Metabolomics Study of Serum Samples of β-YAC Transgenic Mice Treated with Tenofovir Disoproxil Fumarate
Abstract
1. Introduction
2. Results
2.1. Metabolic Profiling and Identification
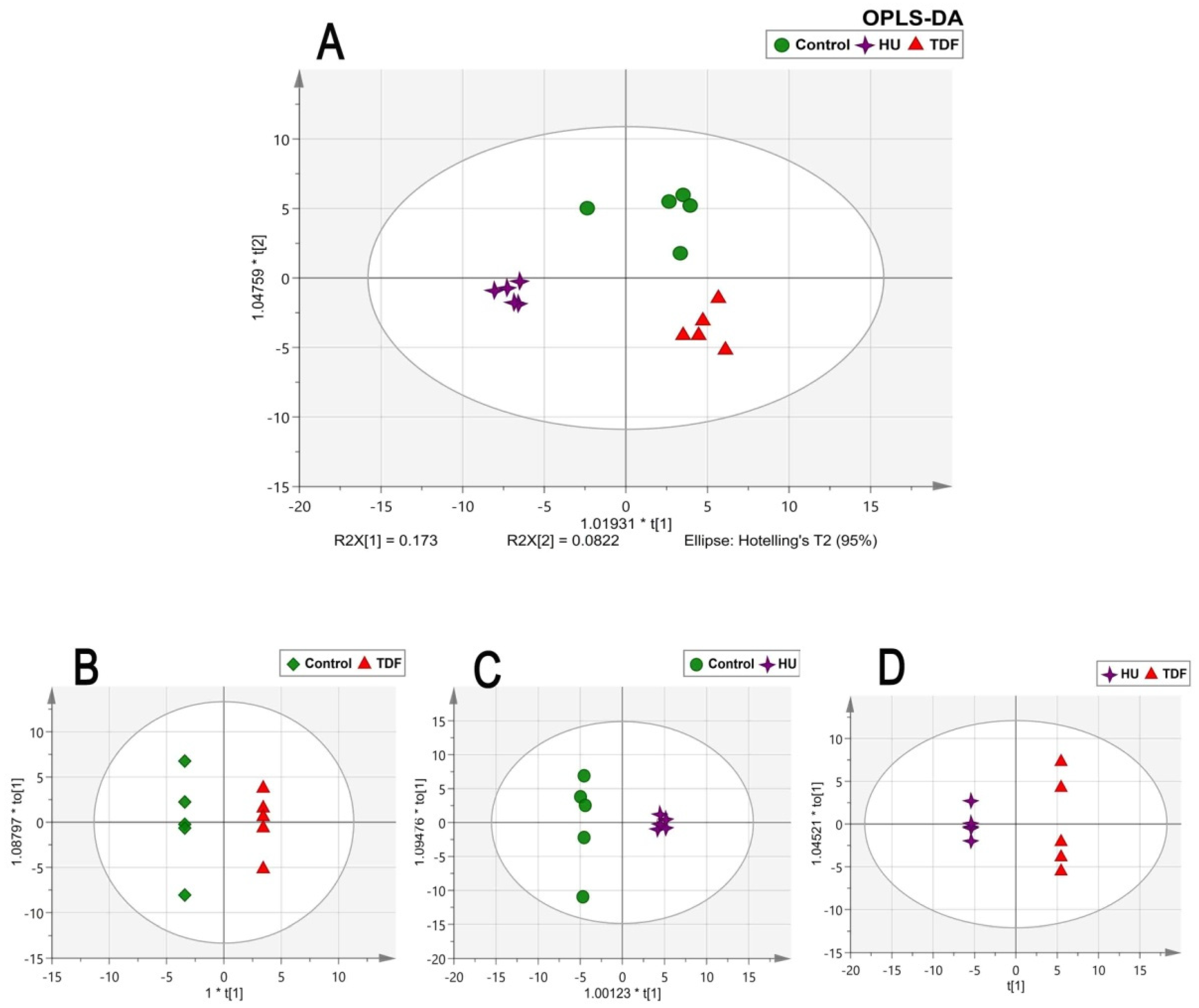
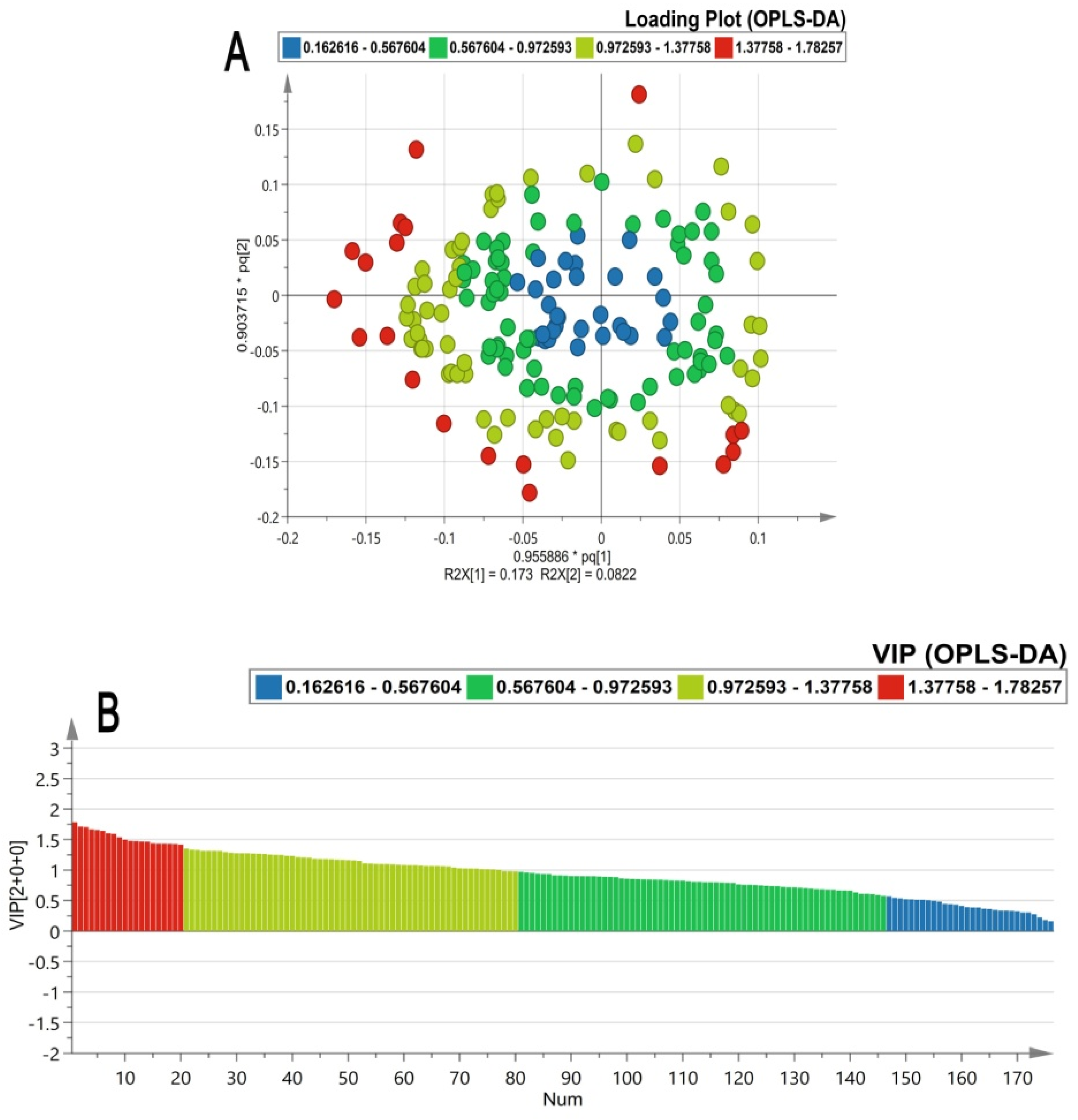
2.2. Chemometric Analysis
2.3. Discriminant Metabolites in β-YAC Transgenic Mice Induced by TDF
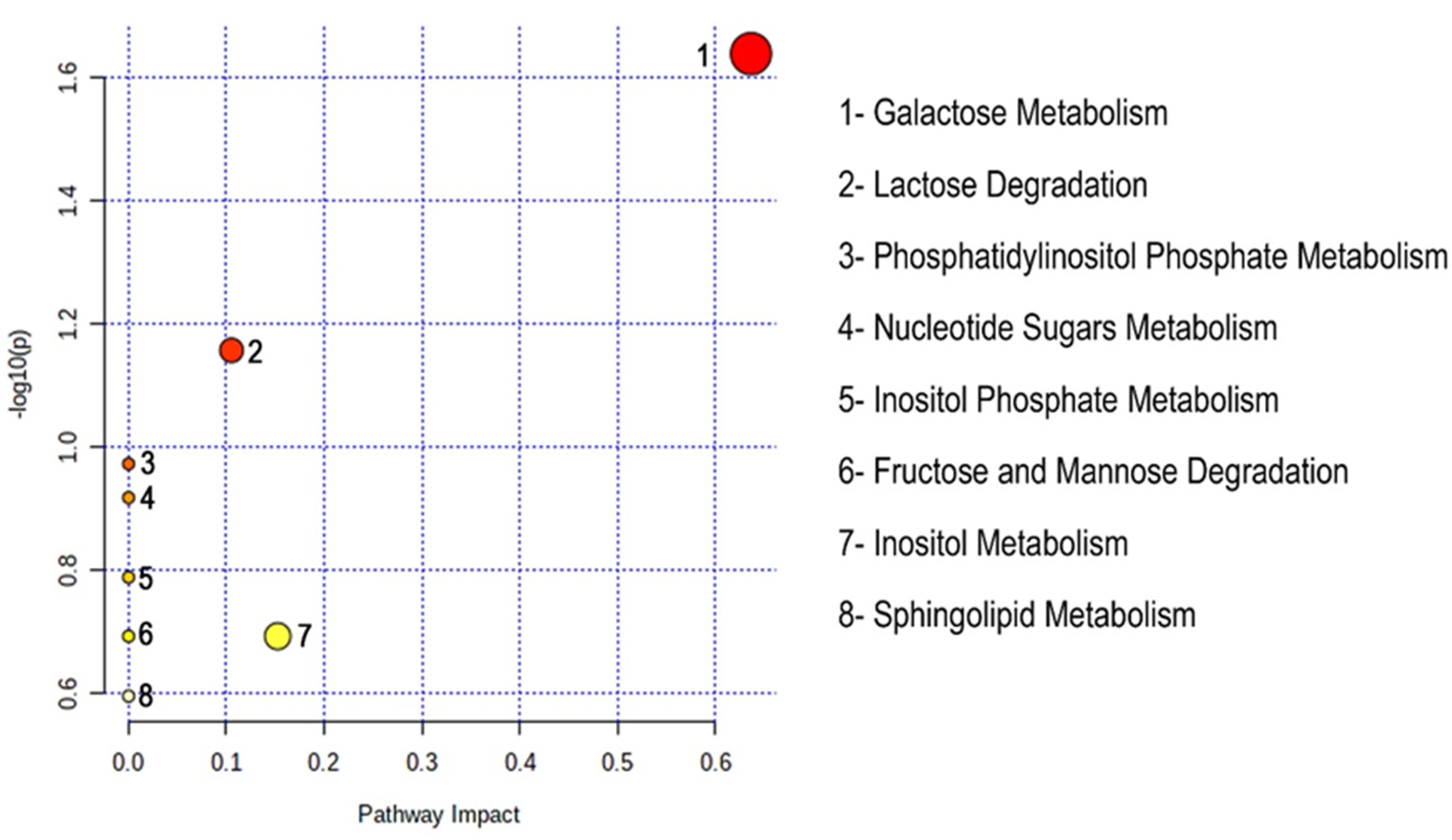
2.4. Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Study
4.3. Sample Collection and Biological Processing
4.4. Sample Preparation and Derivatization
4.5. GC-MS Analysis and Data Processing
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherall, D. Phenotype–genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat. Rev. Rev. Genet. 2001, 2, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.P.; Castaneda, S.A.; Boosalis, M.S.; White, G.L.; Jones, B.M.; Bohacek, R. Induction of Fetal Globin in β-Thalassemia: Cellular Obstacles and Molecular Progress. Ann. N. Y. Acad. Sci. 2005, 1054, 257–265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaewsakulthong, W.; Pongpaksupasin, P.; Nualkaew, T.; Hongeng, S.; Fucharoen, S.; Jearawiriyapaisarn, N.; Sripichai, O. Lysine-Specific Histone Demethylase 1 Inhibition Enhances Robust Fetal Hemoglobin Induction in Human β0-Thalassemia/Hemoglobin E Rrythroid Cells. Hematol. Rep. 2021, 13, 9215. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cosenza, L.C.; Zurlo, M.; Lampronti, I.; Borgatti, M.; Scapoli, C.; Gambari, R.; Finotti, A. Treatment of Erythroid Precursor Cells from β-Thalassemia Patients with Cinchona Alkaloids: Induction of Fetal Hemoglobin Production. Int. J. Mol. Sci. 2021, 22, 13433. [Google Scholar] [CrossRef] [PubMed]
- Bou-Fakhredin, R.; De Franceschi, L.; Motta, I.; Cappellini, M.D.; Taher, A.T. Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals 2022, 15, 753. [Google Scholar] [CrossRef]
- Pourfarzad, F.; von Lindern, M.; Azarkeivan, A.; Hou, J.; Kia, S.K.; Esteghamat, F.; van IJcken, W.; Philipsen, S.; Najmabadi, H.; Grosveld, F. Hydroxyurea responsiveness in β-thalassemic patients is determined by the stress response adaptation of erythroid progenitors and their differentiation propensity. Haematologica 2013, 98, 696. [Google Scholar] [CrossRef][Green Version]
- Jang, E.; Lee, J.K.; Inn, K.-S.; Chung, E.K.; Lee, K.-T.; Lee, J.-H. Renal Dysfunction and Tubulopathy Induced by High-Dose Tenofovir Disoproxil Fumarate in C57BL/6 Mice. Healthcare 2020, 8, 417. [Google Scholar] [CrossRef]
- Italia, K.; Chandrakala, S.; Ghosh, K.; Colah, R. Can hydroxyurea serve as a free radical scavenger and reduce iron overload in β-thalassemia patients? Free Radic. Res. 2016, 50, 959–965. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, S.-G.; Jeong, S.-W.; Jang, J.-Y.; Yoo, J.-J.; Lee, S.-H.; Kim, Y.-S.; Kim, H.-S.; Lee, H.-W.; Park, S. Efficacy and Safety of Tenofovir Disoproxil Orotate in Chronic Hepatitis B Patients Previously Treated with Tenofovir Disoproxil Fumarate: Multicenter, Open-Label, Prospective Study. J. Clin. Med. 2021, 10, 5628. [Google Scholar] [CrossRef]
- Khan, F.; Ali, H.; Musharraf, S.G. Tenofovir disoproxil fumarate induces fetal hemoglobin production in K562 cells and β-YAC transgenic mice: A therapeutic approach for gamma-globin induction. Exp. Cell Res. 2020, 394, 112168. [Google Scholar] [CrossRef]
- Monni, G.; Murgia, F.; Corda, V.; Peddes, C.; Iuculano, A.; Tronci, L.; Balsamo, A.; Atzori, L. Metabolomic Investigation of β-Thalassemia in Chorionic Villi Samples. J. Clin. Med. 2019, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-w.; Li, Q.-h.; Dou, J.-j. Mass spectrometry-based metabolomics in health and medical science: A systematic review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Mazhar, S.; Siddiqui, A.J.; Choudhary, M.I. Metabolite profiling of human plasma by different extraction methods through gas chromatography–mass spectrometry—An objective comparison. Anal. Chim. Acta 2013, 804, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I. Bioenergetics in environmental adaptation and stress tolerance of aquatic ectotherms: Linking physiology and ecology in a multi-stressor landscape. J. Exp. Biol. 2021, 224, jeb236802. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, E.; Moriwaki, K.; Terao, N.; Tan, C.-C.; Terao, M.; Nakagawa, T.; Matsumoto, H.; Shinzaki, S.; Kamada, Y. Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules 2012, 2, 34–45. [Google Scholar] [CrossRef]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Al-Hakeem, T.; Hasan, H.G.; Ali, Z.A. Levels of Total Fucose and Total Protein in Sera of Blood Groups and RBCs of Control, Minor and Major Thalassemic Patients. Middle East J. Intern. Med. 2013, 6, 13–17. [Google Scholar] [CrossRef]
- Assi, M.A. Estimation of α-L-fucose and vitamin D 3 levels in β thalassemia patients in Al-Najaf Province. J. Glob. Pharma Technol. 2019, 11, 192–195. [Google Scholar]
- Vucenik, I. Anticancer properties of inositol hexaphosphate and inositol: An overview. J. Nutr. Sci. Vitaminol. 2019, 65, S18–S22. [Google Scholar] [CrossRef]
- Unfer, V.; Facchinetti, F. Editorial–Update on inositol(s). Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1–3. [Google Scholar] [PubMed]
- Bhowmik, A.; Ojha, D.; Goswami, D.; Das, R.; Chandra, N.S.; Chatterjee, T.K.; Chakravarty, A.; Chakravarty, S.; Chattopadhyay, D. Inositol hexa phosphoric acid (phytic acid), a nutraceuticals, attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed. Pharmacother. 2017, 87, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, F.; Khan, M.B.N.; Musharraf, S.G. Monoterpenes as therapeutic candidates to induce fetal hemoglobin synthesis and up-regulation of gamma-globin gene: An in vitro and in vivo investigation. Eur. J. Pharmacol. 2021, 891, 173700. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Iqbal, A.; Ansari, S.H.; Parveen, S.; Khan, I.A.; Siddiqui, A.J. β-Thalassemia Patients Revealed a Significant Change of Untargeted Metabolites in Comparison to Healthy Individuals. Sci. Rep. 2017, 7, 42249. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Schneider, Y.; Ceraline, J.; Duranton, B.; Gosse, F.; Seiler, N.; Raul, F. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J. Pharmacol. Exp. Ther. 2001, 298, 197–200. [Google Scholar] [PubMed]
- Galle, M.; Crespo, R.; Rodenak Kladniew, B.; Montero Villegas, S.; Polo, M.; de Bravo, M.G. Suppression by geraniol of the growth of A549 human lung adenocarcinoma cells and inhibition of the mevalonate pathway in culture and in vivo: Potential use in cancer chemotherapy. Nutr. Cancer 2014, 66, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.P.; Heidor, R.; de Conti, A.; Dagli, M.L.Z.; Moreno, F.S. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis 2006, 27, 1194–1203. [Google Scholar] [CrossRef]
- Duncan, R.E.; Lau, D.; El-Sohemy, A.; Archer, M.C. Geraniol and β-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem. Pharmacol. 2004, 68, 1739–1747. [Google Scholar] [CrossRef]
- Tekleyes, B.; Huluka, S.A.; Wondu, K.; Wondmkun, Y.T. Wound Healing Activity of 80% Methanol Leaf Extract of Zehneria scabra (Lf) Sond (Cucurbitaceae) in Mice. J. Exp. Pharmacol. 2021, 13, 537. [Google Scholar] [CrossRef]
- Doerfler, P.A.; Feng, R.; Li, Y.; Palmer, L.E.; Porter, S.N.; Bell, H.W.; Crossley, M.; Pruett-Miller, S.M.; Cheng, Y.; Weiss, M.J. Activation of γ-globin gene expression by GATA1 and NF-Y in hereditary persistence of fetal hemoglobin. Nat. Genet. 2021, 53, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
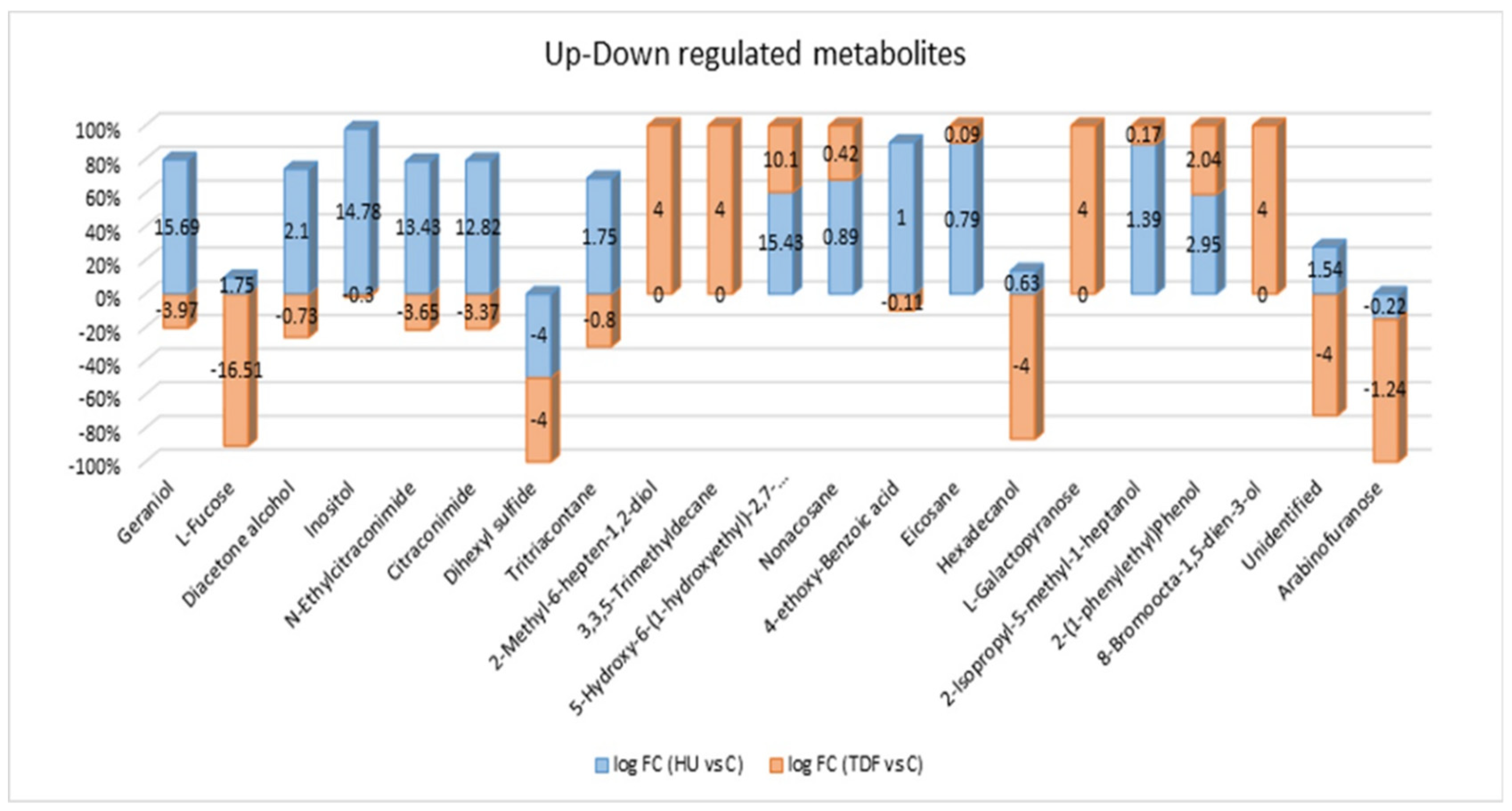

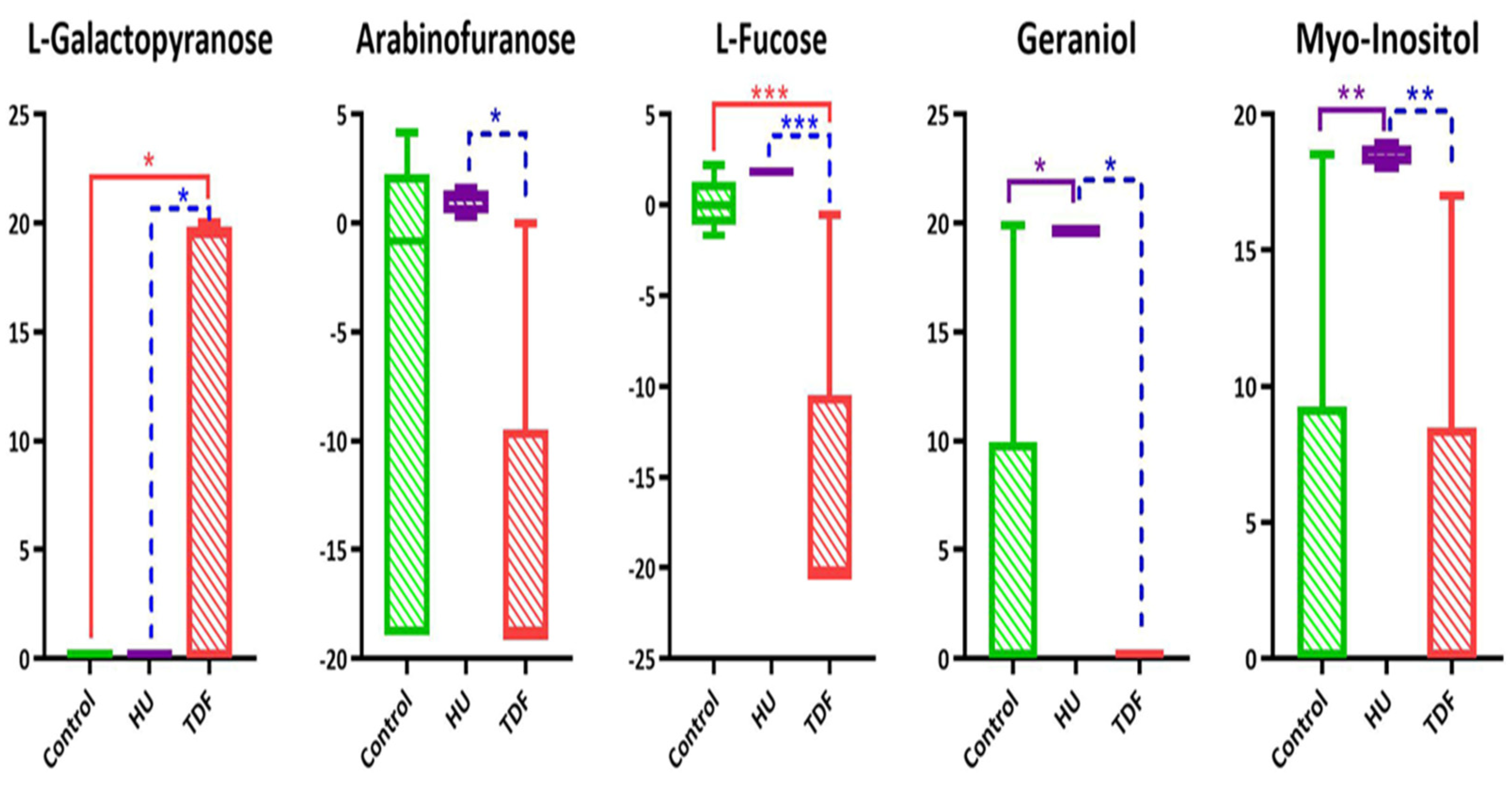
| S.No. | Name | Molar Mass | RT | VIP Value | log (FC) | Regulation | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TDF vs. C | HU vs. C | TDF vs. C | HU vs. C | TDF vs. C | HU vs. C | TDF vs. HU | |||||
| 1 | Geraniol | 154.25 | 25.05 | 1.7825 | −3.97 | 15.69 | ↓ | ↑ | ns | * | * |
| 2 | L-Fucose | 164.16 | 30.83 | 1.7098 | −16.51 | 1.75 | ↓ | ↑ | *** | ns | *** |
| 3 | Diacetone alcohol | 116.16 | 11.04 | 1.6653 | −0.73 | 2.10 | ↑ | ns | ns | ns | |
| 4 | Myo-Inositol | 180.16 | 32.76 | 1.6540 | −0.30 | 14.78 | ↑ | ns | ** | ** | |
| 5 | N-Ethylcitraconimide | 139.15 | 9.30 | 1.3157 | −3.65 | 13.43 | ↓ | ↑ | ns | * | ** |
| 6 | Citraconimide | 111.10 | 11.49 | 1.2955 | −3.37 | 12.82 | ↓ | ↑ | ns | * | ** |
| 7 | Dihexyl sulfide | 202.40 | 18.35 | 1.6420 | −4.00 | −4.00 | ↓ | ↓ | * | * | ns |
| 8 | Tritriacontane | 464.90 | 34.56 | 1.6000 | −0.80 | 1.75 | ↑ | ns | * | *** | |
| 9 | 2-Methyl-6-hepten-1,2-diol | 144.21 | 9.51 | 1.5899 | 4.00 | 0 | ↑ | * | ns | * | |
| 10 | 3,3,5-Trimethyldecane | 184.36 | 19.16 | 1.5363 | 4.00 | 0 | ↑ | * | ns | * | |
| 11 | 5-Hydroxy-6-(1-hydroxyethyl)-2,7-dimethoxy-1,4-naphthoquinone | 278.26 | 21.33 | 1.4968 | 10.10 | 15.43 | ↑ | ↑ | ns | ** | ns |
| 12 | Nonacosane | 408.80 | 38.39 | 1.4755 | 0.42 | 0.89 | ns | ns | ns | ||
| 13 | 4-ethoxy-Benzoic acid | 166.17 | 21.11 | 1.4720 | −0.11 | 1.00 | ↑ | ns | ns | * | |
| 14 | Eicosane | 282.50 | 33.23 | 1.4655 | 0.09 | 0.79 | ns | ns | ns | ||
| 15 | Hexadecanol | 242.44 | 29.28 | 1.4639 | −4.00 | 0.63 | ↓ | ns | ns | * | |
| 16 | L-Galactopyranose | 180.16 | 30.83 | 1.4379 | 4.00 | 0 | ↑ | * | ns | * | |
| 17 | 2-Isopropyl-5-methyl-1-heptanol | 172.31 | 16.28 | 1.4338 | 0.17 | 1.39 | ↑ | ns | ns | ns | |
| 18 | 2-(1-phenylethyl)Phenol | 198.26 | 23.40 | 1.4331 | 2.04 | 2.95 | ↑ | ↑ | ns | ns | ns |
| 19 | 8-Bromoocta-1,5-dien-3-ol | 205.09 | 48.03 | 1.4309 | 4.00 | 0 | ↑ | * | ns | * | |
| 20 | Arabinofuranose | 150.13 | 26.86 | 1.4164 | −1.24 | −0.22 | ↓ | ns | ns | * | |
| 21 | Unidentified | -- | 9.37 | 1.4271 | −4.00 | 1.54 | ↓ | ↑ | ns | ns | * |
| Pathways | Total | Hits | Hits Name | Raw p | FDR | Impact |
|---|---|---|---|---|---|---|
| Galactose Metabolism | 31 | 2 | L-Galactose, Myo-inositol | 0.022974 | 1 | 0.63665 |
| Lactose Degradation | 9 | 1 | L-Galactose | 0.069671 | 1 | 0.10526 |
| Phosphatidylinositol Phosphate Metabolism | 14 | 1 | Myo-inositol | 0.10651 | 1 | 0 |
| Nucleotide Sugars Metabolism | 16 | 1 | L-Galactose | 0.12089 | 1 | 0 |
| Inositol Phosphate Metabolism | 22 | 1 | Myo-inositol | 0.1628 | 1 | 0 |
| Fructose and Mannose Degradation | 28 | 1 | Fucose | 0.20296 | 1 | 0 |
| Inositol Metabolism | 28 | 1 | Myo-inositol | 0.20296 | 1 | 0.15251 |
| Sphingolipid Metabolism | 36 | 1 | L-Galactose | 0.25388 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Khan, F.; Siddiqui, A.J.; Adil, N.; Uddin, J.; Asmari, M.; Musharraf, S.G. Metabolomics Study of Serum Samples of β-YAC Transgenic Mice Treated with Tenofovir Disoproxil Fumarate. Int. J. Mol. Sci. 2022, 23, 15750. https://doi.org/10.3390/ijms232415750
Kumari S, Khan F, Siddiqui AJ, Adil N, Uddin J, Asmari M, Musharraf SG. Metabolomics Study of Serum Samples of β-YAC Transgenic Mice Treated with Tenofovir Disoproxil Fumarate. International Journal of Molecular Sciences. 2022; 23(24):15750. https://doi.org/10.3390/ijms232415750
Chicago/Turabian StyleKumari, Sindhia, Faisal Khan, Amna Jabbar Siddiqui, Nurmeen Adil, Jalal Uddin, Mufarreh Asmari, and Syed Ghulam Musharraf. 2022. "Metabolomics Study of Serum Samples of β-YAC Transgenic Mice Treated with Tenofovir Disoproxil Fumarate" International Journal of Molecular Sciences 23, no. 24: 15750. https://doi.org/10.3390/ijms232415750
APA StyleKumari, S., Khan, F., Siddiqui, A. J., Adil, N., Uddin, J., Asmari, M., & Musharraf, S. G. (2022). Metabolomics Study of Serum Samples of β-YAC Transgenic Mice Treated with Tenofovir Disoproxil Fumarate. International Journal of Molecular Sciences, 23(24), 15750. https://doi.org/10.3390/ijms232415750






