Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases
Abstract
1. Introduction
2. Results
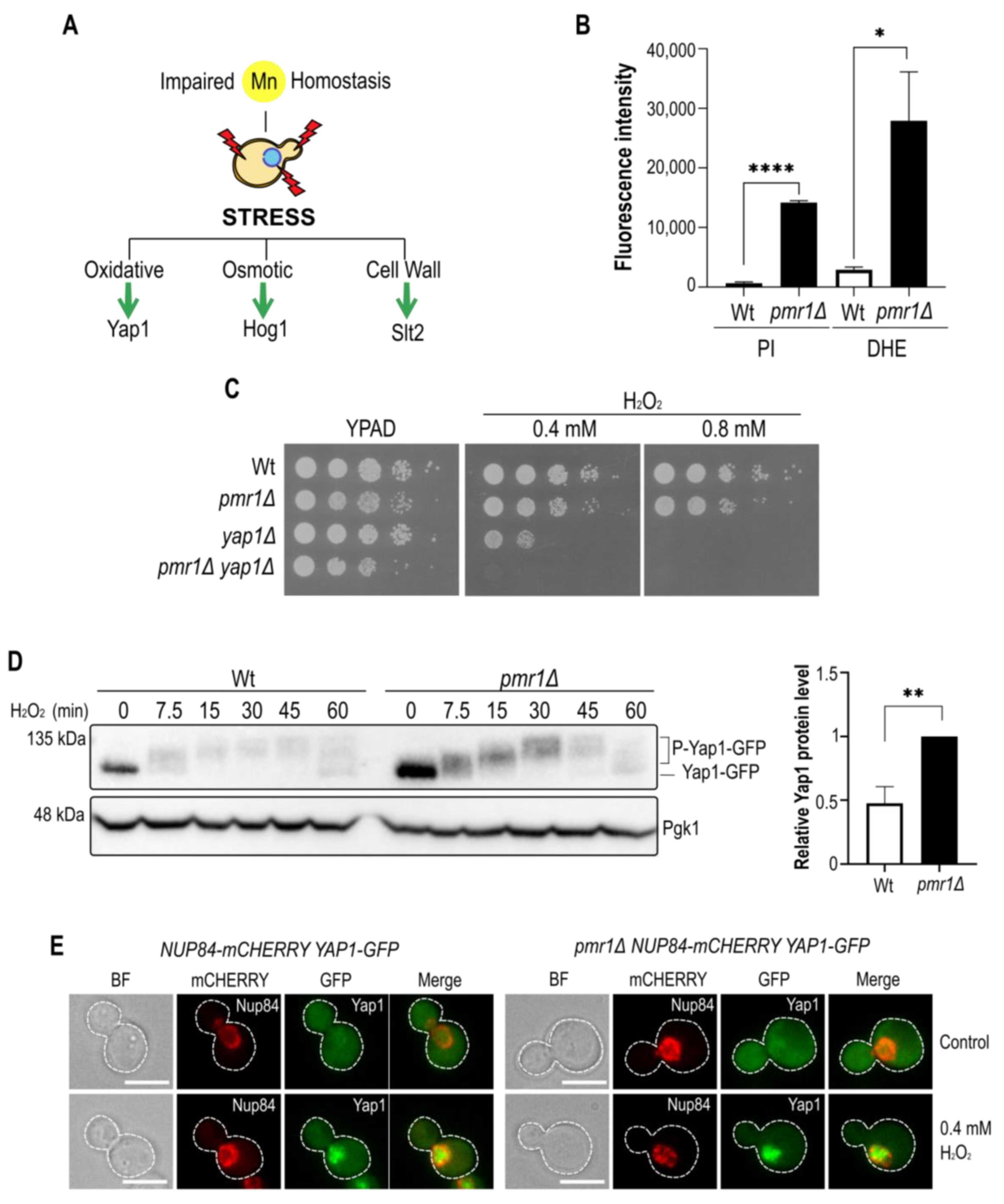
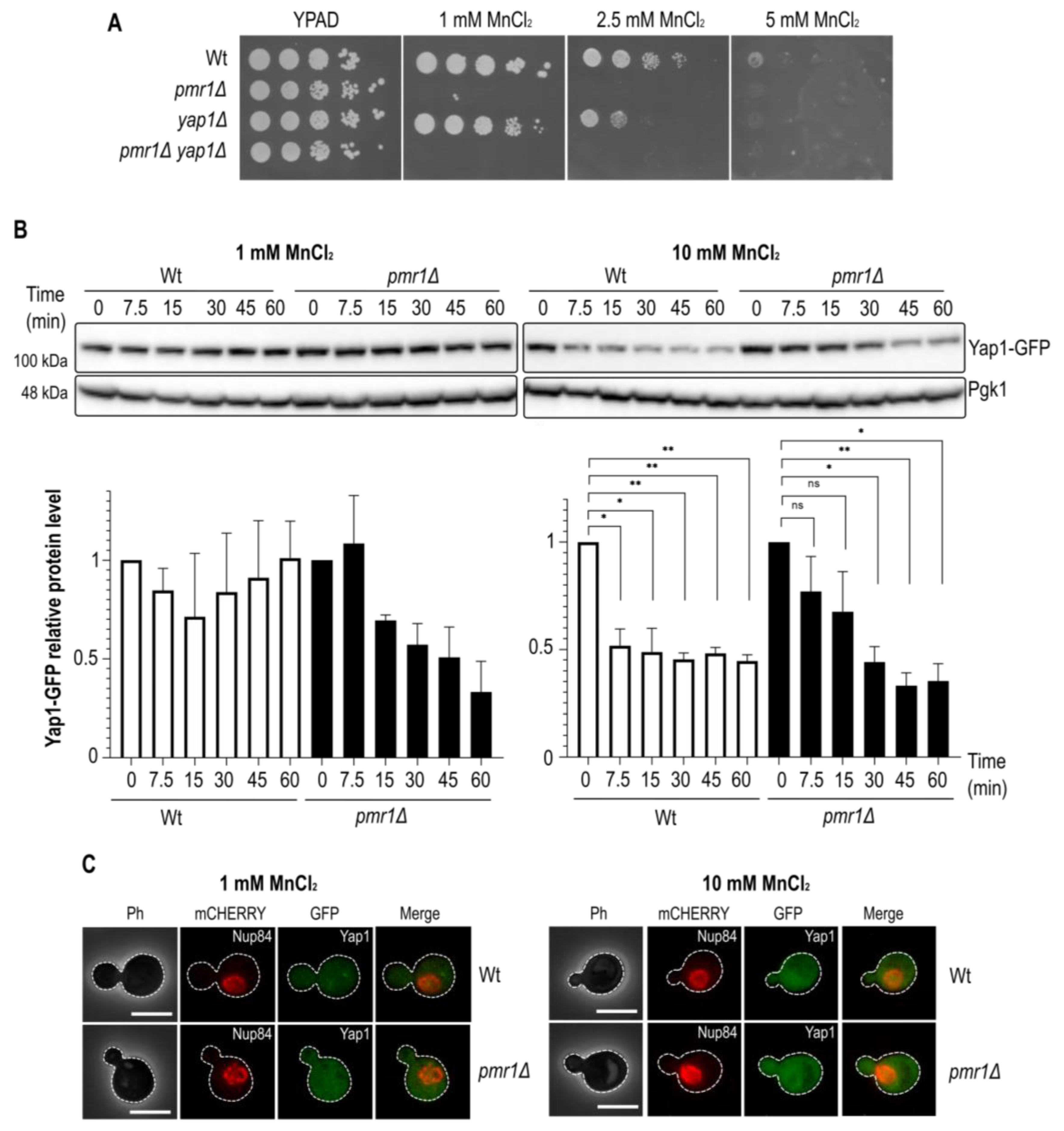
2.1. Impaired Manganese Homeostasis Leads to Oxidative Stress
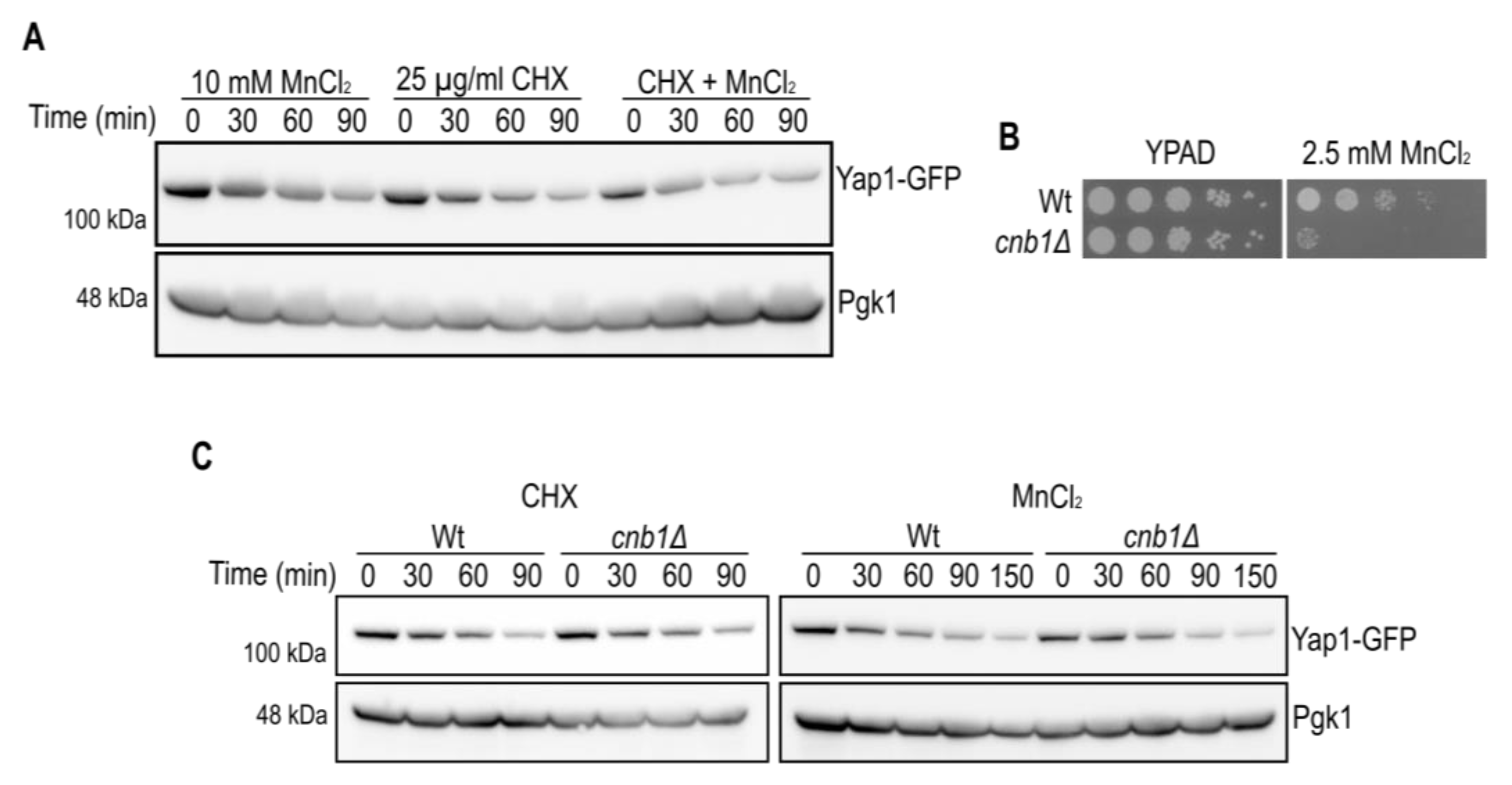
2.2. Manganese-Dependent Yap1 Decay Is Calcineurin B-Independent
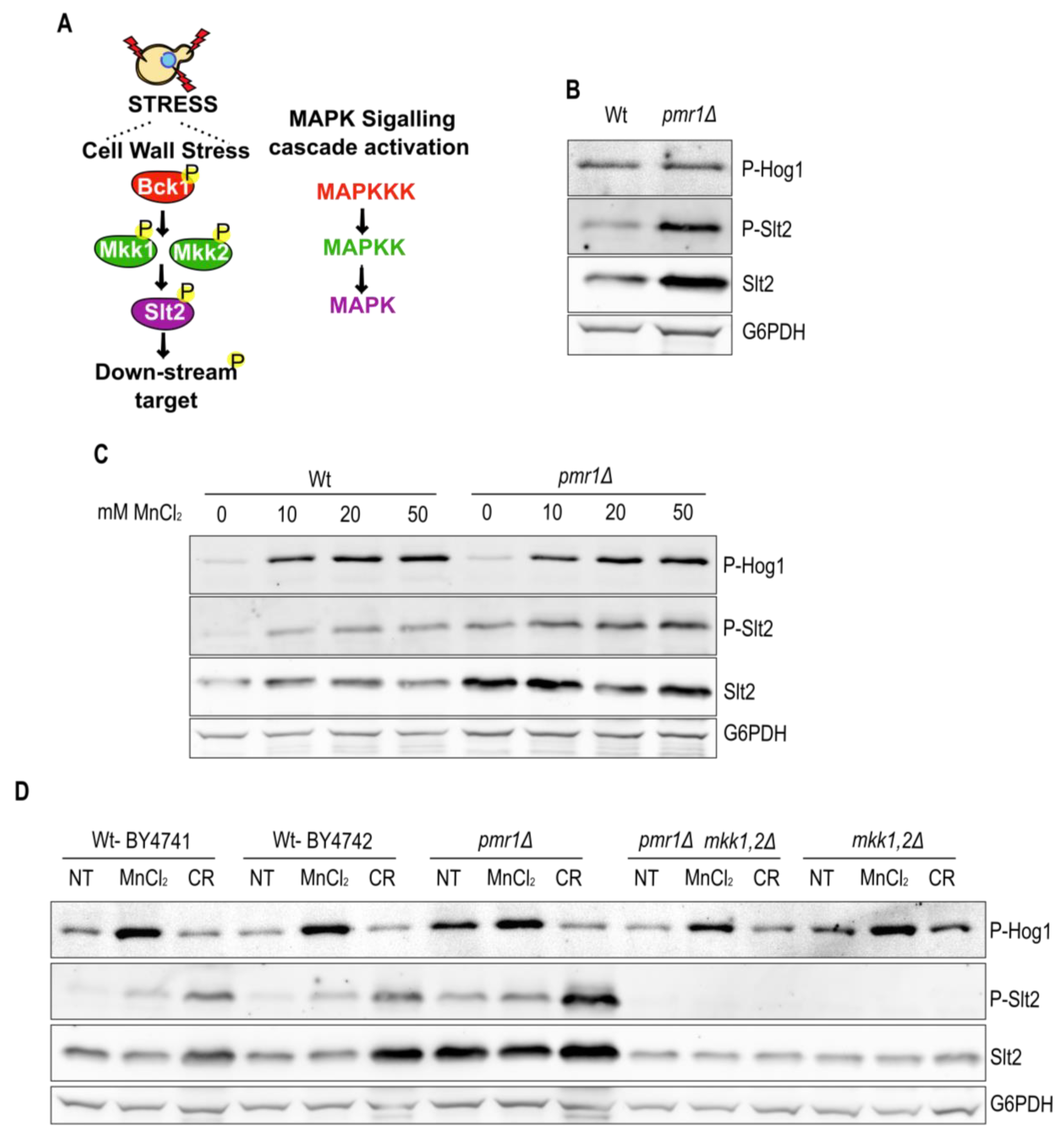
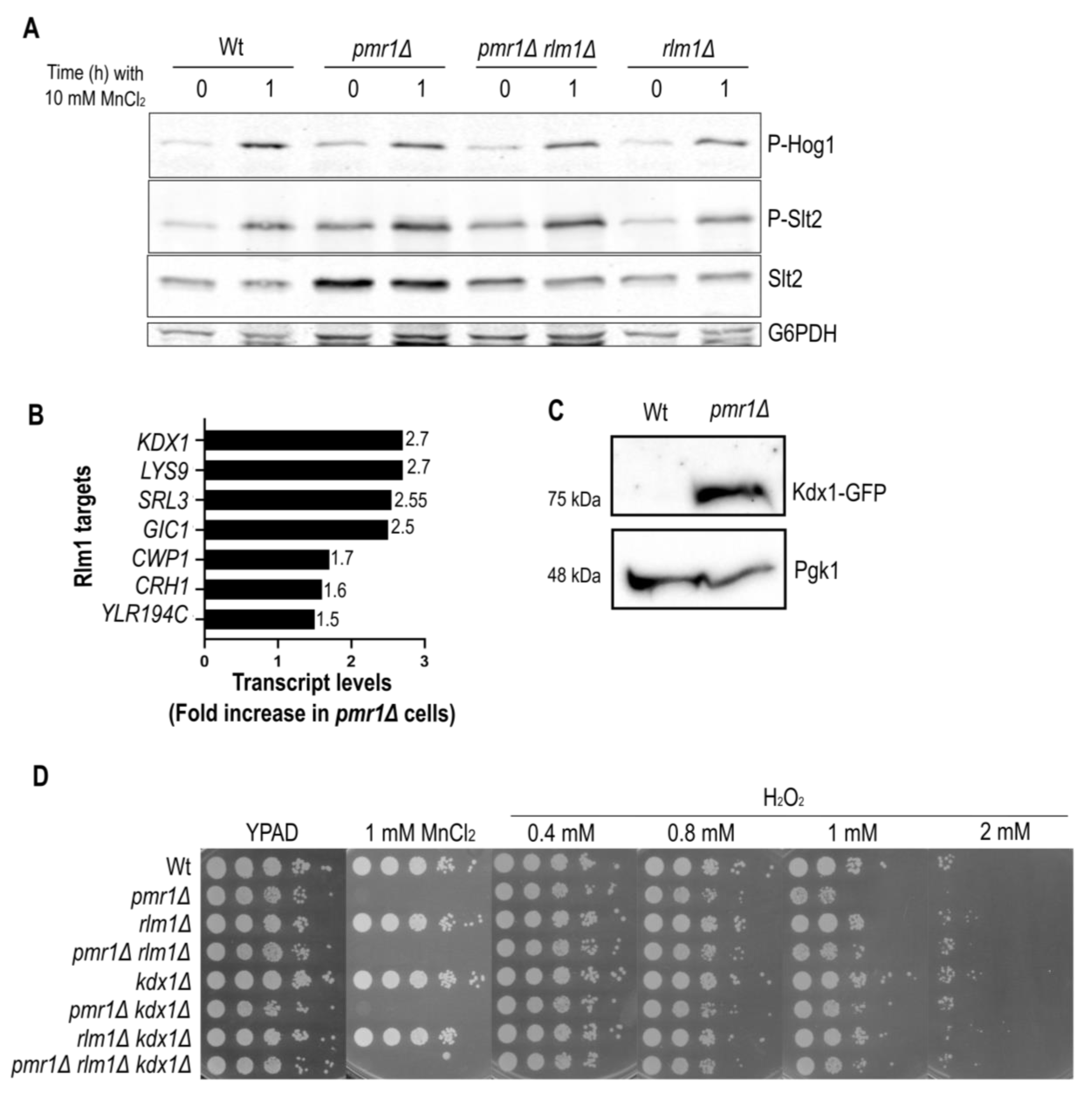
2.3. Pmr1 Depletion Leads to Constitutive Activation of the Slt2 MAP Kinase Pathway
2.4. The Slt2 Target Rlm1 Is Up-Regulated in Cells Lacking Pmr1
2.5. Slt2 Contributes to Oxidative Stress Resistance of pmr1Δ Mutants
3. Discussion
3.1. Yap1 Has a Hitherto Unknown Role in Manganese Stress Response
3.2. SAPKs Activation Is a Read-Out for Manganese Stress
3.3. Activation of the CWI Stress Response in the Absence of Yap1
3.4. Concluding Remarks and Perspectives
4. Materials and Methods
4.1. Yeast Strains, Plasmids, and Growth Conditions
4.2. Drug Sensitivity Assays
4.3. Yap1-GFP Localization
4.4. Preparation of Yeast Extracts and Immunoblot Analysis
4.5. Microarray Analysis
4.6. ROS Detection Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, T.V.; Schettinger, M.R.C.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Muend, S.; Zhang, Y.; Rao, R. A phenomics approach in yeast links proton and calcium pump function in the Golgi. Mol. Biol. Cell 2007, 18, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, R. Hailey–Hailey disease as an orthodisease of PMR1 deficiency in Saccharomyces cerevisiae. FEBS Lett. 2005, 579, 2021–2025. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Healy, E.; Strachan, T.; Sudbrak, R.; Perrussel, M.; Lehrach, H.; Munro, C.; Burge, S.; Hovnanian, A.; Monaco, A. Hailey-Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum. Mol. Genet. 2000, 9, 1131–1140. [Google Scholar]
- Ando, A.; Nakamura, T.; Murata, Y.; Takagi, H.; Shima, J. Identification and classification of genes required for tolerance to freeze–thaw stress revealed by genome-wide screening of Saccharomyces cerevisiae deletion strains. FEMS Yeast Res. 2007, 7, 244–253. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Cunningham, K.W.; Liu, X.F.; Fink, G.R.; Culotta, V.C. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 1995, 15, 1382–1388. [Google Scholar] [CrossRef]
- Nadal Clanchet, E.D.; Posas Garriga, F. Osmostress-induced gene expression--a model to understand how stress-activated protein kinases (SAPKs) regulate transcription. FEBS J. 2015, 282, 3275–3285. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Duch, A.; De Nadal, E.; Posas, F. The p38 and Hog1 SAPKs control cell cycle progression in response to environmental stresses. FEBS Lett. 2012, 586, 2925–2931. [Google Scholar] [CrossRef]
- Yan, C.; Luo, H.; Lee, J.D.; Abe, J.; Berk, B.C. Molecular cloning of mouse ERK5/BMK1 splice variants and characterization of ERK5 functional domains. J. Biol. Chem. 2001, 276, 10870–10878. [Google Scholar] [CrossRef]
- Duch, A.; Felipe-Abrio, I.; Barroso, S.; Yaakov, G.; García-Rubio, M.; Aguilera, A.; de Nadal, E.; Posas, F. Coordinated control of replication and transcription by a SAPK protects genomic integrity. Nature 2013, 493, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, M.; Jaquenoud, M.; De Virgilio, C. TORC1 controls G1–S cell cycle transition in yeast via Mpk1 and the greatwall kinase pathway. Nat. Commun. 2015, 6, 8256. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Pousada, C.; Devaux, F.; Caetano, S.M.; Pimentel, C.; da Silva, S.; Cordeiro, A.C.; Amaral, C. Yeast AP-1 like transcription factors (Yap) and stress response. Microb. Cell 2019, 6, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Jeong, S.-G.; Jin, S.H.; Mishra, R.; Peter, M.; Lee, C.-S.; Lee, S.S. Quantitative analysis of yeast MAPK signaling networks and crosstalk using a microfluidic device. Lab Chip 2020, 20, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.-i.; Izawa, S.; Inoue, Y. The Yap1p-dependent induction of glutathione synthesis in heat shock response of Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 15535–15540. [Google Scholar] [CrossRef]
- Wu, A.-L.; Moye-Rowley, W.S. GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol. Cell. Biol. 1994, 14, 5832–5839. [Google Scholar] [PubMed]
- Garcia-Rodriguez, N.; Diaz de la Loza, M.C.; Andreson, B.; Monje-Casas, F.; Rothstein, R.; Wellinger, R.E. Impaired manganese metabolism causes mitotic misregulation. J. Biol. Chem. 2012, 287, 18717–18729. [Google Scholar] [CrossRef]
- Bolton, E.C.; Mildvan, A.S.; Boeke, J.D. Inhibition of reverse transcription in vivo by elevated manganese ion concentration. Mol. Cell 2002, 9, 879–889. [Google Scholar] [CrossRef]
- Cialfi, S.; Le Pera, L.; De Blasio, C.; Mariano, G.; Palermo, R.; Zonfrilli, A.; Uccelletti, D.; Palleschi, C.; Biolcati, G.; Barbieri, L.; et al. The loss of ATP2C1 impairs the DNA damage response and induces altered skin homeostasis: Consequences for epidermal biology in Hailey-Hailey disease. Sci. Rep. 2016, 6, 31567. [Google Scholar] [CrossRef]
- Ficociello, G.; Zanni, E.; Cialfi, S.; Aurizi, C.; Biolcati, G.; Palleschi, C.; Talora, C.; Uccelletti, D. Glutathione S-transferase ϴ-subunit as a phenotypic suppressor of pmr1Δ strain, the Kluyveromyces lactis model for Hailey-Hailey disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2650–2657. [Google Scholar] [CrossRef]
- Moye-Rowley, W.; Harshman, K.; Parker, C. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989, 3, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Proft, M.; Mas, G.; de Nadal, E.; Vendrell, A.; Noriega, N.; Struhl, K.; Posas, F. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol. Cell 2006, 23, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Krasley, E.; Cooper, K.F.; Mallory, M.J.; Dunbrack, R.; Strich, R. Regulation of the oxidative stress response through Slt2p-dependent destruction of cyclin C in Saccharomyces cerevisiae. Genetics 2006, 172, 1477–1486. [Google Scholar] [CrossRef]
- Neklesa, T.K.; Davis, R.W. Superoxide anions regulate TORC1 and its ability to bind Fpr1: Rapamycin complex. Proc. Natl. Acad. Sci. USA 2008, 105, 15166–15171. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.T.; Epping, E.A.; Steggerda, S.M.; Moye-Rowley, W.S. Yap1p activates gene transcription in an oxidant-specific fashion. Mol. Cell. Biol. 1999, 19, 8302–8313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. Yap1: A DNA damage responder in Saccharomyces cerevisiae. Mech. Ageing Dev. 2012, 133, 147–156. [Google Scholar] [CrossRef]
- Delaunay, A.; Isnard, A.-D.; Toledano, M.B. H2O2 sensing through oxidation of the Yap1 transcription factor. EMBO J. 2000, 19, 5157–5166. [Google Scholar] [CrossRef]
- Yokoyama, H.; Mizunuma, M.; Okamoto, M.; Yamamoto, J.; Hirata, D.; Miyakawa, T. Involvement of calcineurin-dependent degradation of Yap1p in Ca2+-induced G2 cell-cycle regulation in Saccharomyces cerevisiae. EMBO Rep. 2006, 7, 519–524. [Google Scholar] [CrossRef]
- Cyert, M.S.; Philpott, C.C. Regulation of cation balance in Saccharomyces cerevisiae. Genetics 2013, 193, 677–713. [Google Scholar] [CrossRef]
- García, R.; Sanz, A.B.; Rodríguez-Peña, J.M.; Nombela, C.; Arroyo, J. Rlm1 mediates positive autoregulatory transcriptional feedback that is essential for Slt2-dependent gene expression. J. Cell Sci. 2016, 129, 1649–1660. [Google Scholar] [CrossRef]
- Chang, M.; Kang, H.-J.; Baek, I.-J.; Kang, C.-M.; Park, Y.-S.; Yun, C.-W. Kdx1 regulates RCK1 gene expression by interacting with Rlm1 in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2013, 435, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Kang, H.-J.; Baek, I.-J.; Kang, C.-M.; Park, Y.-S.; Yun, C.-W. Rck1 up-regulates Hog1 activity by down-regulating Slt2 activity in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2013, 440, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Pujol, N.; Petkova, M.I.; Serrano, L.; Torre-Ruiz, M.A.D.L. The MAP kinase Slt2 is involved in vacuolar function and actin remodeling in Saccharomyces cerevisiae mutants affected by endogenous oxidative stress. Appl. Environ. Microbiol. 2013, 79, 6459–6471. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.; Roy, J.; Bodenmiller, B.; Wanka, S.; Landry, C.R.; Aebersold, R.; Cyert, M.S. The calcineurin signaling network evolves via conserved kinase-phosphatase modules that transcend substrate identity. Mol. Cell 2014, 55, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.P.; Lennon, P.J.; Neumann, W.L.; Weiss, R.H. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalytic activity of macrocyclic manganese (II) complexes. J. Am. Chem. Soc. 1997, 119, 6522–6528. [Google Scholar] [CrossRef]
- Delaunay, A.; Pflieger, D.; Barrault, M.-B.; Vinh, J.; Toledano, M.B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 2002, 111, 471–481. [Google Scholar] [CrossRef]
- Okazaki, S.; Tachibana, T.; Naganuma, A.; Mano, N.; Kuge, S. Multistep disulfide bond formation in Yap1 is required for sensing and transduction of H2O2 stress signal. Mol. Cell 2007, 27, 675–688. [Google Scholar] [CrossRef]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 system: An evolutionary journey through stressful space and time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef]
- Gulshan, K.; Thommandru, B.; Moye-Rowley, W.S. Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J. Biol. Chem. 2012, 287, 26796–26805. [Google Scholar] [CrossRef]
- Luo, X.; Li, S.; Lu, L.; Liu, B.; Kuang, X.; Shao, G.; Yu, S. Gene expression of manganese-containing superoxide dismutase as a biomarker of manganese bioavailability for manganese sources in broilers. Poult. Sci. 2007, 86, 888–894. [Google Scholar] [CrossRef]
- Vilela, C.; Linz, B.; Rodrigues-Pousada, C.; McCarthy, J.E. The yeast transcription factor genes YAP1 and YAP2 are subject to differential control at the levels of both translation and mRNA stability. Nucleic Acids Res. 1998, 26, 1150–1159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, B.L.; Jurado, L.A.; Hengge, A.C. Comparison of the reaction progress of calcineurin with Mn2+ and Mg2+. Biochemistry 1999, 38, 3386–3392. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, R.; Gaillard, H.; Zarzuela, L.; Péli-Gulli, M.-P.; Fernández-García, E.; Tomé, M.; García-Rodríguez, N.; Durán, R.V.; De Virgilio, C.; Wellinger, R.E. Manganese is a physiologically relevant TORC1 activator in yeast and mammals. elife 2022, 11, e80497. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Li, H.; Hogan, P.G.; Cyert, M.S. A conserved docking site modulates substrate affinity for calcineurin, signaling output, and in vivo function. Mol. Cell 2007, 25, 889–901. [Google Scholar] [CrossRef][Green Version]
- Ferreira, R.T.; Silva, A.R.C.; Pimentel, C.; Batista-Nascimento, L.; Rodrigues-Pousada, C.; Menezes, R.A. Arsenic stress elicits cytosolic Ca2+ bursts and Crz1 activation in Saccharomyces cerevisiae. Microbiology 2012, 158, 2293–2302. [Google Scholar] [CrossRef]
- García-Rodríguez, N.; Manzano-López, J.; Muñoz-Bravo, M.; Fernández-García, E.; Muñiz, M.; Wellinger, R.E. Manganese redistribution by calcium-stimulated vesicle trafficking bypasses the need for P-type ATPase function. J. Biol. Chem. 2015, 290, 9335–9347. [Google Scholar] [CrossRef]
- Durr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Truman, A.W.; Levin, D.E. Yeast Mpk1 MAPK activates transcription through Swi4/Swi6 by a non-catalytic mechanism that requires upstream signal. Mol. Cell. Biol. 2008, 28, 2579–2589. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Levin, D.E. Mpk1 MAPK association with the Paf1 complex blocks Sen1-mediated premature transcription termination. Cell 2011, 144, 745–756. [Google Scholar] [CrossRef]
- Yurko, N.; Liu, X.; Yamazaki, T.; Hoque, M.; Tian, B.; Manley, J.L. MPK1/SLT2 links multiple stress responses with gene expression in budding yeast by phosphorylating Tyr1 of the RNAP II CTD. Mol. Cell 2017, 68, 913–925.e3. [Google Scholar] [CrossRef]
- Liu, L.; Levin, D.E. Intracellular mechanism by which genotoxic stress activates yeast SAPK Mpk1. Mol. Biol. Cell 2018, 29, 2898–2909. [Google Scholar] [CrossRef] [PubMed]

| Strain | Genotype | Source |
|---|---|---|
| BY4741 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 | Euroscarf |
| BY4742 | MATα ura3Δ0 leu2Δ0 his3Δ0 lys2Δ0 | Euroscarf |
| YGL167C | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 pmr1Δ::kan | Euroscarf |
| YHR030C | BY4741 slt2Δ::kan | Euroscarf |
| YKL161C | BY4741 kdx1Δ::kan | Euroscarf |
| YML007W | BY4741 yap1Δ::kan | Euroscarf |
| YPL089C | BY4741 rlm1Δ::kan | Euroscarf |
| YKL190W | BY4741 cnb1Δ::kan | Euroscarf |
| RWY160 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 NUP84mCHERRY::hph YAP1GFP::nat | This study |
| RWY213 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 NUP84mCHERRY::hph YAP1GFP::nat pmr1Δ::kan | This study |
| yWH1464 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 | Woo-Hyun Chung |
| RWY258 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 pmr1Δ::kan | This study |
| RWY230 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 pmr1Δ::nat slt2Δ::kan | This study |
| RWY223 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 pmr1Δ::kan kdx1Δ::hph | This study |
| ASY003 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 pmr1Δ::nat rlm1Δ::kan | This study |
| YMJ29 | MATα ura3Δ0 his3Δ0 lys2Δ0 mkk1Δ::S.p.HIS5 mkk2Δ::kan | M. Molina |
| RWY219 | MATα ura3Δ0 his3Δ0 lys2Δ0 pmr1Δ::nat mkk1Δ::S.p.HIS5 mkk2Δ::kan | This study |
| RWY225 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 pmr1Δ::nat yap1Δ::kan | This study |
| RWY204 | MATα ura3Δ0 leu2Δ0 his3Δ0 lys2Δ0 KDX1GFP::nat | This study |
| RWY208 | MATα ura3Δ0 leu2Δ0 his3Δ0 lys2Δ0 KDX1GFP::nat pmr1Δ::kan | This study |
| RWY244 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 rlm1Δ::kan | This study |
| RWY246 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 rlm1Δ::kan pmr1Δ::nat | This study |
| RWY248 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 slt2Δ::kan | This study |
| RWY249 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 slt2Δ::kan pmr1Δ::kan | This study |
| RWY252 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 kdx1Δ::kan | This study |
| RWY253 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 kdx1Δ::kan pmr1Δ::nat | This study |
| RWY254 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 YAP1GFP::HIS3 rlm1Δ::kan kdx1Δ::hph | This study |
| RWY256 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 lys2Δ0 YAP1GFP::HIS3 pmr1Δ::nat rlm1Δ::kan kdx1Δ::hph | This study |
| RWY260 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 lys2Δ0 YAP1GFP::HIS3 rlm1Δ::kan slt2Δ::hph pmr1Δ::nat | This study |
| RWY278 | MATa ura3Δ0 leu2Δ0 his3Δ0 met15Δ0 lys2Δ0 YAP1GFP::HIS3 cnb1Δ::kan | This study |
| Plasmid | Description | Reference |
|---|---|---|
| pAG25-natMX4 | DEL-MARKER-SET nourseothricin (nat) | Euroscarf |
| pFA6a-kanMX4 | DEL-MARKER-SET geneticin G418 (kan) | Euroscarf |
| pAG32-hygMX4 | DEL-MARKER-SET hygromycin (hph) | Euroscarf |
| GFP tagging | pYM-GFP-natNT2 | Helle Ulrich |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oya, I.G.; Jiménez-Gutiérrez, E.; Gaillard, H.; Molina, M.; Martín, H.; Wellinger, R.E. Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases. Int. J. Mol. Sci. 2022, 23, 15706. https://doi.org/10.3390/ijms232415706
de Oya IG, Jiménez-Gutiérrez E, Gaillard H, Molina M, Martín H, Wellinger RE. Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases. International Journal of Molecular Sciences. 2022; 23(24):15706. https://doi.org/10.3390/ijms232415706
Chicago/Turabian Stylede Oya, Inés G., Elena Jiménez-Gutiérrez, Hélène Gaillard, María Molina, Humberto Martín, and Ralf Erik Wellinger. 2022. "Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases" International Journal of Molecular Sciences 23, no. 24: 15706. https://doi.org/10.3390/ijms232415706
APA Stylede Oya, I. G., Jiménez-Gutiérrez, E., Gaillard, H., Molina, M., Martín, H., & Wellinger, R. E. (2022). Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases. International Journal of Molecular Sciences, 23(24), 15706. https://doi.org/10.3390/ijms232415706









