.G.; Lim, B.O. Neuroprotective Effects of Sodium Butyrate through Suppressing Neuroinflammation and Modulating Antioxidant Enzymes. Neurochem. Res. 2021, 46, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between Microbes and Host Intestinal Health: Modulation by Dietary Nutrients and Gut-Brain-Endocrine-Immune Axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune Mechanisms of Alcohol and Drug Addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namba, M.D.; Leyrer-Jackson, J.M.; Nagy, E.K.; Olive, M.F.; Neisewander, J.L. Neuroimmune Mechanisms as Novel Treatment Targets for Substance Use Disorders and Associated Comorbidities. Front. Neurosci. 2021, 15, 650785. [Google Scholar] [CrossRef]
- Smiley, C.E.; Wood, S.K. Stress- and Drug-Induced Neuroimmune Signaling as a Therapeutic Target for Comorbid Anxiety and Substance Use Disorders. Pharmacol. Ther. 2022, 239, 108212. [Google Scholar] [CrossRef]
- Lowe, P.P.; Morel, C.; Ambade, A.; Iracheta-Vellve, A.; Kwiatkowski, E.; Satishchandran, A.; Furi, I.; Cho, Y.; Gyongyosi, B.; Catalano, D.; et al. Chronic Alcohol-Induced Neuroinflammation Involves CCR2/5-Dependent Peripheral Macrophage Infiltration and Microglia Alterations. J. Neuroinflammation 2020, 17, 296. [Google Scholar] [CrossRef]
- Tracey, K.J. Understanding Immunity Requires More than Immunology. Nat. Immunol. 2010, 11, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; He, J.; Hanes, R.N.; Pluzarev, O.; Hong, J.-S.; Crews, F.T. Increased Systemic and Brain Cytokine Production and Neuroinflammation by Endotoxin Following Ethanol Treatment. J. Neuroinflammation 2008, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Kirson, D.; Wolfe, S.A.; Khom, S.; D’Ambrosio, S.R.; Spierling Bagsic, S.R.; Bajo, M.; Vlkolinský, R.; Hoang, N.K.; Singhal, A.; et al. Importance of Sex and Trauma Context on Circulating Cytokines and Amygdalar GABAergic Signaling in a Comorbid Model of Posttraumatic Stress and Alcohol Use Disorders. Mol. Psychiatry 2021, 26, 3093–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, G.; Manwani, D.; Mortha, A.; Xu, C.; Faith, J.J.; Burk, R.D.; Kunisaki, Y.; Jang, J.-E.; Scheiermann, C.; et al. Neutrophil Ageing Is Regulated by the Microbiome. Nature 2015, 525, 528–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, S.A.; Casachahua, J.D.; Rinker, J.A.; Blose, A.K.; Lysle, D.T.; Thiele, T.E. IL-1 Receptor Signaling in the Basolateral Amygdala Modulates Binge-like Ethanol Consumption in Male C57BL/6J Mice. Brain Behav. Immun. 2016, 51, 258–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, S.P.; Skulberg, A.; Skulberg, K.R.; Aass, H.C.D.; Bramness, J.G. Cytokine Changes Following Acute Ethanol Intoxication in Healthy Men: A Crossover Study. Mediat. Inflamm. 2016, 2016, 3758590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential Activation of Microglia and Astrocyte Cytokine Expression Precedes Increased Iba-1 or GFAP Immunoreactivity Following Systemic Immune Challenge. Glia 2016, 64, 300–316. [Google Scholar] [CrossRef] [Green Version]
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G.J. The Role of Neuroimmune Signaling in Alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef]
- McClain, J.A.; Morris, S.A.; Deeny, M.A.; Marshall, S.A.; Hayes, D.M.; Kiser, Z.M.; Nixon, K. Adolescent Binge Alcohol Exposure Induces Long-Lasting Partial Activation of Microglia. Brain Behav. Immun. 2011, 25 (Suppl. 1), S120–S128. [Google Scholar] [CrossRef] [Green Version]
- Minghetti, L.; Levi, G. Microglia as Effector Cells in Brain Damage and Repair: Focus on Prostanoids and Nitric Oxide. Prog. Neurobiol. 1998, 54, 99–125. [Google Scholar] [CrossRef]
- Adermark, L.; Bowers, M.S. Disentangling the Role of Astrocytes in Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 2016, 40, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kamiya, T.; Tsuboi, T. Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front. Neurosci. 2015, 9, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhya, R.; Zingg, W.; Shetty, S.; Shetty, A.K. Astrocyte-Derived Extracellular Vesicles: Neuroreparative Properties and Role in the Pathogenesis of Neurodegenerative Disorders. J. Control. Release 2020, 323, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.E.N.; Al Omran, A.J.; Davies, D.L.; Asatryan, L. Antibiotic-Induced Disruption of Commensal Microbiome Linked to Increases in Binge-like Ethanol Consumption Behavior. Brain Res. 2020, 1747, 147067. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.E.; Gao, L.; Zhang, Z.; Davies, D.L.; Asatryan, L. Supplementation with Sodium Butyrate Protects against Antibiotic-Induced Increases in Ethanol Consumption Behavior in Mice. Alcohol 2022, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stragier, E.; Martin, V.; Davenas, E.; Poilbout, C.; Mongeau, R.; Corradetti, R.; Lanfumey, L. Brain Plasticity and Cognitive Functions after Ethanol Consumption in C57BL/6J Mice. Transl. Psychiatry 2015, 5, e696. [Google Scholar] [CrossRef] [Green Version]
- Harry, G.J.; Kraft, A.D. Neuroinflammation and Microglia: Considerations and Approaches for Neurotoxicity Assessment. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1265–1277. [Google Scholar] [CrossRef]
- Barney, T.M.; Vore, A.S.; Gano, A.; Mondello, J.E.; Deak, T. The Influence of Central Interleukin-6 on Behavioral Changes Associated with Acute Alcohol Intoxication in Adult Male Rats. Alcohol 2019, 79, 37–45. [Google Scholar] [CrossRef]
- Coleman, L.G.J.; Crews, F.T.; Vetreno, R.P. The Persistent Impact of Adolescent Binge Alcohol on Adult Brain Structural, Cellular, and Behavioral Pathology: A Role for the Neuroimmune System and Epigenetics. Int. Rev. Neurobiol. 2021, 160, 1–44. [Google Scholar] [CrossRef]
- Lutz, T.A. The Brain Needs Interleukin-6 (IL-6) to Maintain a “Healthy” Energy Balance. Focus on “IL-6 Ameliorates Defective Leptin Sensitivity in DIO Ventromedial Hypothalamic Nucleus Neurons”. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R989–R991. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Dong, Y.; Zhu, T.; Luo, Y.; Kang, X.; Pang, M.; Li, H.; Xu, H.; Gu, C.; Pan, S.; et al. Semen Hoveniae Extract Ameliorates Alcohol-Induced Chronic Liver Damage in Rats via Modulation of the Abnormalities of Gut-Liver Axis. Phytomedicine 2019, 52, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lousberg, E.L.; Moldenhauer, L.M.; Hayball, J.D.; Coller, J.K.; Rice, K.C.; Watkins, L.R.; Somogyi, A.A.; Hutchinson, M.R. Inhibiting the TLR4-MyD88 Signalling Cascade by Genetic or Pharmacological Strategies Reduces Acute Alcohol-Induced Sedation and Motor Impairment in Mice: TLR4 and MyD88 Contribute to Alcohol Actions. Br. J. Pharmacol. 2012, 165, 1319–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and Levofloxacin Attenuate Microglia Inflammatory Response via TLR4/NF-KB Pathway. J. Neuroinflammation 2019, 16, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Zhang, Y.; Tang, X.; Jing, C.; Qiu, S.; Li, B.; Li, Y. IL-6 and IL-1β Upregulation and Tau Protein Phosphorylation in Response to Chronic Alcohol Exposure in the Mouse Hippocampus. Neuroreport 2021, 32, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Zuo, T.; Zuo, Z.-C. Impact of IL-10 Gene Polymorphisms and Its Interaction with Environment on Susceptibility to Systemic Lupus Erythematosus. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420945916. [Google Scholar] [CrossRef]
- Rangaraju, S.; Dammer, E.B.; Raza, S.A.; Rathakrishnan, P.; Xiao, H.; Gao, T.; Duong, D.M.; Pennington, M.W.; Lah, J.J.; Seyfried, N.T.; et al. Identification and Therapeutic Modulation of a Pro-Inflammatory Subset of Disease-Associated-Microglia in Alzheimer’s Disease. Mol. Neurodegener. 2018, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- Loane, D.J.; Byrnes, K.R. Role of Microglia in Neurotrauma. Neurotherapeutics 2010, 7, 366–377. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef] [Green Version]
- Rueda-Carrasco, J.; Martin-Bermejo, M.J.; Pereyra, G.; Mateo, M.I.; Borroto, A.; Brosseron, F.; Kummer, M.P.; Schwartz, S.; López-Atalaya, J.P.; Alarcon, B.; et al. SFRP1 Modulates Astrocyte-to-Microglia Crosstalk in Acute and Chronic Neuroinflammation. EMBO Rep. 2021, 22, e51696. [Google Scholar] [CrossRef]
- Huang, Y.H.; Sinha, S.R.; Tanaka, K.; Rothstein, J.D.; Bergles, D.E. Astrocyte Glutamate Transporters Regulate Metabotropic Glutamate Receptor-Mediated Excitation of Hippocampal Interneurons. J. Neurosci. 2004, 24, 4551–4559. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-Y.; Chen, H.; Hamada, K.; Gatta, E.; Chen, Y.; Zhang, H.; Drnevich, J.; Krishnan, H.R.; Maienschein-Cline, M.; Grayson, D.R.; et al. Transcriptomics Identifies STAT3 as a Key Regulator of Hippocampal Gene Expression and Anhedonia during Withdrawal from Chronic Alcohol Exposure. Transl. Psychiatry 2021, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Castanon, N.; Luheshi, G.; Layé, S. Role of Neuroinflammation in the Emotional and Cognitive Alterations Displayed by Animal Models of Obesity. Front. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, P.T.; Kipp, B.T.; Reitz, N.L.; Savage, L.M. Aging with Alcohol-Related Brain Damage: Critical Brain Circuits Associated with Cognitive Dysfunction. Int. Rev. Neurobiol. 2019, 148, 101–168. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.E.N.; Zhang, Z.; Gao, L.; Asatryan, L. Microbiome Meets Microglia in Neuroinflammation and Neurological Disorders. Neuroimmunol. Neuroinflammation 2020, 2020, 215–233. [Google Scholar] [CrossRef]
- Okumura, T.; Nozu, T.; Ishioh, M.; Igarashi, S.; Kumei, S.; Ohhira, M. Centrally Administered Butyrate Improves Gut Barrier Function, Visceral Sensation and Septic Lethality in Rats. J. Pharmacol. Sci. 2021, 146, 183–191. [Google Scholar] [CrossRef]
- Xu, C.; Lee, S.K.; Zhang, D.; Frenette, P.S. The Gut Microbiome Regulates Psychological-Stress-Induced Inflammation. Immunity 2020, 53, 417–428.e4. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e1-3. [Google Scholar] [CrossRef] [Green Version]
- Kiraly, D.D.; Walker, D.M.; Calipari, E.S.; Labonte, B.; Issler, O.; Pena, C.J.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar] [CrossRef] [Green Version]
- Barkley-Levenson, A.M.; Crabbe, J.C. High Drinking in the Dark Mice: A Genetic Model of Drinking to Intoxication. Alcohol 2014, 48, 217–223. [Google Scholar] [CrossRef]



| Target Gene | Forward Primer (5′ > 3′) | Reverse Primer (5′ > 3′) |
|---|---|---|
| MCP-1 | GCAGCAGGTGTCCCAAAGAA | ATTTACGGGTCAACTTCACATTCAA |
| TNF-α | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| IL-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| IL-6 | ACAACCACGGCCTTCCCTACT T | CACGATTTCCCAGAGAACATGTG |
| IL-10 | CGGGAAGACAATAACTGCACCC | CGGTTAGCAGTATGTTGTCCAGC |
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Davies, D.L.; Asatryan, L. Sodium Butyrate Supplementation Modulates Neuroinflammatory Response Aggravated by Antibiotic Treatment in a Mouse Model of Binge-like Ethanol Drinking. Int. J. Mol. Sci. 2022, 23, 15688. https://doi.org/10.3390/ijms232415688
Gao L, Davies DL, Asatryan L. Sodium Butyrate Supplementation Modulates Neuroinflammatory Response Aggravated by Antibiotic Treatment in a Mouse Model of Binge-like Ethanol Drinking. International Journal of Molecular Sciences. 2022; 23(24):15688. https://doi.org/10.3390/ijms232415688
Chicago/Turabian StyleGao, Lei, Daryl L. Davies, and Liana Asatryan. 2022. "Sodium Butyrate Supplementation Modulates Neuroinflammatory Response Aggravated by Antibiotic Treatment in a Mouse Model of Binge-like Ethanol Drinking" International Journal of Molecular Sciences 23, no. 24: 15688. https://doi.org/10.3390/ijms232415688
APA StyleGao, L., Davies, D. L., & Asatryan, L. (2022). Sodium Butyrate Supplementation Modulates Neuroinflammatory Response Aggravated by Antibiotic Treatment in a Mouse Model of Binge-like Ethanol Drinking. International Journal of Molecular Sciences, 23(24), 15688. https://doi.org/10.3390/ijms232415688





