Abstract
Auxin plays a critical role in organogenesis in plants. The classical auxin signaling pathway holds that auxin initiates downstream signal transduction by degrading Aux/IAA transcription repressors that interact with ARF transcription factors. In this study, 23 MoIAA genes were identified in the drumstick tree genome. All MoIAA genes were located within five subfamilies based on phylogenetic evolution analysis; the gene characteristics and promoter cis-elements were also analyzed. The protein interaction network between the MoIAAs with MoARFs was complex. The MoIAA gene family responded positively to NAA treatment, exhibiting different patterns and degrees, notably for MoIAA1, MoIAA7 and MoIAA13. The three genes expressed and functioned in the nucleus; only the intact encoding protein of MoIAA13 exhibited transcriptional activation activity. The shoot regeneration capacity in the 35S::MoIAA13-OE transgenic line was considerably lower than in the wild type. These results establish a foundation for further research on MoIAA gene function and provide useful information for improved tissue culture efficiency and molecular breeding of M. oleifera.
1. Introduction
Moringa oleifera Lam. is a fast-growing tree from southern Asia and has since spread throughout the world’s tropical and subtropical regions; it is commonly known as the drumstick tree [1]. M. oleifera has gained attention because of its rich nutritional composition [2], potential medicinal applications [3], and considerable industrial value [4]. In previous studies, we successfully established an induction system for drumstick trees using vitro young leaves of sterilized seedlings [5]. However, the induction rate is genotype-dependent. Furthermore, we found that hormones had a significant effect on drumstick tree shoot regeneration, especially auxin. A study of auxin early response genes has revealed that the Aux/IAA (Auxin/Indole-3-Acetic), ARF (Auxin Response Factor), GH3 (Gretchen Hagen 3), and SAUR (Small Auxin Up-regulated RNA) family genes are closely involved in the embryogenic transformation of upland cotton callus; Aux/IAAs, in particular, display a high level of expression [6]. Global transcriptomic analysis implied that Aux/IAA gene family is involved in the molecular regulatory network during drumstick tree shoot regeneration in our previous research [7].
The Aux/IAA family comprises auxin early response genes, responding to auxin levels rapidly and precisely to participate in cellular processes, such as cell differentiation and division [8]. As a repressor of auxin-inducible gene expression, the Aux/IAA family is pivotal in the auxin signaling pathway [9]. The increasing popularity of whole genome sequencing has led to Aux/IAA genes of several species being identified, including A. thaliana [10], O. sativa [11], P. trichocarpa [12] and E. grandis [13]. In the auxin signaling pathway, Aux/IAA proteins are a type of short-lived protein, repressing the activity of ARFs (auxin response factors) in the absence of auxin [14]. In contrast, at high levels of auxin, Aux/IAA proteins bind with SCFTIR1/AFB and subsequent degradation via the ubiquitination pathway, thus activating ARF proteins and inducing downstream gene expression [15]. Aux/IAA proteins engage in the spatial-temporal distribution of auxin by regulating the auxin signaling pathway [16].
Aux/IAA proteins usually have four highly conserved domains, designated I-IV, that deliver distinct functions [10]. Domain I consists of a LxLxLx motif that interacts with TPL (TOPLESS) and is responsible for transcriptional repression [17]. Domain II is a key region for protein degradation with a GWPPV motif that interacts with SCFTIR1/AFB [18]. Domains III and IV contain a Phox and Bem 1 (PB1 domain), which share homology with domains of ARFs, and thus are responsible for transcriptional repression between ARFs and other Aux/IAA proteins [19]. In recent years, molecular and genetic studies have uncovered the functions of Aux/IAA proteins [8]. These studies emphasized that Aux/IAA genes display diverse functions in developmental processes, including embryo development, root and shoot tropisms, and leaf patterns [20].
It has been confirmed that Aux/IAA proteins were involved in shoot regeneration by interacting with ARF proteins. IAA12 controls shoot regeneration in combination with ARF4 and ARF5 in Arabidopsis [21]. In this study, 23 Aux/IAA genes were identified and characterized in M. oleifera. The expression patterns of MoIAA genes were examined at three stages in shoot regeneration. Moreover, MoIAA13 functional roles were investigated for the first time in N. benthamiana. Our findings provide useful information to help to unravel the mechanisms of the function of Aux/IAA in shoot regeneration, and establish the foundation for improved tissue culture efficiency and molecular breeding for M. oleifera.
2. Results and Discussion
2.1. Genome-Wide Identification of the Aux/IAA Gene Family of M. oleifera
The Aux/IAA genes of Arabidopsis thaliana were used to search for Aux/IAA genes in the genomes of M. oleifera using TBtools [22]. Their deduced peptides were confirmed after domain analysis using Pfam and NCBI-CDD online search platforms. A total of 23 members were finally found and designated MoIAA1 to MoIAA23 (Table 1). Compared with A. thaliana (29 AtIAAs), O. sativa (31 OsIAAs) and P. trichocarpa (35 PopIAAs), the number of M. oleifera IAAs is low (23 MoIAAs), but the same as Prunus persica (23 PpIAAs) [23]. The amino acid sequences of MoIAAs were further analyzed. The amino acid length ranged from 135 aa to 506 aa in MoIAAs (Table 1). The MWs ranged from 14.69–54.62 kDa, and the pI varied from 4.67 to 9.54 (Table 1). It was predicted that all MoIAAs were located in the nucleus. The characterization of MoIAAs has similarities with other plants [24].

Table 1.
Molecular characteristics of MoIAA genes in M. oleifera.
2.2. Comprehensive Analysis of MoIAA Gene Structure
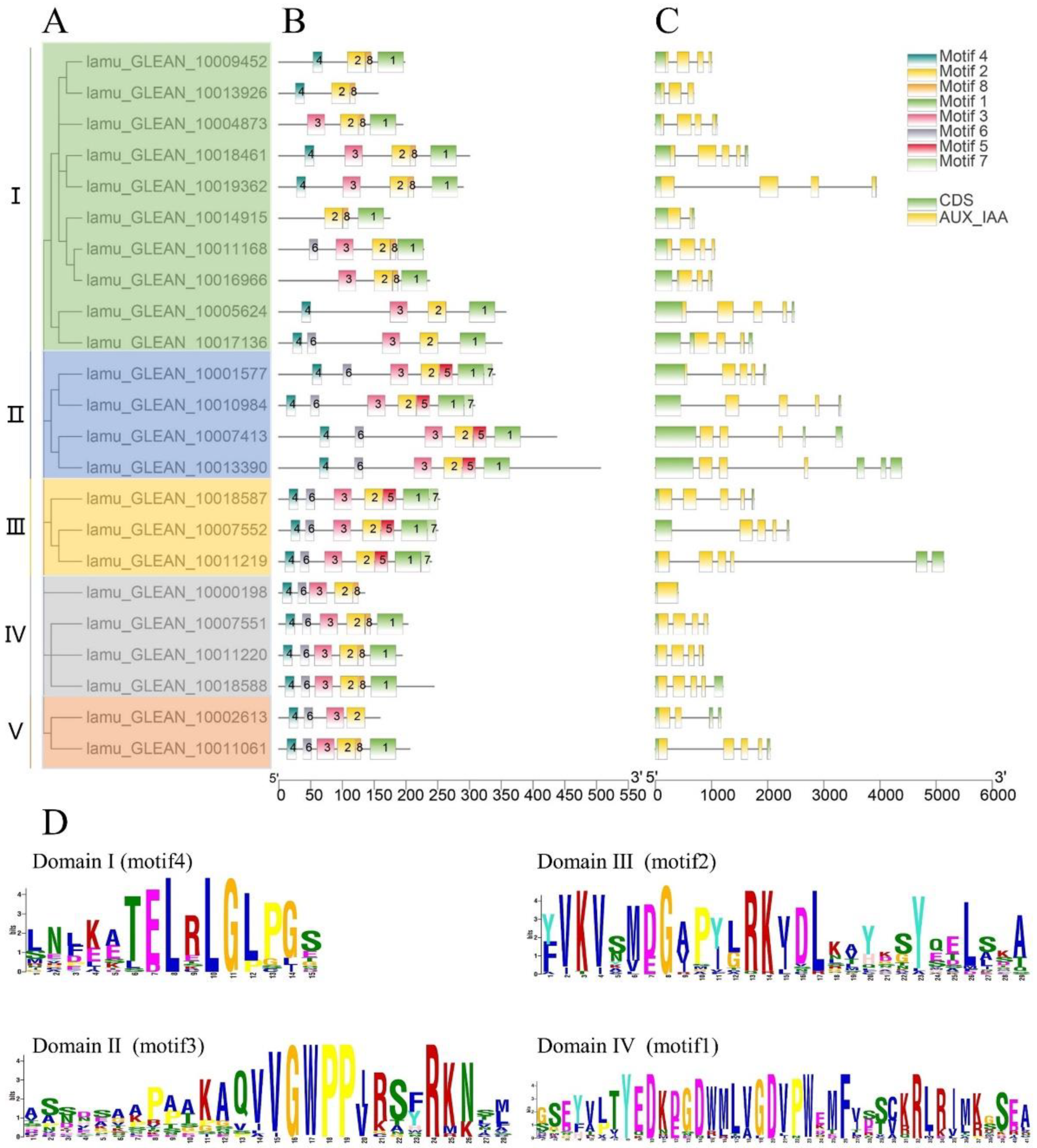
In a previous study, the phylogenetic analysis of 434 Aux/IAA genes in 17 plant species showed that the Aux/IAA proteins can be broadly categorized into five primary clades [18]. The 23 MoIAA’s protein sequences were also divided into five subfamilies (I, II, Ⅲ, IV and Ⅴ) by phylogenetic analysis (Figure 1A). We then used the alignment of genomic DNA with full-length cDNA of MoIAAs to explore structures of exons/introns. All of the MoIAA genes except MoIAA1 had introns (lamu_GLEAN_10000198). Most members had a typical gene structure pattern of five exons and four introns; only MoIAA17 (lamu_GLEAN_10014915) had one intron (Figure 1C). Meanwhile, the conserved motifs of MoIAAs were closely identified, and the MEME website found eight. The C-terminus was composed of motif 4, motif 6, motif 3, and motif 2, and the N-terminus consisted of motif 7, motif 1, motif 5 and motif 8. The C-terminus is conserved, and the N termini of Aux/IAA proteins interact with ubiquitin ligase receptors through motif-based interactions [25]. MoIAA proteins have four highly conserved domains, including domain I (motif 4), domain II (motif 3), domain Ⅲ (motif 2) and domain IV (motif 1) (Figure 1D). Recent studies have shown that deficiency in domains may lead to changes in protein properties, and the functions of individual domains in Aux/IAA proteins have been revealed [18]. Only 15 MoIAAs had four complete domains. Several motifs were common to all MoIAAs, such as motif 2 (domain Ⅲ). Domain III was the PB1 domain, which is a dimerization region involved with other PB1 domain proteins, such as ARFs [26]. In addition, members of the same subfamily showed generally similar gene structures, which suggested they were highly conserved (Figure 1B).
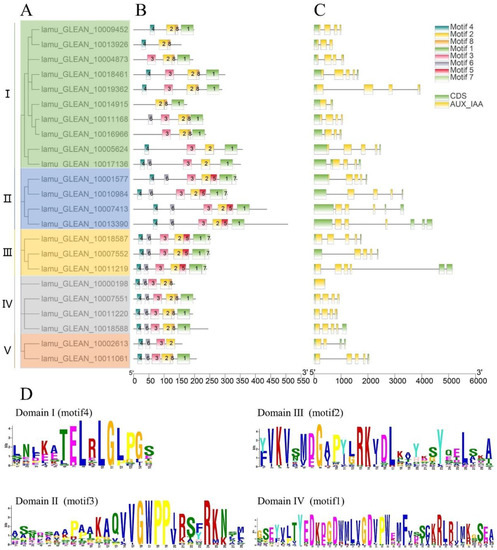
Figure 1.
Gene structure and motif analysis of MoIAA proteins from M. oleifera (A). The phylogenetic tree of all MoIAA proteins (B). The motifs in the MoIAA proteins (C). The exon–intron structure distribution of MoIAAs (D). The abscissa of sequence logos refers to the amino acid with the highest frequency, and the ordinate represents the relative frequency of the corresponding amino acid.
Furthermore, we undertook a multiple alignment analysis of the 23 MoIAA protein sequences in the drumstick tree. Most of the MoIAAs were found to contain nuclear localization signals (NLSs), indicating that the nuclear localization signal in Aux/IAA proteins is conserved, and Aux/IAAs are probably localized in the nucleus [24]. The motif (one β sheet and two α helices) acting in Aux/IAA dimerization was also found in domain III of most MoIAAs. Meanwhile, some members were missing domains; for example, MoIAA1 and MoIAA3 were without domain IV (VKVxMxG), and MoIAA17 and MoIAA18 were without domain I (LxLxL). A similar pattern of results was obtained in different plant species; for example, the Aux/IAA proteins in tomato (SlIAA13 SlIAA16 and SlIAA20) and in peach (PpIAA26 and PpIAA33) also lack domain I [27]. Meanwhile, there is a mutation of domain I in AtIAA7, AtIAA17 and AtIAA19, which partially reduces the inhibition of auxin-modulated transcription [28]. “V” was mutated to “I” in domain II (GWPPV) in some MoIAAs (Figure 2). In addition, MoIAA9, MoIAA16 and MoIAA17 were without domain II, implying that these proteins may not be quickly degraded at high auxin levels.

Figure 2.
Multiple sequence alignment of conserved domains in MoIAAs’ proteins.
2.3. Phylogenetic and Evolution Analysis of MoIAAs
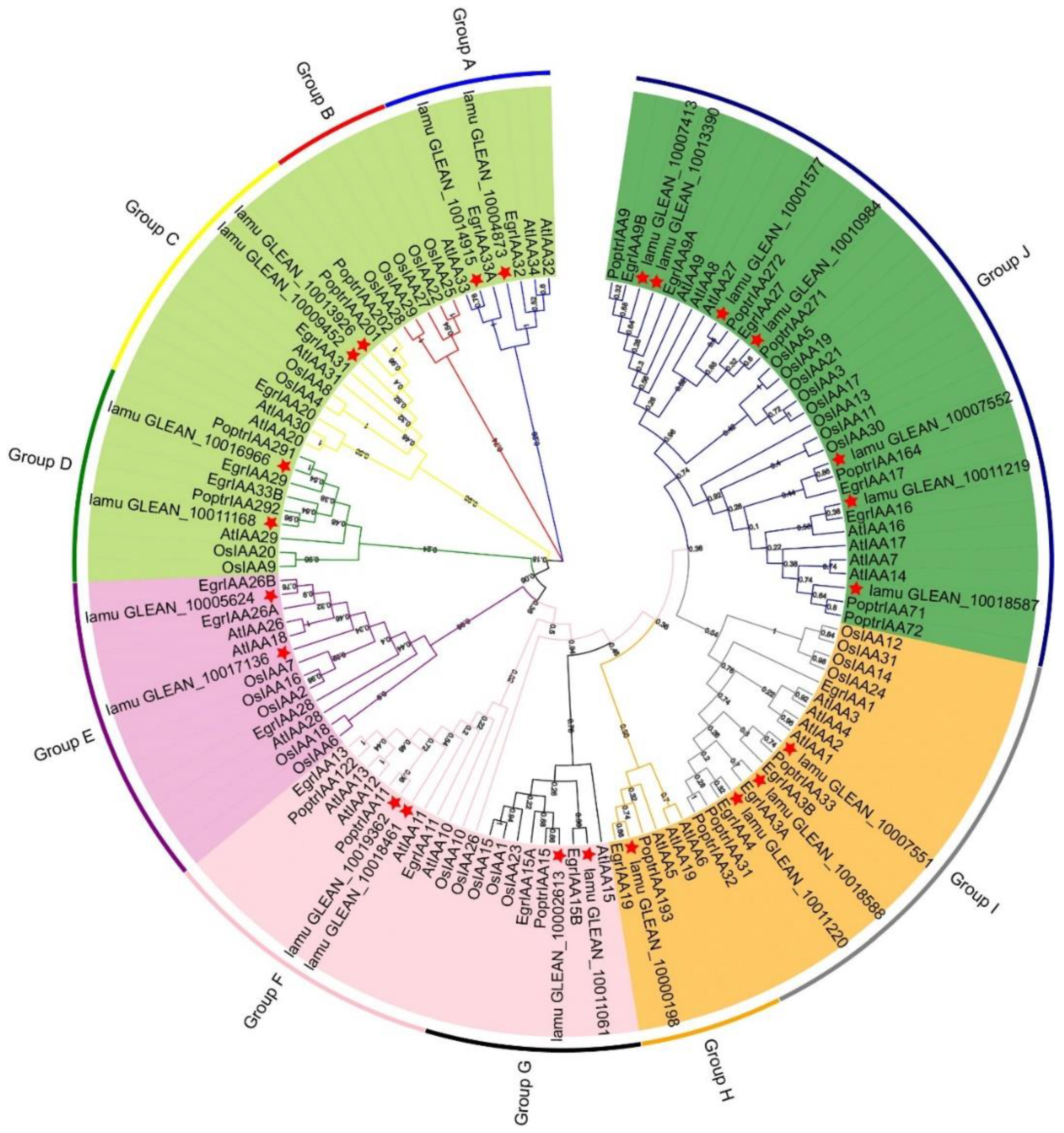
Previous studies have revealed that phylogenetic analysis can help elucidate evolutionary relationships as well as predict the potential functions of various genes [29]. Here, all Aux/IAA proteins in M. oleifera (23 MoIAAs), A. thaliana (29 AtIAAs), O. sativa (31 OsIAAs), P. trichocarpa (35 PoptrIAAs) and E. grandis (24 EgrIAAs) were compared using ClustalW. A phylogenetic tree was generated with the maximum likelihood method to reveal their evolutionary relationships (Figure 3). All the Aux/IAA protein sequences are listed in Supplemental Table S3. All the Aux/IAA proteins were classified into five subfamilies, including 10 groups (Groups A to J). This was similar to the phylogenetic analysis of the Eucalyptus Aux/IAA gene family, which is divided into 11 groups [13]. There were slightly fewer Aux/IAA proteins in M. oleifera—only 23. MoIAAs were present in all groups except B (Figure 3). The highest number of members were placed in group J, and these have four complete domains. Group H had only one M. oleifera member, MoIAA1 (lamu_GLEAN_10000198), which has a higher homology than EgrIAA19. MoIAA14 (lamu_GLEAN_10011220) has a close relationship with EgrIAA4, and a previous study revealed that overexpressing EgrlIAA4 in Arabidopsis impaired its growth and auxin-sensitive ability, suggesting that MoIAA14 may also impede the auxin response ability. MoIAA15 (lamu_GLEAN_10013390) has higher homology to EgrIAA9A and PoptrIAA9, implying MoIAA15 is also involved in wood formation [15].
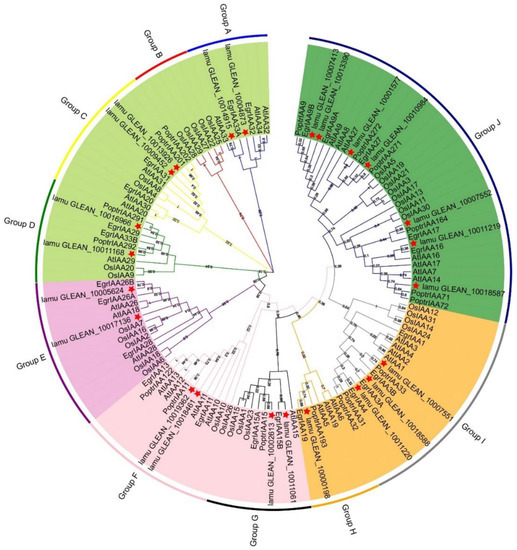
Figure 3.
Phylogenetic tree of the Aux/IAA proteins family for M. oleifera, A. thaliana, O. sativa, P. trichocarpa, and E. grandis. The phylogenetic tree was constructed with 1000 bootstrap replications in MEGA7.0. Five colors represent the five subfamilies, and a red star indicates MoIAAs.
Moreover, MoIAAs are in a different branch than OsIAAs, indicating that M. oleifera is not closely related to O. sativa (Figure 3). During plant evolution, gene expansion and duplication events often occur among members of the same gene family, which make gene functions diverse and specific [30]. It has been demonstrated that segmental and tandem duplications occur most frequently in plants, and these have played a crucial role in expanding gene families [31]. We detected only two pairs of tandemly duplicated genes in the 23 genes of the MoIAA gene family, namely MoIAA13-MoIAA14 and MoIAA21-MoIAA22. Most gene pairs were identified as segmentally duplicated genes; it appears that segmental duplication events were the main cause of the expansion of the Aux/IAA gene family in M. oleifera. The Ka/Ks ratios of duplicate gene pairs (Table 2) were all less than 0.5, suggesting that MoIAAs were under strong purifying selection. These basic results are consistent with a previous study reporting that MdAux/IAAs had undergone purifying selection [32], implying the Aux/IAA genes tended to lose mutations by purifying selection because they adapted to their current environment.

Table 2.
Analysis of Ka/Ks for the MoIAAs.
2.4. Promoter Cis-Element Analysis
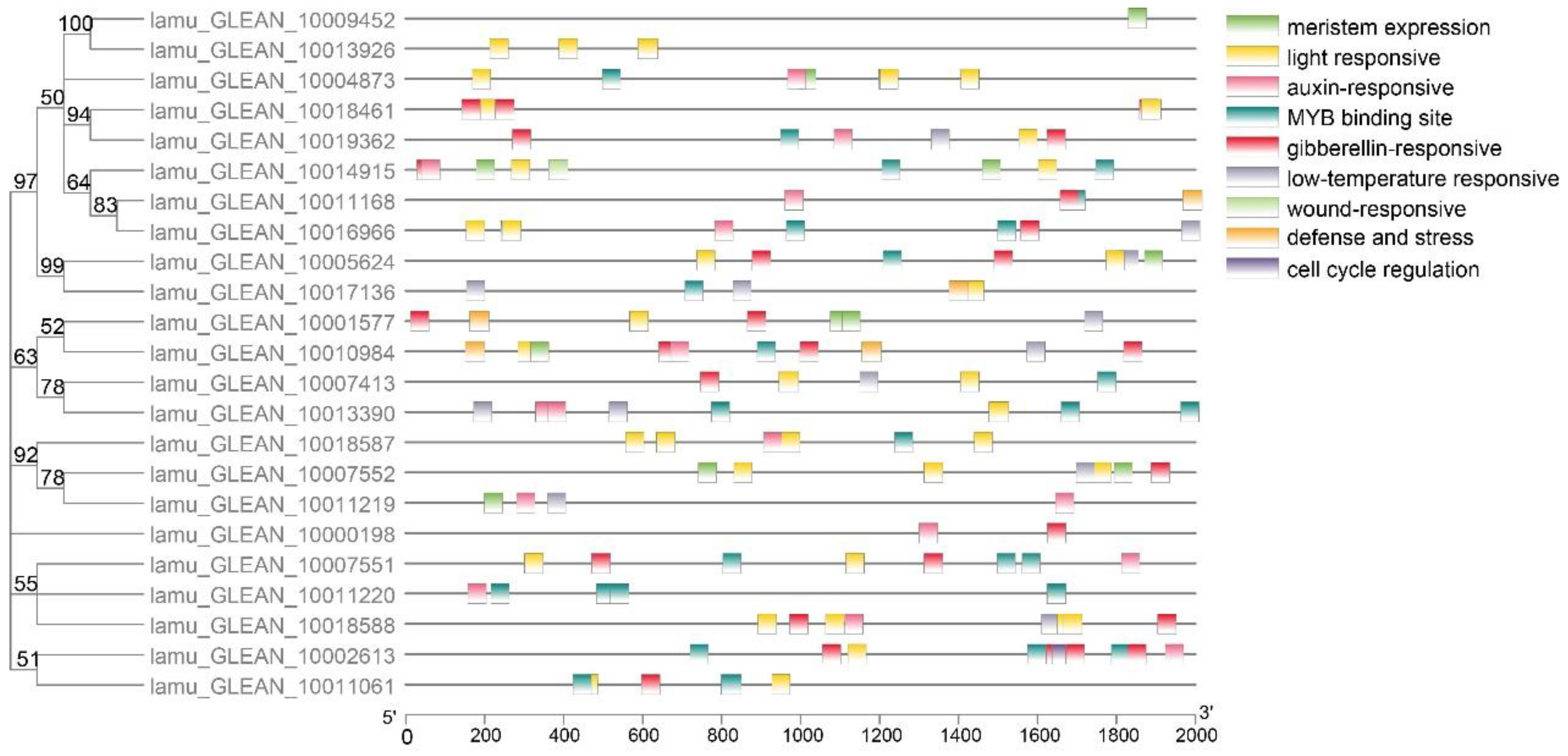
Several studies have indicated that promoters influence the temporal and spatial expression of genes, and cis-elements within promoters are key to regulating gene function through interplay with trans-acting factors [33]. Multiple Aux/IAA genes with promoter elements associated with abiotic stress and hormones have been found to participate in these abiotic stress responses [34]. For example, OsIAA20 and most of the MtIAA genes exhibited wide-ranging responses to salt stress, and more than half of the FveIAA genes responded to IAA treatment [35]. The promoter analysis of MoIAAs detected nine major cis-elements, including low-temperature-responsive, light-responsive and hormone-responsive elements (Figure 4). Auxin-responsive and light-responsive related elements were abundant. In addition, there were also more MYB binding sites, indicating that Aux/IAA genes may be related to MYB transcription factors and light-induced plant growth regulation. There is evidence that CpAux/IAA gene expression decreases when in vitro Carica papaya plantlets are exposed to high light intensity, even though no auxin has been added [36]. Notably, a wound-responsive element was identified in the MoIAA17 (lamu_GLEAN_10014915) gene promoter, which may be involved in the dedifferentiation process induced by wound signaling. Furthermore, meristem-expression and cell-cycle-regulation elements were also identified, suggesting that MoIAAs may be involved in regulating cell division.
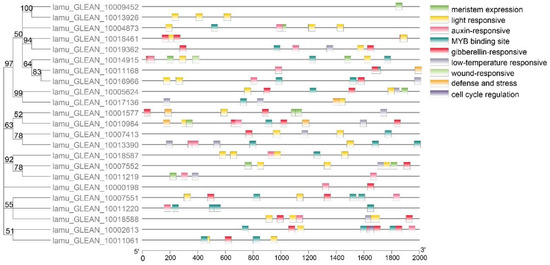
Figure 4.
Prediction and visualization of cis-elements in MoIAA promoters.
2.5. Predicted MoIAA and MoARF Protein Interaction Network
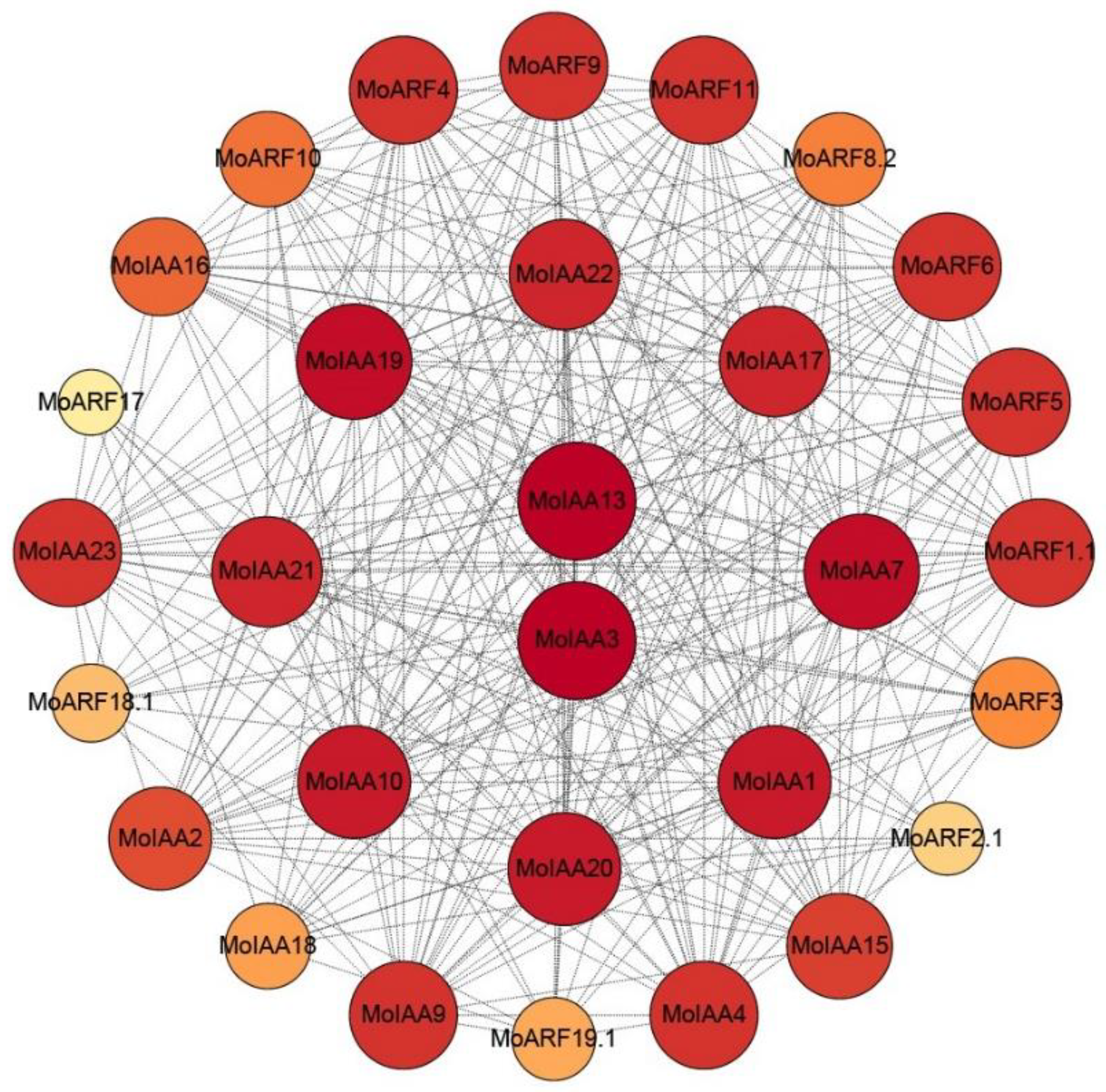
It has been found that the Aux/IAA proteins negatively regulate the ARF proteins, so there is a high potential for interaction between them. A total of 19 ARF members were identified in M. oleifera (Supplemental Table S4). Therefore, the protein–protein interaction network for the 23 MoIAA and 19 MoARF proteins was constructed using the STRING online tool [37]. The results indicated that 17 MoIAAs and 13 MoARFs interact closely (Figure 5). Generally, the interaction between MoIAAs and MoARFs was complex; some MoIAA proteins may interact with several MoARFs and vice versa, representing functional redundancy within the Aux/IAA proteins [38]. For example, MtIAA12 and MtIAA21 interacted with MtARF29, which also interacts with most of the MtARFs [34]. Notably, MoIAA3 and MoIAA13 proteins were at the center of the network, suggesting that they interact strongly with other proteins, while MoARF2.1 and MoARF17 exhibit the least interaction with others. Therefore, MoIAA3 and MoIAA13 may be the key proteins in regulating the auxin signaling pathway by interacting with other MoIAA and MoARF proteins.
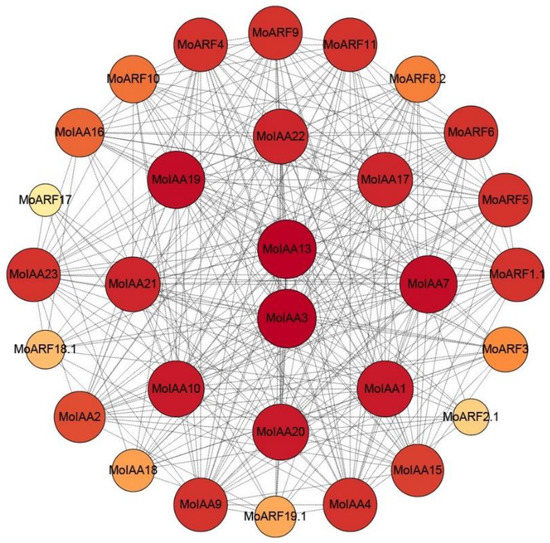
Figure 5.
Protein–protein interaction network for the MoIAA and MoARF gene family; the network contains 30 nodes, and darker colors and larger nodes represent more interaction with others.
2.6. Expression Analysis of MoIAA Genes in Response to NAA Treatment
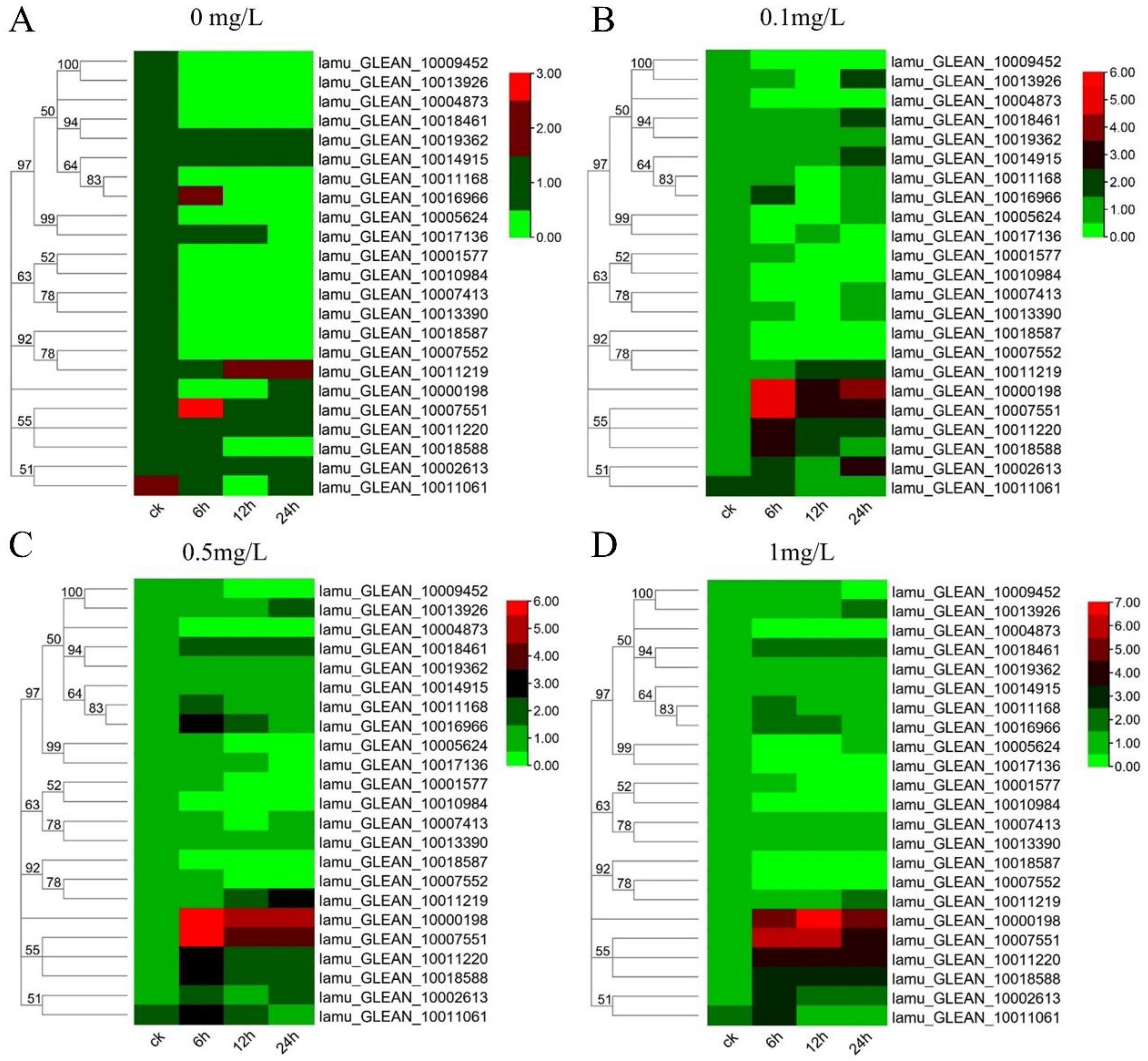
Auxin is essential for callus formation in isolated tissues of plants. As a primary auxin-responsive gene family, Aux/IAA genes respond quickly to auxin treatment to regulate downstream genes [39]. Previous studies have focused on tissue expression patterns and plant development of Aux/IAA genes [40]. In this study, to understand how the MoIAAs respond to NAA treatment in callus induction and shoot regeneration of M. oleifera, the leaf explants were inoculated with different NAA concentrations. The leaves were then collected after 0 h, 6 h, 12 h, and 24 h to study all MoIAA gene expression patterns using qRT-PCR. When compared to the expression of these genes in untreated controls, we discovered that NAA stimulated most MoIAA genes. However, NAA had little effect on the gene expression of MoIAA4 (lamu_GLEAN_10004873) and MoIAA21 (lamu_GLEAN_10018587), which remained steady (Figure 6). With the rise in NAA concentration, the expression of some members increased across all time points, while the expression levels of almost all members remained constant over time with 0 mg/L NAA (Supplemental Figure S1). Of the entire MoIAA gene family, MoIAA1 (lamu_GLEAN_10000198) and MoIAA7 (lamu_GLEAN_10007551) have the highest expression, responding strongly to NAA (Figure 6). MoIAA1 expression was dramatically upregulated after NAA treatment, increasing nearly 50-fold in just 6 h.
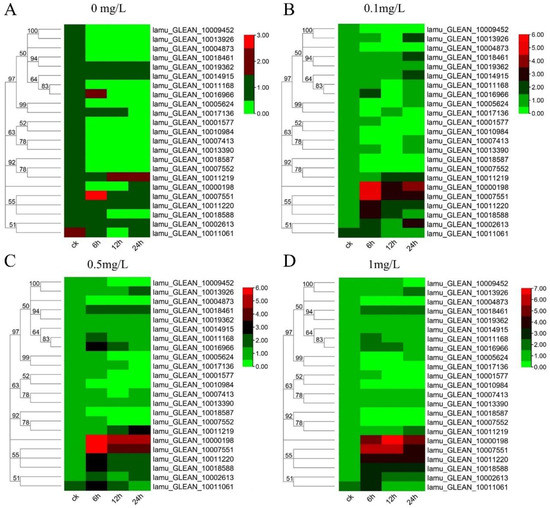
Figure 6.
Heatmap of MoIAA gene expression level under 0 mg/L (A), 0.1 mg/L (B), 0.5mg/L (C), 1.0 mg/L (D) NAA treatment. The scale represents the logarithm of the value of gene expression.
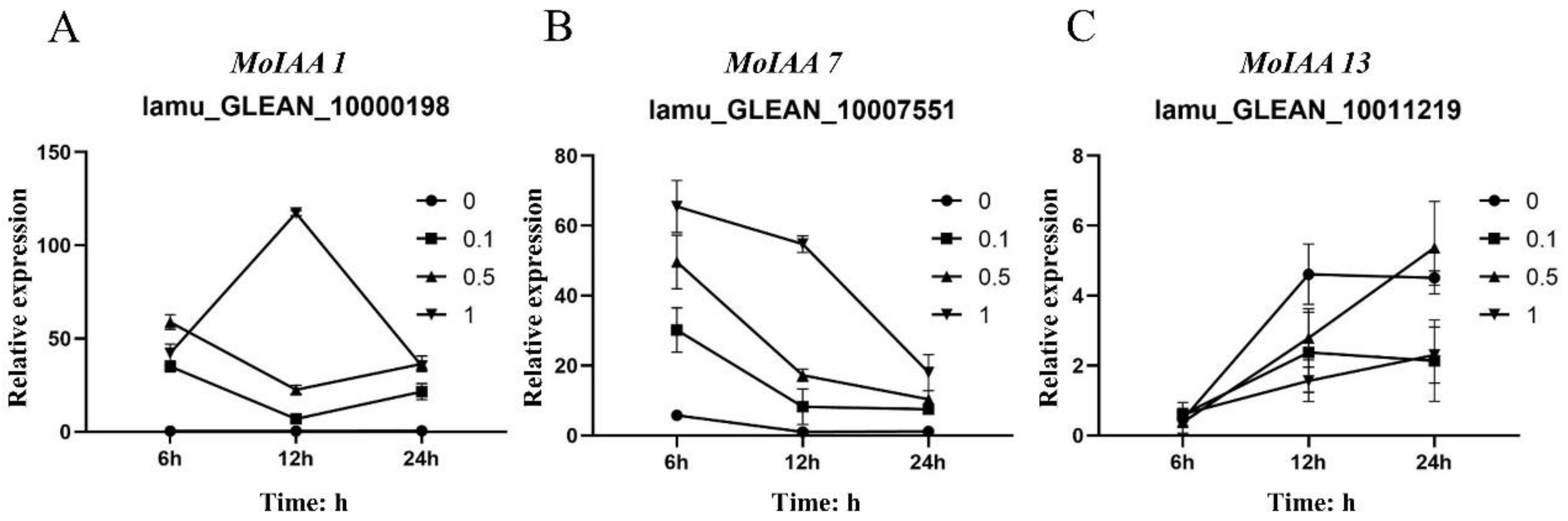
Moreover, MoIAA7 expression reached its highest value after 6 h of NAA treatment, then continued to decline, and the expression level had a corresponding relationship with NAA concentration. In contrast, the expression of MoIAA13 (lamu_GLEAN_10011219) increased with increasing treatment time (Figure 7). IAA11 and IAA14 gene expression exhibits a general downward trend throughout the embryogenic callus induction process in Tamarillo, while IAA17 expression appears greater than that in the initial explant [41]. Other research has revealed that shoot regeneration ability was hampered by increased IAA12 expression [21]. These results showed that the MoIAA gene family responds positively to NAA treatment, exhibiting different patterns and to different degrees, indicating that MoIAAs are involved in the regulation of adventitious shoot regeneration.
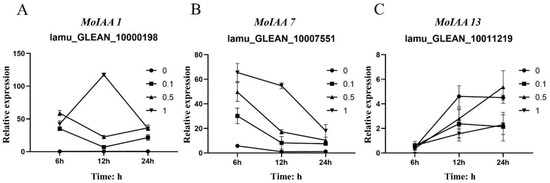
Figure 7.
Relative expression level of MoIAA1 (A), MoIAA7 (B) and MoIAA13 (C) under NAA treatment at 6, 12, and 24 h. The expression value was calculated relative to 0 h.
2.7. Subcellular Location of MoIAA1, MoIAA7 and MoIAA13 Proteins
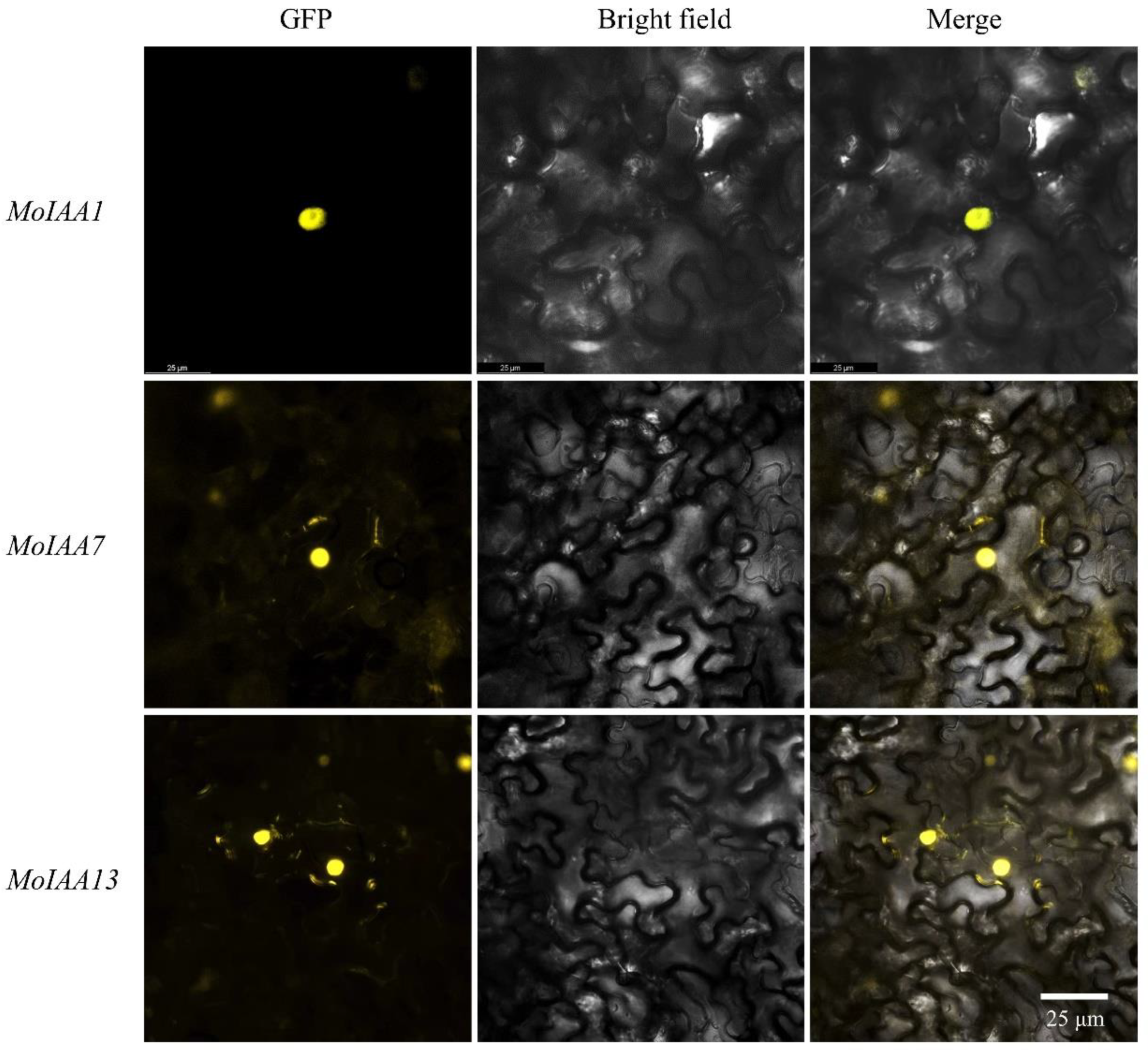
Aux/IAA proteins act as transcription factors in the nucleus [42]. Full-length coding sequences from MoIAA1, MoIAA7 and MoIAA13 genes were cloned to validate the protein subcellular location prediction results. The three MoIAA genes without a stop codon were then cloned into the pEarleyGate101 vector with the YFP protein (Supplemental Figure S2). The results of subcellular localization revealed that YFP fused separately with these three MoIAA proteins are expressed in the nucleus (Figure 8), proving that the MoIAA1, MoIAA7 and MoIAA13 genes are functional in the nucleus.
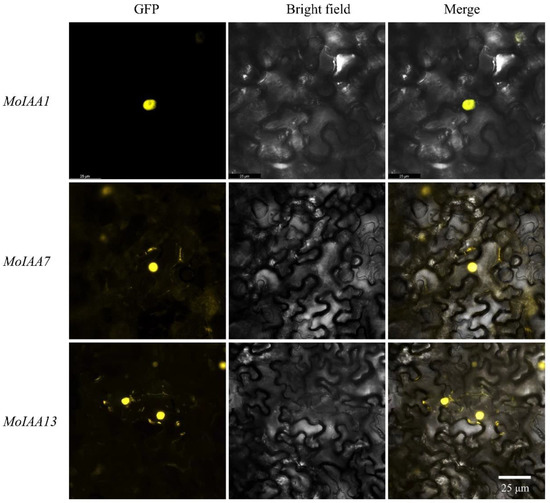
Figure 8.
Subcellular localization of the MoIAA1, MoIAA7 and MoIAA13 proteins in N. benthamiana leaves.
2.8. Validation of MoIAA1, MoIAA7 and MoIAA13 Transcriptional Activation Activity
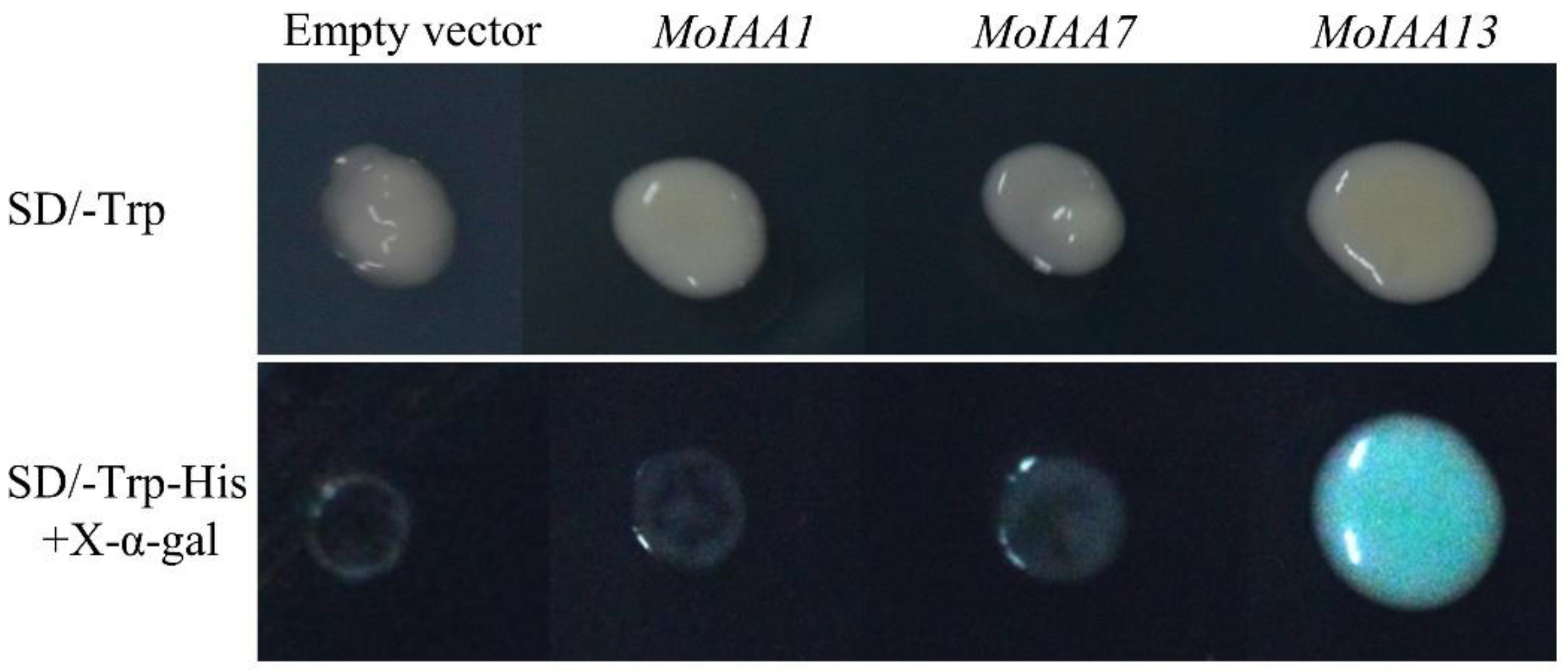
To verify the transcriptional activation activity of the MoIAA1, MoIAA7 and MoIAA13 encoding proteins, the CDSs of the three genes were ligated into the pGBKT7 vector. The vectors were transformed into yeast cells using the PEG/LiAc-mediated transformation method [43]. The results showed that the yeasts transformed with the empty vector, and the recombinant vector grew normally on SD/-Trp solid medium. However, the yeasts transformed with pGBKT7 (empty vector), pGBKT7-MoIAA1, and pGBKT7-MoIAA7 do not grow properly on the medium with SD/-Trp-His and X-α-gal, while the yeast transformed with the pGBKT7-MoIAA13 grows normally and turns blue (Figure 9). It was demonstrated that only the intact MoIAA13 encoding protein exhibits transcriptional activation activity in these three genes.
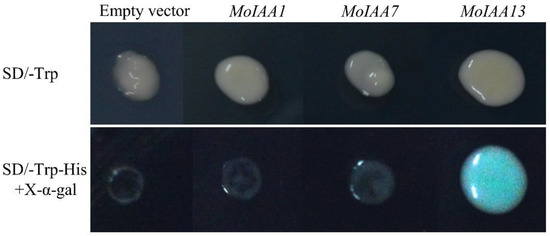
Figure 9.
Analyses of transcriptional activation activity of the MoIAA1, MoIAA7 and MoIAA13 encoding proteins.
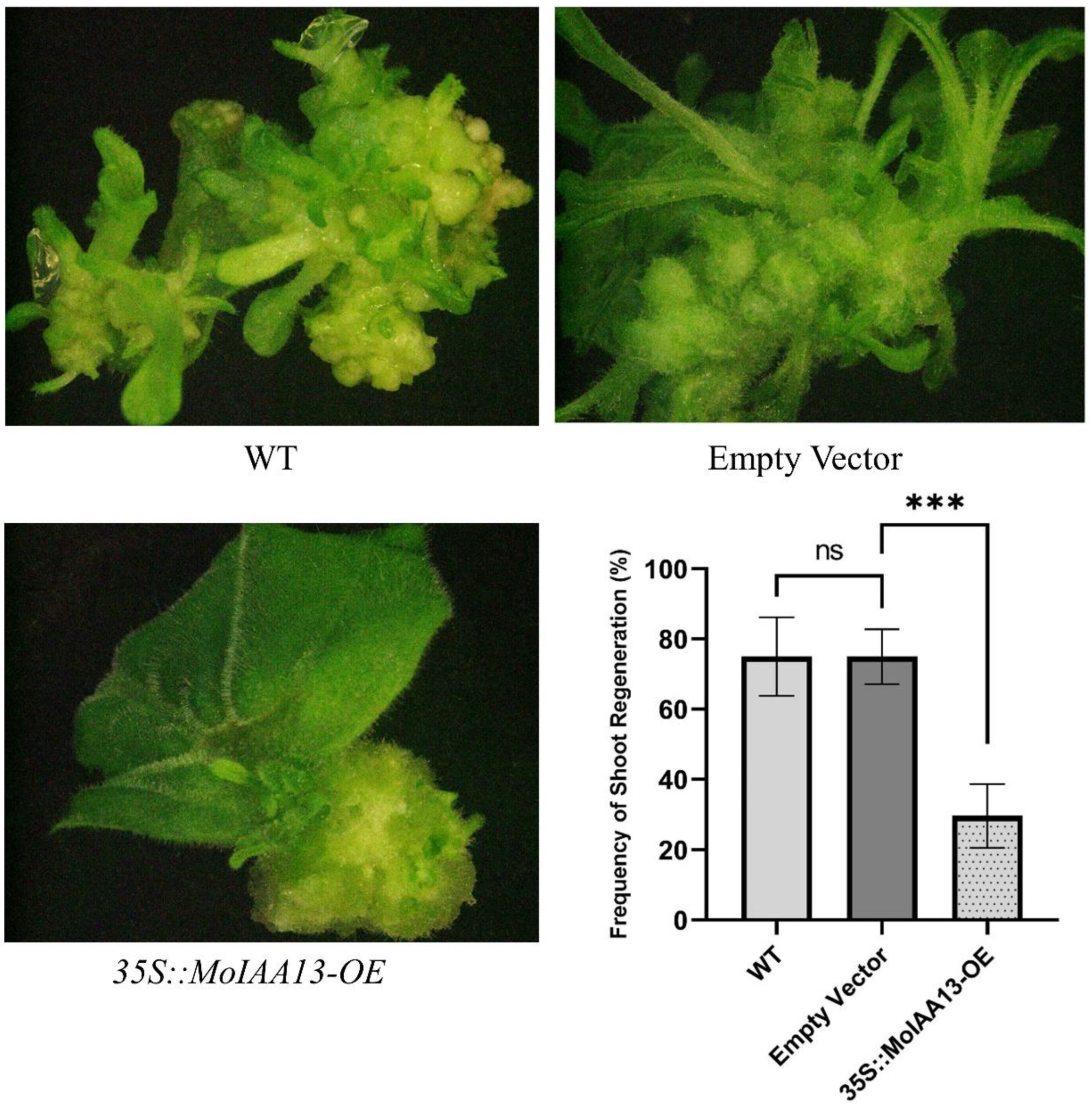
2.9. MoIAA13 Regulates the Process of Shoot Regeneration
The special expression patterns of MoIAA1, MoIAA7 and MoIAA13 in association with NAA treatment suggest their involvement in adventitious shoot regeneration. Further experiments revealed that they were all expressed in the nucleus, but only MoIAA13 shows transcriptional activation activity. Consequently, we are interested in the effect of MoIAA13 on adventitious shoot regeneration. We established transgenic lines of Overexpression MoIAA13 in N. benthamiana to investigate the shoot regeneration capacity. We found that the regeneration frequency in the 35S::MoIAA13-OE transgenic line was considerably lower than in the wild type (Figure 10). The occurrence of adventitious buds is dependent on the formation of primordia resembling the lateral root primordia (LRP) [44]. The specification of founder cells is the most important event in pluripotent primordia formation. Cell specification within cells with local auxin maxima will act as founder cells and subsequently develop into pluripotent primordia [45]. Therefore, the expression of genes related to the auxin signaling pathway is crucial for founder cell specification and primordia formation [46]. There is a population of stem cells in the primordia that can be induced to differentiate by inducing changes in gene expression [47]. Several phytohormones deliver a specific molecular signal and play key roles in plant cell division and differentiation [7]. Auxin works by directly binding to the nuclear receptor of TIR1/AFB proteins; the auxin-TIR1/AFB co-receptors inhibit the Aux/IAA transcriptional repressors. The release of ARF transcription factors leads to the activation of downstream genes [46]. Aux/IAAs and ARFs are important regulators of all stages of shoot regeneration. ARFs activate the initiation of the LRP by activating the transcription factor of LBDs (Lateral Organ Boundaries Domains), while ARFs are negatively regulated by Aux/IAAs. The MoLBD gene family was found to be differently expressed at the callus differentiation stage in our previous study [7]. ARF7 activates SAUR19 expression by binding its promoter directly, and several SAUR genes have been found to exhibit highly increased expression across the coffee somatic embryogenesis process [48]. There are studies revealing that Auxin-induced callus formation is retarded when the repressive interaction of IAA19 with ARF7 is destabilized [49]. IAA12 overexpression hampered shoot regeneration capacity, which can be complemented by ARF4 overexpression, and ARF4 regulates shoot regeneration by collaborating with ARF5 and IAA12 [21]. In this study, our results are consistent with these findings, implying that MoIAA13 interacts with MoARFs in response to auxin signaling, thereby regulating shoot regeneration. It is expected that future studies will reveal the specific details of this signaling mechanism.
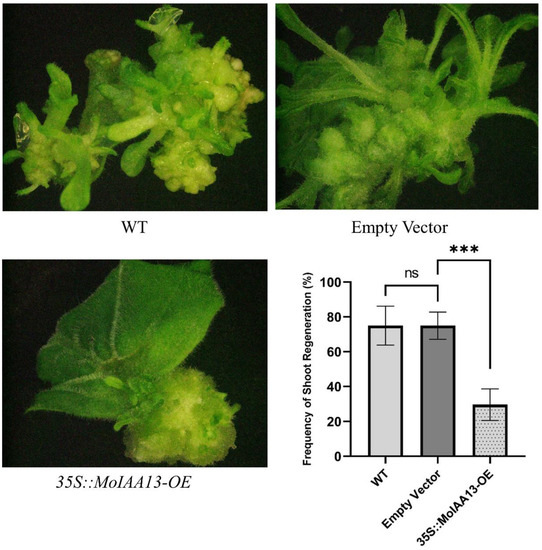
Figure 10.
Overexpression MoIAA13 in N. benthamiana compromised the shoot regeneration capacity. *** p < 0.001 between the EV (empty vector) and 35S::MoIAA13-OE transgenic lines and ns represent no significance.
3. Material and Methods
3.1. Identification of the Aux/IAA Genes in Drumstick Tree
The complete protein sequences of Moringa oleifera Lam. are available from NCBI under the accession number PRJNA268707. We used Arabidopsis thaliana Aux/IAA proteins [10] as queries in BLASTP searches with an E value lower than 1 × 10−10 for predicted proteins in the drumstick tree genome. The candidate proteins were then verified for the presence of the Aux/IAA domain using NCBI-CDD [50]. Ultimately, 23 Aux/IAA protein sequences of the drumstick tree were obtained.
3.2. Analysis of Physicochemical Properties of Amino Acids
The number of amino acids, molecular weight, and theoretical isoelectric points of all MoIAAs were analyzed using the online ProtParam tool provided by ExPASY (https://web.expasy.org/protparam/) (accessed on 31 January 2022). Then, their subcellular locations were predicted using Plant-mPLo (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) (accessed on 31 January 2022).
3.3. Gene Structure and Phylogenetic Analysis
The structures of MoIAAs were visualized using Tbtools software, with the gene annotation described in the GFF3 format [22]. Motifs of the protein sequences were investigated with the MEME tool (http://meme-suite.org/index.html) (accessed on 31 January 2022). The multiple alignments of protein sequences were performed using the ClustalW function of MEGA 7., and visualized with the Jalview software [51]. Phylogenetic relationships were established using MEGA 7.0 by the maximum likelihood (ML) method [51]. A non-parametric bootstrapping was performed with 1000 replicates.
3.4. Analysis of Aux/IAA Gene Duplication and Evolution
MCScanX software was used to analyze the gene duplication and syntenic events based on full-length MoIAA sequences with the default parameters [52]. The Ka/Ks ratio between gene pairs was calculated using TBtools software [22]. A Ka/Ks ratio less than 1 indicates ‘‘negative selection’’ and greater than 1 represents ‘‘positive selection’’ [53].
3.5. Analysis of Cis-Acting Elements in Aux/IAA Gene Promoters
The 2.0-kb upstream sequences from the translation start sites of the MoIAAs were extracted from the drumstick tree genome, then submitted to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 31 January 2022). The cis-acting elements were visualized using TBtools software [22].
3.6. Predicted Protein–Protein Interaction Network
First, we used AtARF proteins as queries for the predicted MoARF proteins in the drumstick tree genome. Then, analysis of the interaction network of the 23 MoIAAs and 19 MoARFs was performed using the STRING functional protein association network (https://string-db.org) (accessed on 8 September 2022), calculated and visualized by Cytoscape software [54].
3.7. Expression Analysis
The leaf explants with consistent growth status were inoculated onto MS medium containing NAA concentrations of 0 mg/L, 0.1 mg/L, 0.5 mg/L, and 1 mg/L. Samples were taken at three time points—6 h, 12 h, and 24 h—and three biological replicates were examined. The leaves treated at 0 h were used as the control (CK). These samples were frozen in liquid nitrogen and then stored at −80 °C. The forward primers and reverse primers listed in Supplemental Table S1 were designed using Primer 5.0. ACP2 was amplified as a reference gene [55]. Gene expression was detected with RT-qPCR, and the relative expression level for each gene was estimated using 2−ΔΔCT [7].
3.8. Subcellular Localization
The coding sequences without the stop codon of MoIAA1 (lamu_GLEAN_10000198), MoIAA7 (lamu_GLEAN_10007551) and MoIAA13 (lamu_GLEAN_10011219) were cloned into the pEarley Gate101 vector in a frame with EYFP and the CaMV 35 S promoter. The recombinant vector was transformed into Agrobacterium (GV3101) for the transformation of N. benthamiana. Then, subcellular localization was analyzed by N. benthamiana leaves [56]. The YFP signals were detected using a fluorescence microscope.
3.9. Transactivation Activity Analysis in Yeast
The full-length coding sequences of MoIAA1, MoIAA7 and MoIAA13 were amplified using the primers listed in Supplemental Table S2 and cloned into the pGBKT7 vector. The recombinant and control vectors were transformed into the yeast strain Y2HGold [43]. Then, the transformed strains were cultured on SD/-Trp and SD/-Trp/-His/x-α-Gal media at 30 °C for four days.
3.10. Generation of Overexpressing MoIAA13 Transgenic N. benthamiana Lines
The full-length coding sequences of MoIAA13 were cloned into the pEarley Gate101 vector, which is a binary vector with a CaMV 35S promoter. Then, the empty vector and recombinant vector were transferred into Agrobacterium (GV3101) for the transformation of N. benthamiana. The transgenic plants were identified by PCR amplification and qPCR. The leaf explants of WT, the empty vector, and the 35S::MoIAA13-OE transgenic lines were inoculated on MS medium containing 1.0 mg/L 6-BA and 0.1 mg/L NAA. After 25 days, the regeneration frequency (ratio of explants with regenerated buds to total explants) was determined from three independent replicates containing 30 explants. The data presented are mean ± SD from the replicates.
4. Conclusions
This study identified and characterized the Aux/IAA gene family of M. oleifera. Bioinformatics analysis suggested the MoIAAs were highly conserved. Meanwhile, the structure of MoIAA proteins was different, indicating the changes in protein properties and functions. Auxin-responsive and abiotic stress-related elements were detected in the MoIAA genes promoter. MoIAA and MoARF protein interaction network predicted analysis indicated that they interact closely. The results of the preliminary experimental analysis demonstrated that the MoIAA13s were sensitive to NAA treatment and exhibited transcriptional activation activity, and may have significant effects on shoot regeneration capacity during plant tissue culture. The findings provide a reference for future functional studies of this gene and establish the foundation for improved tissue culture efficiency and molecular breeding for the drumstick tree.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415729/s1.
Author Contributions
E.Y. and H.Y. conducted the experiments; C.L. and M.Z. analyzed the data; H.S. and X.Z. performed the subcellular localization experiment; E.Y. wrote the draft; J.Z. revised the manuscript; X.C. and J.Z. designed the research and obtained funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Forestry Technology Innovation Program, the Department of Forestry of Guangdong Province (2018KJCX001) and the Guangzhou Science and Technology Bureau (202201011354).
Conflicts of Interest
No potential conflict of interest is reported by the authors.
References
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Si, B.; Deng, K.; Tu, Y.; Zhou, C.; Diao, Q. Effects of feeding a Moringa oleifera rachis and twig preparation to dairy cows on their milk production and fatty acid composition, and plasma antioxidants. J. Sci. Food Agric. 2018, 98, 661–666. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.E.A.; Alqhtani, A.H.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; Babalghith, A.O.; Taha, A.E.; Ahmed, O.; Abdo, M.; El-Tarabily, K.A. Pharmacological, nutritional and antimicrobial uses of Moringa oleifera Lam. leaves in poultry nutrition: An updated knowledge. Poult. Sci. 2022, 101, 102031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Z.; Cai, J.; Wang, J.; Wang, G.; Zhu, Z.; Cao, F. Effects of dietary fish meal replacement by fermented moringa (Moringa oleifera Lam.) leaves on growth performance, nonspecific immunity and disease resistance against Aeromonas hydrophila in juvenile gibel carp (Carassius auratus gibelio var. CAS III). Fish Shellfish. Immunol. 2020, 102, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pian, R.; Yang, E.; Zhou, W.; He, Q.; Chen, X. In vitro induction and characterisation of tetraploid drumstick tree (Moringa oleifera Lam.). Open Life Sci. 2020, 15, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, S.; Ma, D.; Li, Y.; Liu, C. Genome-Wide Analysis of Cotton Auxin Early Response Gene Families and Their Roles in Somatic Embryogenesis. Genes 2019, 10, 730. [Google Scholar] [CrossRef]
- Yang, E.; Zheng, M.; Zou, X.; Huang, X.; Yang, H.; Chen, X.; Zhang, J. Global Transcriptomic Analysis Reveals Differentially Expressed Genes Involved in Embryogenic Callus Induction in Drumstick (Moringa oleifera Lam.). Int. J. Mol. Sci. 2021, 22, 12130. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA Gene Family in Plants: Molecular Structure, Regulation, and Function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Liao, S.; Wang, L.; Li, J.; Ruan, Y.-L. Cell Wall Invertase Is Essential for Ovule Development through Sugar Signaling Rather Than Provision of Carbon Nutrients. Plant Physiol. 2020, 183, 1126–1144. [Google Scholar] [CrossRef]
- Overvoorde, P.J.; Okushima, Y.; Alonso, J.M.; Chan, A.; Chang, C.; Ecker, J.R.; Hughes, B.; Liu, A.; Onodera, C.; Quach, H.; et al. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 2005, 17, 3282–3300. [Google Scholar] [CrossRef]
- Jain, M.; Kaur, N.; Garg, R.; Thakur, J.K.; Tyagi, A.K.; Khurana, J.P. Structure and expression analysis of early auxin-responsive Aux/IAA gene family in rice (Oryza sativa). Funct. Integr. Genom. 2006, 6, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, U.C.; DiFazio, S.P.; Brunner, A.M.; Tuskan, G.A. Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 2007, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Soler, M.; Clemente, H.S.; Mila, I.; Paiva, J.A.P.; Myburg, A.A.; Bouzayen, M.; Grima-Pettenati, J.; Cassan-Wang, H. Comprehensive Genome-Wide Analysis of the Aux/IAA Gene Family in Eucalyptus: Evidence for the Role of EgrIAA4 in Wood Formation. Plant Cell Physiol. 2015, 56, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, H.; Zhai, L.; Xie, X.; Li, X.; Bian, S. Identification and functional characterization of the Aux/IAA gene VcIAA27 in blueberry. Plant Signal. Behav. 2020, 15, 1700327. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, F.; Friml, J.; Ding, Z. Auxin signaling: Research advances over the past 30 years. J. Integr. Plant Biol. 2022, 64, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Su, H.; Cao, H.; Wei, H.; Fu, X.; Jiang, X.; Song, Q.; He, X.; Xu, C.; Luo, K. AUXIN RESPONSE FACTOR7 integrates gibberellin and auxin signaling via interactions between DELLA and AUX/IAA proteins to regulate cambial activity in poplar. Plant Cell 2022, 34, 2688–2707. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Y.; Hou, J.; Yang, Y.; Yin, T. Aux/IAA and ARF Gene Families in Salix suchowensis: Identification, Evolution, and Dynamic Transcriptome Profiling During the Plant Growth Process. Front. Plant Sci. 2021, 12, 666310. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Wang, Y.; Li, H.; Liu, J.; Tan, J.; He, J.; Bai, J.; Ma, H. Evolution Analysis of the Aux/IAA Gene Family in Plants Shows Dual Origins and Variable Nuclear Localization Signals. Int. J. Mol. Sci. 2017, 18, 2107. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Vinh-Trieu, T.; Shi, J.; Zhang, D.; Cai, W. Genome-wide characterization and expression analyses of the auxin/indole-3-acetic acid (Aux/IAA) gene family in barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 10242. [Google Scholar] [CrossRef]
- Yang, X.Q.; Lee, S.; So, J.H.; Dharmasiri, S.; Dharmasiri, N.; Ge, L.; Jensen, C.; Hangarter, R.; Hobbie, L.; Estelle, M. The IAA1 protein is encoded by AXR5 and is a substrate of SCFTIR1. Plant J. 2004, 40, 772–782. [Google Scholar] [CrossRef]
- Zhang, M.M.; Zhang, H.K.; Zhai, J.F.; Zhang, X.S.; Sang, Y.L.; Cheng, Z.J. ARF4 regulates shoot regeneration through coordination with ARF5 and IAA12. Plant Cell Rep. 2021, 40, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Hu, X.; Diao, D.; Wang, F.; Liu, Y. Genome-Wide Analysis and Identification of the Aux/IAA Gene Family in Peach. Int. J. Mol. Sci. 2019, 20, 4703. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, M.; Li, J.; Zhao, H.; Ge, W.; Zhang, K. Identification and Analysis of Aux/IAA Family in Acer rubrum. Evol. Bioinform. 2021, 17, 1176934321994127. [Google Scholar] [CrossRef]
- Figueiredo, M.R.A.d.; Strader, L.C. Intrinsic and extrinsic regulators of Aux/IAA protein degradation dynamics. Trends Biochem. Sci. 2022, 47, 865–874. [Google Scholar] [CrossRef]
- Guilfoyle, T.J. The PB1 Domain in Auxin Response Factor and Aux/IAA Proteins: A Versatile Protein Interaction Module in the Auxin Response. Plant Cell 2015, 27, 33–43. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z.; Liu, S.; He, Y.; Cheng, L.; Kong, F.; Wang, J.; Lu, G. Genome-wide analysis of Aux/IAA gene family in Solanaceae species using tomato as a model. Mol. Genet. Genom. 2012, 287, 295–311. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Wang, X.J.; Hagen, G.; Guilfoyle, T.J. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef]
- Begum, T.; Robinson-Rechavi, M. Special Care Is Needed in Applying Phylogenetic Comparative Methods to Gene Trees with Speciation and Duplication Nodes. Mol. Biol. Evol. 2021, 38, 1614–1626. [Google Scholar] [CrossRef]
- Li, X.; Cai, K.; Pei, X.; Li, Y.; Hu, Y.; Meng, F.; Song, X.; Tigabu, M.; Ding, C.; Zhao, X. Genome-Wide Identification of NAC Transcription Factor Family in Juglans mandshurica and Their Expression Analysis during the Fruit Development and Ripening. Int. J. Mol. Sci. 2021, 22, 12414. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, L.; Wu, R.; Chen, Y.; Lu, C. Genome-wide identification and characterization of UDP-glucose dehydrogenase family genes in moso bamboo and functional analysis of PeUGDH4 in hemicellulose synthesis. Sci. Rep. 2020, 10, 10124. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; He, H.; Wang, P.; Ma, Z.; Mao, J.; Chen, B. Genome-wide characterization and expression analyses of the auxin/indole-3-acetic acid (Aux/IAA) gene family in apple (Malus domestica). Gene 2021, 768, 145302. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, Y.; Pan, J.; Li, Z.; Wang, Q.; Mastouri, F.; Li, Y.; Yang, S.; Liu, M.; Dai, S.; Liu, W. Genome-wide identification of PTI1 family in Setaria italica and salinity-responsive functional analysis of SiPTI1-5. BMC Plant Biol. 2021, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, Z.; Lu, S. Genome-Wide Identification and Expression Analysis of the Aux/IAA and Auxin Response Factor Gene Family in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 10494. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, X.; Lu, J.; Song, F.; Sun, J.; Wang, C.; Lian, J.; Zhao, L.; Zhao, B. OsIAA20, an Aux/IAA protein, mediates abiotic stress tolerance in rice through an ABA pathway. Plant Sci. 2021, 308, 110903. [Google Scholar] [CrossRef]
- Estrella-Maldonado, H.; Chan-Leon, A.; Fuentes, G.; Giron-Ramirez, A.; Desjardins, Y.; Santamaria, J.M. The interaction between exogenous IBA with sucrose, light and ventilation alters the expression of ARFs and Aux/IAA genes in Carica papaya plantlets. Plant Mol. Biol. 2022, 110, 107–130. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Weijers, D.; Benkova, E.; Jager, K.E.; Schlereth, A.; Hamann, T.; Kientz, M.; Wilmoth, J.C.; Reed, J.W.; Jurgens, G. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J. 2005, 24, 1874–1885. [Google Scholar] [CrossRef]
- Dreher, K.A.; Brown, J.; Saw, R.E.; Callis, J. The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 2006, 18, 699–714. [Google Scholar] [CrossRef]
- Iqbal, S.; Hayat, F.; Mushtaq, N.; Khalil-Ur-Rehman, M.; Khan, U.; Yasoob, T.B.; Khan, M.N.; Ni, Z.; Ting, S.; Gao, Z. Bioinformatics Study of Aux/IAA Family Genes and Their Expression in Response to Different Hormones Treatments during Japanese Apricot Fruit Development and Ripening. Plants 2022, 11, 1898. [Google Scholar] [CrossRef]
- Caeiro, A.; Caeiro, S.; Correia, S.; Canhoto, J. Induction of Somatic Embryogenesis in Tamarillo (Solanum betaceum Cav.) Involves Increases in the Endogenous Auxin Indole-3-Acetic Acid. Plants 2022, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Zhang, T.; Chen, X. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Hortic. Res. 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Liang, X.; Zhou, X.; Yang, F.; Liu, J.; He, S.Y.; Guo, Z. Alternative Splicing of Rice WRKY62 and WRKY76 Transcription Factor Genes in Pathogen Defense. Plant Physiol. 2016, 171, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.; Mangano, S.; Gonzalez-Garcia, M.P.; Contreras, R.; Sauer, M.; de Rybel, B.; Weijers, D.; Sanchez-Serrano, J.J.; Sanmartin, M.; Rojo, E. RIMA-Dependent Nuclear Accumulation of IYO Triggers Auxin-Irreversible Cell Differentiation in Arabidopsis. Plant Cell 2017, 29, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Benkova, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertova, D.; Jurgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Raspor, M.; Motyka, V.; Kaleri, A.R.; Ninkovic, S.; Tubic, L.; Cingel, A.; Cosic, T. Integrating the Roles for Cytokinin and Auxin in De Novo Shoot Organogenesis: From Hormone Uptake to Signaling Outputs. Int. J. Mol. Sci. 2021, 22, 8554. [Google Scholar] [CrossRef]
- Rosspopoff, O.; Chelysheva, L.; Saffar, J.; Lecorgne, L.; Gey, D.; Caillieux, E.; Colot, V.; Roudier, F.; Hilson, P.; Berthome, R.; et al. Direct conversion of root primordium into shoot meristem relies on timing of stem cell niche development. Development 2017, 144, 1187–1200. [Google Scholar] [CrossRef]
- Zanin, F.C.; Freitas, N.C.; Pinto, R.T.; Maximo, W.P.F.; Diniz, L.E.C.; Paiva, L.V. The SAUR gene family in coffee: Genome-wide identification and gene expression analysis during somatic embryogenesis. Mol. Biol. Rep. 2022, 49, 1973–1984. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, R.; Yu, D.; Chang, P.; Guo, S.; Yang, X.; Liu, X.; Xu, C.; Hu, Y. The calcium signaling module CaM-IQM destabilizes IAA-ARF interaction to regulate callus and lateral root formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2202669119. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Bennetzen, J.L. Mechanisms and rates of genome expansion and contraction in flowering plants. Genetica 2002, 115, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Deng, L.-T.; Wu, Y.-L.; Li, J.-C.; OuYang, K.-X.; Ding, M.-M.; Zhang, J.-J.; Li, S.-Q.; Lin, M.-F.; Chen, H.-B.; Hu, X.-S.; et al. Screening Reliable Reference Genes for RT-qPCR Analysis of Gene Expression in Moringa oleifera. PLoS ONE 2016, 11, e0159458. [Google Scholar] [CrossRef]
- Sparkes, A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).