m6A-Mediated PPARA Translational Suppression Contributes to Corticosterone-Induced Visceral Fat Deposition in Chickens
Abstract
:1. Introduction
2. Results
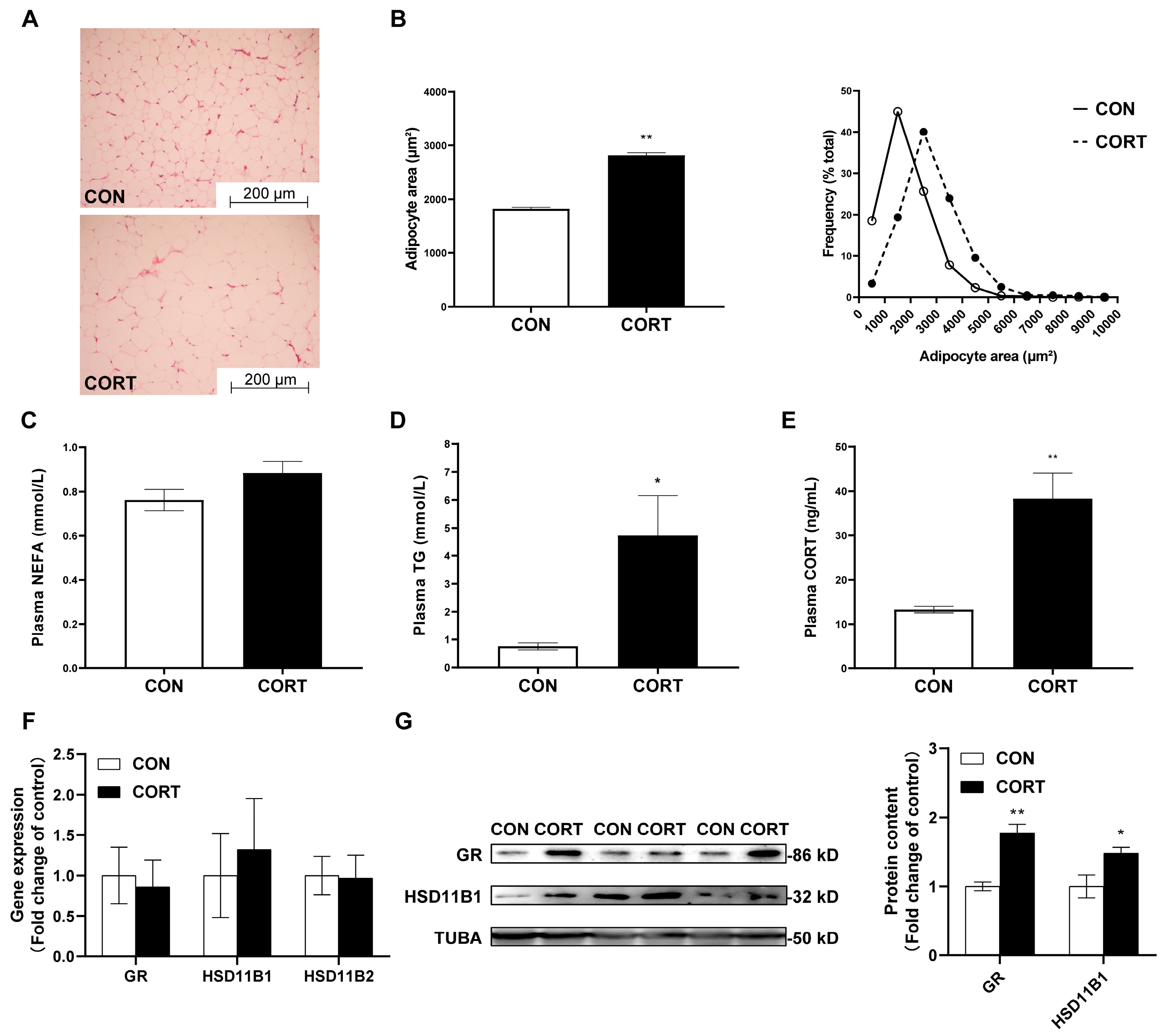
2.1. Chronic CORT Exposure Induces Visceral Fat Deposition
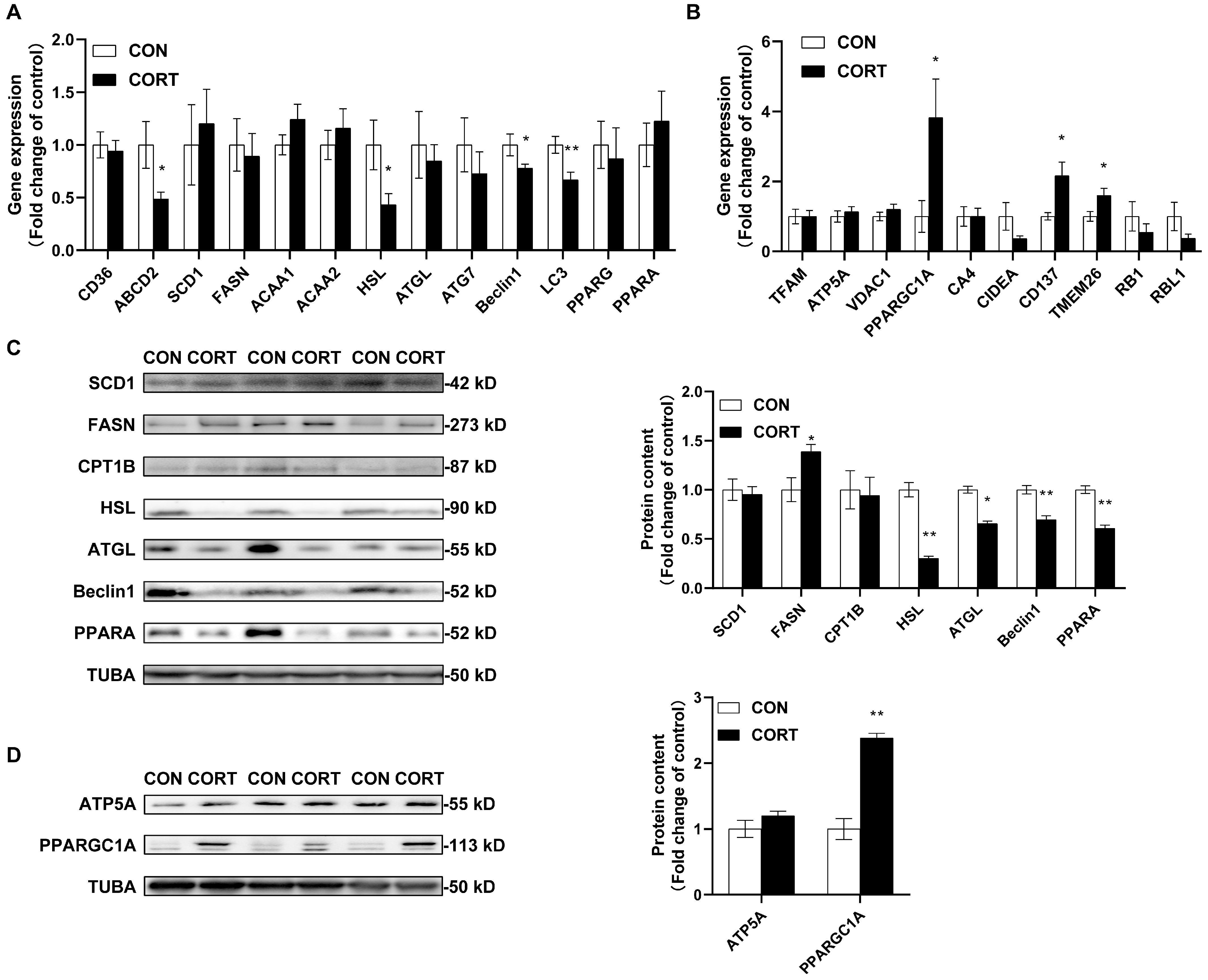
2.2. Chronic CORT Exposure Decreases Lipolytic Functions in Visceral Fat Tissue
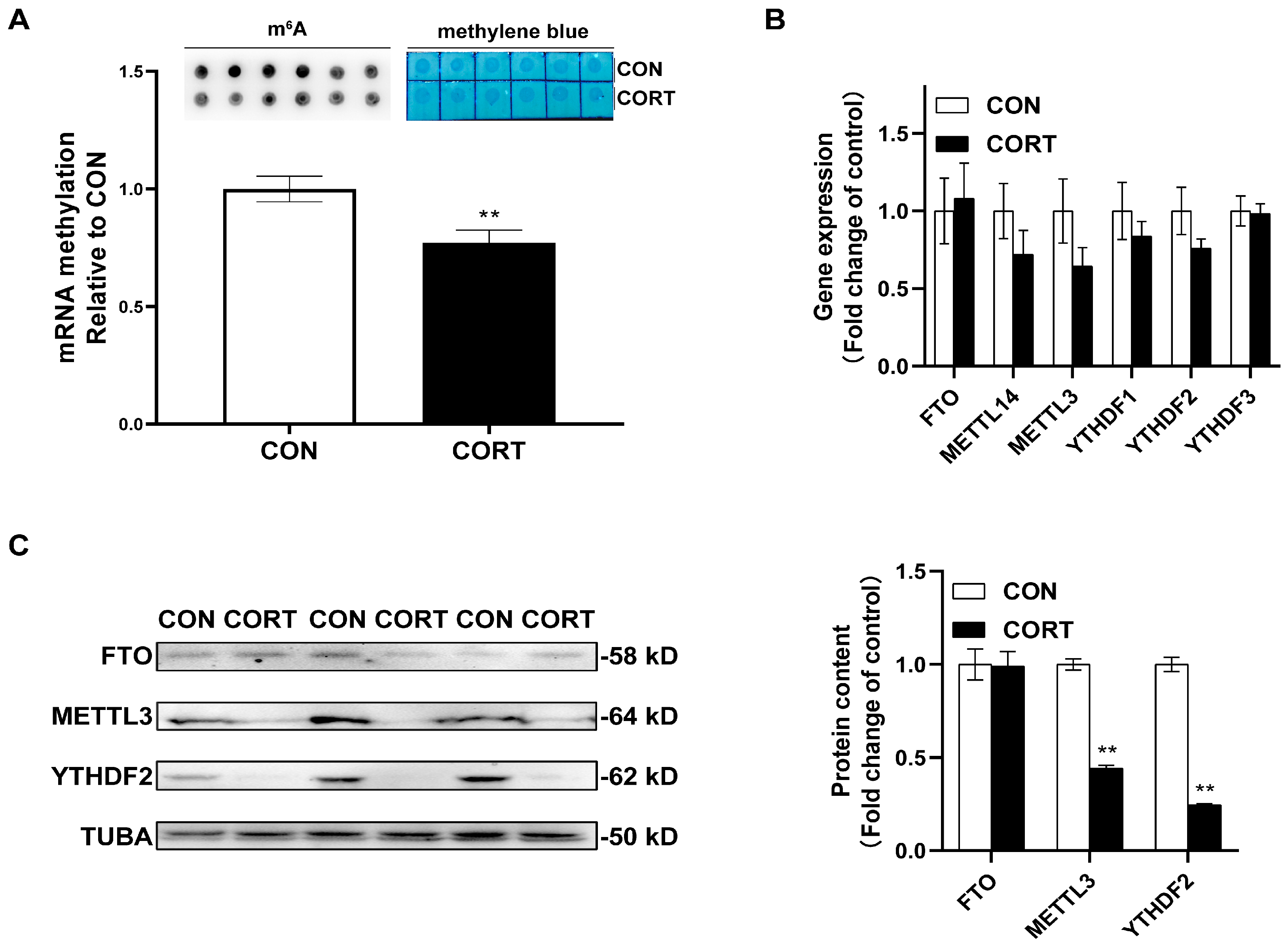
2.3. Chronic CORT Exposure Inhibits Global m6A Methylation Levels in Visceral Fat Tissue
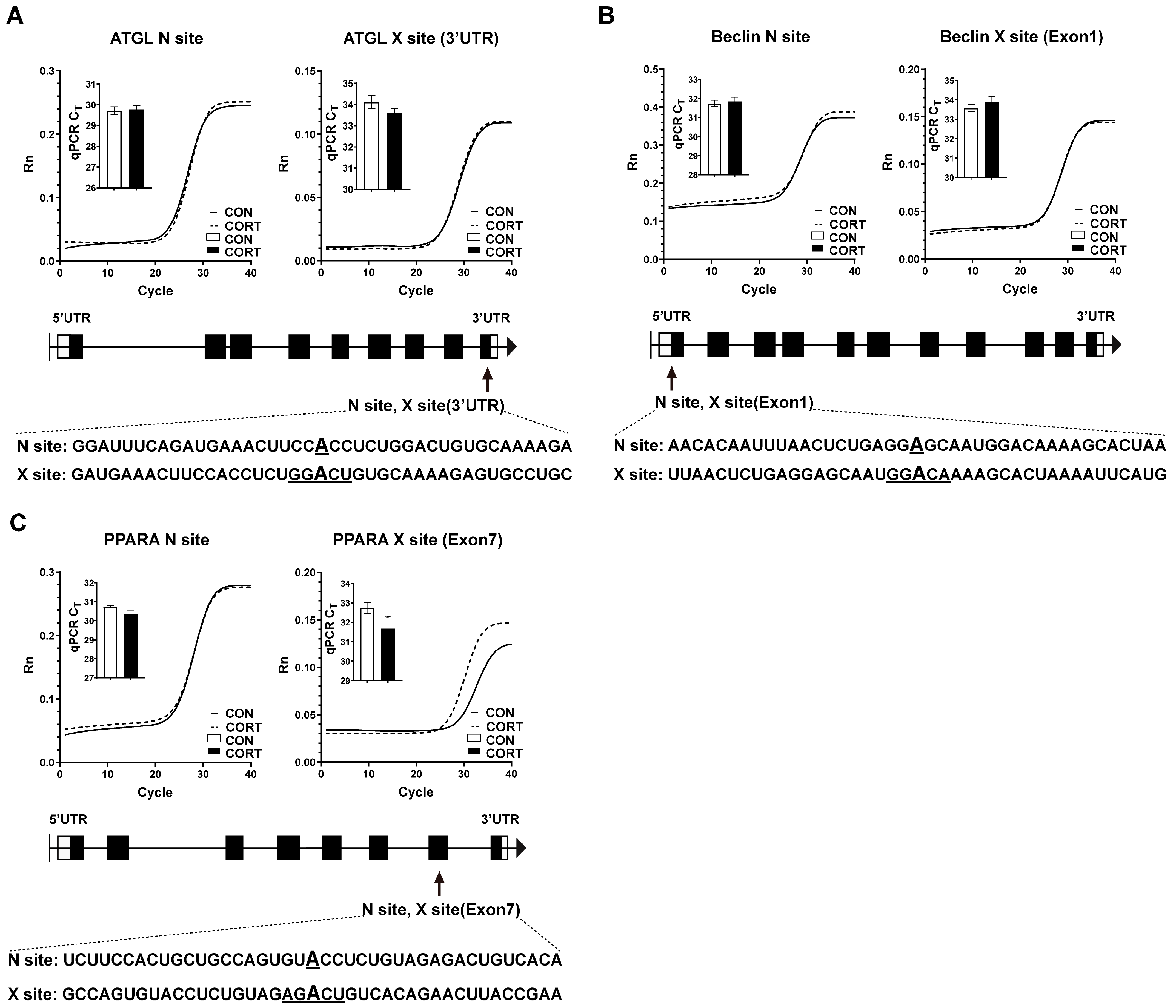
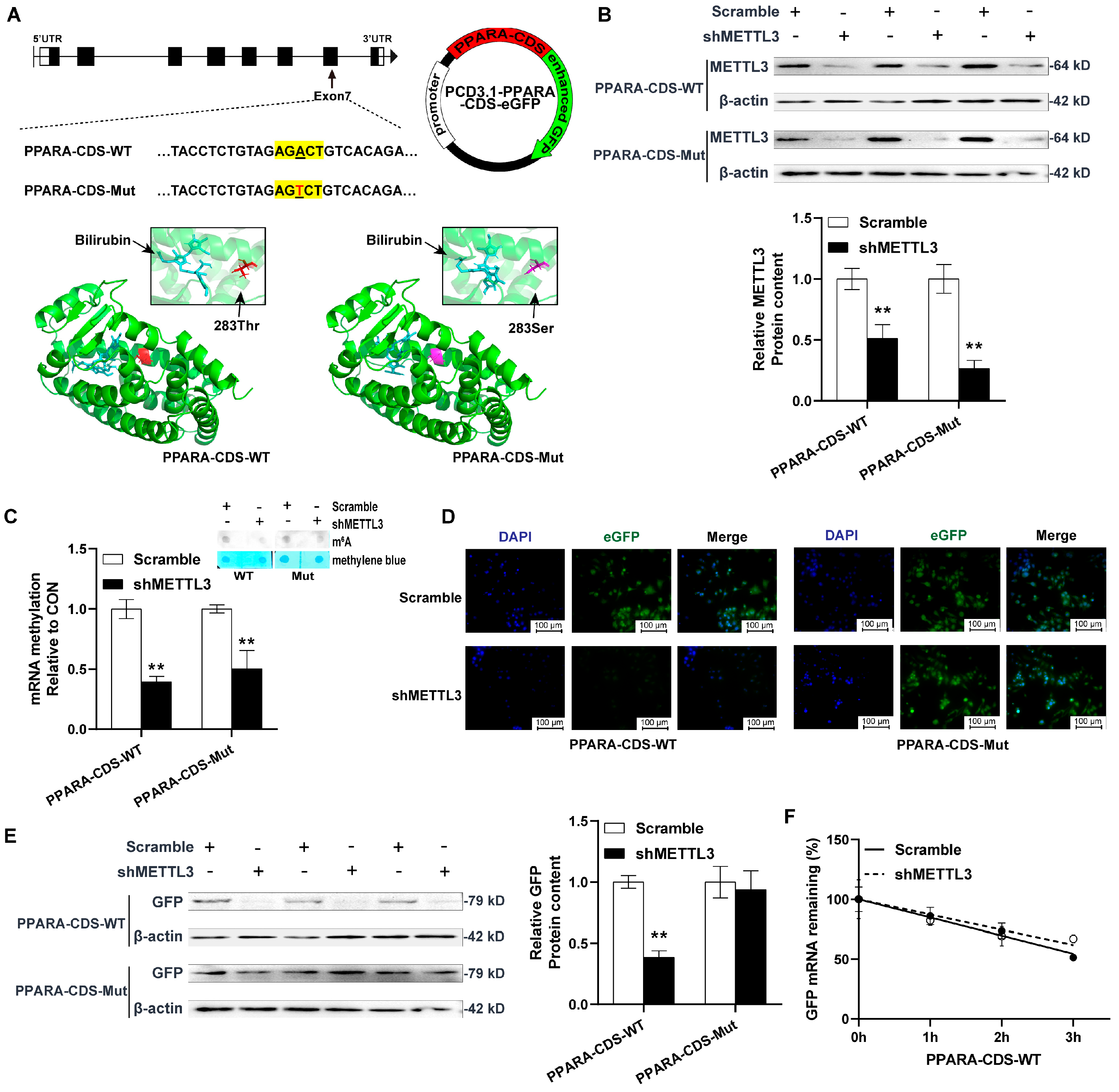
2.4. Chronic CORT Exposure Decreased Site-Specific RNA Methylation in Exon7 of PPARA
2.5. METTL3 Knockdown Decreased PPARA Protein Content via m6A Demethylation in CDS Region
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. HE Staining
4.3. Plasma Parameters Determination
4.4. RNA Isolation and Real-Time PCR
4.5. Western Blot
4.6. Global and Site-Specific M6A Detection
4.7. Plasmids Construction
4.8. Cell Culture and Transfection
4.9. Immunofluorescence Analysis
4.10. Deadenylation and Decay Assays
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Target Genes | Primer Sequences (5′ to 3′) | PCR Products (bp) | Amplification Efficiency (%) |
|---|---|---|---|
| GR | TGCACAAGCCCCTGTATCTG | 265 | 102.21% |
| NM_001037826.1 | AATGCTTTCCTCCAGGAGCC | ||
| HSD11B1 | TTGGGACTGGTTTTGGCCTT | 238 | 98.84% |
| XM_025143927.3 | TGGCCTTCCTGTGTTTGTGT | ||
| HSD11B2 | AGAGAGTGATCGTGACGGGA | 294 | 107.23% |
| XM_417988.8 | CGTCCCCGTTGAAGAAGTCA | ||
| ABCD2 | CACACAGACCTTCTCTTTGTACC | 136 | 93.43% |
| NM_001199395.2 | CCTGCAAGTTGGGACTCCAG | ||
| ACAA1 | TGGAAAACAGCAAAGCTCGC | 117 | 91.29% |
| NM_001197288.2 | GTTGGGAAGCCAAGGCAAAG | ||
| ACAA2 | TAATGGGCATTGGCCCTGTT | 280 | 91.29% |
| NM_001006571.3 | CCAATGCAAGCTGACCCAAC | ||
| ATG7 | CTGAGGGGCATGGAGGATGT | 100 | 93.43% |
| NM_001030592.2 | CCCAACTCCCCTTCTGGAAC | ||
| TFAM | AAAGCTTCGCTGCGTGAAAG | 242 | 92.71% |
| NM_204100.2 | CACAGCTCAGGTTACACCGT | ||
| ATP5A | TCTGGCTCCTTACTCAGGCT | 143 | 101.78% |
| NM_001001935.3 | AAAGCAGTCAGAGAGCCACC | ||
| VDAC | TGCTGATCTGGGCAAGTCTG | 201 | 103.54% |
| NM_001033869.3 | TTCTCCGTGAACATCAGCCC | ||
| PPARGC1A | AAAACGGAGGGATGGCGAAT | 134 | 98.03% |
| NM_001006457.2 | TCGAGCAGCTGTGTTCATGT | ||
| CA4 | GTGAAGACCCTCGACACTGG | 282 | 100.92% |
| XM_415893.8 | GCTGTTCCTCTTGGACTCCC | ||
| CIDEA | ATGGTTGCAAGCAAAGCTGG | 103 | 104.89% |
| NM_001195123.2 | GTGATGCAAAGCAACAGGCA | ||
| CD137 | TTGCCTTGCTGCTCTACCTC | 117 | 93.80% |
| NM_001025366.3 | AGCTGCGCTGATAGACATCC | ||
| TMEM26 | TGATGGTTCTGCTCAACGCT | 145 | 91.29% |
| NM_001199598.2 | CCTGTCTCCAGGCAAAGGAG | ||
| RB1 | GAAACCTCCACAAACGGCAC | 184 | 99.25% |
| NM_204419.2 | GCCCCAATGCAACACACATT | ||
| RBL1 | CACAAAGTGTGAGCCGGTTG | 285 | 94.17% |
| XM_417312.8 | TCCCATGCAGTCTTCGCATT | ||
| HSL | TATGGGAGGTGTCTCGGGTT | 231 | 109.34% |
| XM_040657096.1 | AAGCTCTTCCAGAAGCCCAC | ||
| ATGL | TCACCTTCAGCGTCCAAGTC | 267 | 108.68% |
| NM_001113291.1 | AGCAGGTAGGGGACCATCAT | ||
| PLIN1 | GACCACAGCAAGGTACACGA | 177 | 102.56% |
| XM_015292005.3 | GATTGCTGCTGGGAGACCTT | ||
| PPARA | ATGCCAAGGTCTGAGAAGGC | 168 | 108.68% |
| XM_025150258.2 | CCCTGCAAGGATGACTCTGG | ||
| PPARG | ACCTCACGAGGAGTCTTCCA | 287 | 94.92% |
| NM_001001460.1 | GCTTCTCCTTCTCCGCTTGT | ||
| CEBPB | CTCCTACCTGGGCTACCAGT | 195 | 95.30% |
| NM_205253.2 | TTGTACTCGTCGCTGTGCTT | ||
| FASN | GCTAAGATGGCATTGCACGG | 300 | 106.28% |
| NM_205155.3 | TGCCAGAGCCTCCACTATCT | ||
| SCD1 | ACACCTTAGGGCTCAATGCC | 171 | 98.84% |
| NM_204890.1 | TGGTGGAGTAGTCGTAGGGG | ||
| CD36 | CTCTTTGTGGCCTTTGCACC | 291 | 107.71% |
| XM_040686380.1 | CAGCACAGCACCAATGACAG | ||
| FATP | GGTGAGAAGTGGACCTTCCG | 165 | 102.21% |
| NM_001039602.2 | CGCTTCGATGCTGACTTTCG | ||
| Beclin1 | ACAGTGGTCAGTTTGGCACA | 285 | 105.82% |
| NM_001006332.1 | AGAAAGCCACCATCGCATGA | ||
| LC3 | GTTTCCAATGCCTGCGTGTT | 244 | 94.54% |
| NM_001031461.1 | AACAGGGTCCTAATGCCAGC | ||
| FTO | TCACCAAGGCGACCTCTACT | 88 | 103.54% |
| XM_040707054.1 | GCTGAACCGAGGTGAAAAGC | ||
| METTL14 | ATTCGACCAGGATGGCTGAC | 155 | 99.25% |
| XM_040698977.1 | GACTTGGGTGGTGGTGACTT | ||
| METTL3 | ATCCTGGAGCTGCTCAACAC | 195 | 108.20% |
| XM_040655036.1 | AGATTCGTCCGTGTGCTTGT | ||
| YTHDF1 | AGTTCAGCATACGGGAGCAG | 236 | 93.07% |
| XM_040650685.1 | CCACCATTACCAGAGAGGGC | ||
| YTHDF2 | AAGGCCAAGGCAACAAAGTG | 110 | 94.54% |
| XM_040690125.1 | ATATGCATTGTTCGGCCGGG | ||
| YTHDF3 | GTGACCCTCCAATGCCATACT | 108 | 105.82% |
| NM_001006391.1 | GGGTGTTTCCTAATGCCCCA | ||
| PPIA | GTCGTGTTCTTCGACATCGC | 265 | 106.76% |
| XM_046903390.1 | CGAAGAGAAAATGGCGGCG |
| Name | X Site | N Site |
|---|---|---|
| ATGL-3’UTR-up | tagccagtaccgtagtgcgtgGCAGGCACTCTTTTGCACAG | tagccagtaccgtagtgcgtgTCTTTTGCACAGTCCAGAGG |
| ATGL-3’UTR-down | CCAGAGGTGGAAGTTTCATCcagaggctgagtcgctgcat | GGAAGTTTCATCTGAAATCCcagaggctgagtcgctgcat |
| PPARA-Exon7-up | tagccagtaccgtagtgcgtgTTCGGTAAGTTCTGTGACAG | tagccagtaccgtagtgcgtgTGTGACAGTCTCTACAGAGG |
| PPARA-Exon7-down | CTCTACAGAGGTACACTGGCcagaggctgagtcgctgcat | ACACTGGCAGCAGTGGAAGAcagaggctgagtcgctgcat |
| PPARA-Lastexon-up | tagccagtaccgtagtgcgtgTTAAAAATCCTTAATACATG | tagccagtaccgtagtgcgtgTGTGACAGTCTCTACAGAGG |
| PPARA-Lastexon-down | CCCTGTAGATTTCCTGCAGTcagaggctgagtcgctgcat | ACACTGGCAGCAGTGGAAGAcagaggctgagtcgctgcat |
| BECN1-Exon1-up | tagccagtaccgtagtgcgtgCATGAATTTTAGTGCTTTTG | tagccagtaccgtagtgcgtgTTAGTGCTTTTGTCCATTGC |
| BECN1-Exon1-down | CCATTGCTCCTCAGAGTTAAcagaggctgagtcgctgcat | CCTCAGAGTTAAATTGTGTTcagaggctgagtcgctgcat |
References
- Nematbakhsh, S.; Pei Pei, C.; Selamat, J.; Nordin, N.; Idris, L.H.; Abdull Razis, A.F. Molecular Regulation of Lipogenesis, Adipogenesis and Fat Deposition in Chicken. Genes 2021, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, H.; Jiang, K.J.; Jiao, H.C.; Song, Z.G. Corticosterone administration and high-energy feed results in enhanced fat accumulation and insulin resistance in broiler chickens. Br. Poult. Sci. 2008, 49, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Hasenmajer, V.; Sbardella, E.; Sciarra, F.; Minnetti, M.; Isidori, A.M.; Venneri, M.A. The Immune System in Cushing’s Syndrome. Trends Endocrinol. Metab. 2020, 31, 655–669. [Google Scholar] [CrossRef]
- Pantoja, C.; Huff, J.T.; Yamamoto, K.R. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell 2008, 19, 4032–4041. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef] [Green Version]
- Mir, N.; Chin, S.A.; Riddell, M.C.; Beaudry, J.L. Genomic and Non-Genomic Actions of Glucocorticoids on Adipose Tissue Lipid Metabolism. Int. J. Mol. Sci. 2021, 22, 8503. [Google Scholar] [CrossRef]
- Ramage, L.E.; Akyol, M.; Fletcher, A.M.; Forsythe, J.; Nixon, M.; Carter, R.N.; van Beek, E.J.; Morton, N.M.; Walker, B.R.; Stimson, R.H. Glucocorticoids Acutely Increase Brown Adipose Tissue Activity in Humans, Revealing Species-Specific Differences in UCP-1 Regulation. Cell Metab. 2016, 24, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Thuzar, M.; Law, W.P.; Ratnasingam, J.; Jang, C.; Dimeski, G.; Ho, K.K.Y. Glucocorticoids suppress brown adipose tissue function in humans: A double-blind placebo-controlled study. Diabetes Obes. Metab. 2018, 20, 840–848. [Google Scholar] [CrossRef]
- Griffin, H.D.; Guo, K.; Windsor, D.; Butterwith, S.C. Adipose tissue lipogenesis and fat deposition in leaner broiler chickens. J. Nutr. 1992, 122, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARalpha in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, J.K. Nonalcoholic steatosis and steatohepatitis. III. Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am. J. Physiol. Gastrointest Liver Physiol. 2001, 281, G1333–G1339. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.C.; Gil-Gomez, G.; Hegardt, F.G.; Haro, D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J. Biol. Chem. 1994, 269, 18767–18772. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mizrachi, C.; Weng, S.; Feng, C.; Finck, B.N.; Knutsen, R.H.; Leone, T.C.; Coleman, T.; Mecham, R.P.; Kelly, D.P.; Semenkovich, C.F. Dexamethasone induction of hypertension and diabetes is PPAR-alpha dependent in LDL receptor-null mice. Nat. Med. 2003, 9, 1069–1075. [Google Scholar] [CrossRef]
- Lee, H.Y.; Gao, X.; Barrasa, M.I.; Li, H.; Elmes, R.R.; Peters, L.L.; Lodish, H.F. PPAR-alpha and glucocorticoid receptor synergize to promote erythroid progenitor self-renewal. Nature 2015, 522, 474–477. [Google Scholar] [CrossRef] [Green Version]
- Regnier, M.; Polizzi, A.; Lippi, Y.; Fouche, E.; Michel, G.; Lukowicz, C.; Smati, S.; Marrot, A.; Lasserre, F.; Naylies, C.; et al. Insights into the role of hepatocyte PPARalpha activity in response to fasting. Mol. Cell Endocrinol. 2018, 471, 75–88. [Google Scholar] [CrossRef]
- Bernal-Mizrachi, C.; Xiaozhong, L.; Yin, L.; Knutsen, R.H.; Howard, M.J.; Arends, J.J.; Desantis, P.; Coleman, T.; Semenkovich, C.F. An afferent vagal nerve pathway links hepatic PPARalpha activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metab. 2007, 5, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Hinds, T.D., Jr.; Kipp, Z.A.; Xu, M.; Yiannikouris, F.B.; Morris, A.J.; Stec, D.F.; Wahli, W.; Stec, D.E. Adipose-Specific PPARalpha Knockout Mice Have Increased Lipogenesis by PASK-SREBP1 Signaling and a Polarity Shift to Inflammatory Macrophages in White Adipose Tissue. Cells 2021, 11, 4. [Google Scholar] [CrossRef]
- Lemberger, T.; Staels, B.; Saladin, R.; Desvergne, B.; Auwerx, J.; Wahli, W. Regulation of the peroxisome proliferator-activated receptor alpha gene by glucocorticoids. J. Biol. Chem. 1994, 269, 24527–24530. [Google Scholar] [CrossRef]
- Lemberger, T.; Saladin, R.; Vazquez, M.; Assimacopoulos, F.; Staels, B.; Desvergne, B.; Wahli, W.; Auwerx, J. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J. Biol. Chem. 1996, 271, 1764–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Liu, B.; Nie, Z.; Duan, L.; Xiong, Q.; Jin, Z.; Yang, C.; Chen, Y. The role of m6A modification in the biological functions and diseases. Signal Transduct. Targe Ther. 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, X. Epigenetic regulation of adipose tissue expansion and adipogenesis by N(6) -methyladenosine. Obes. Rev. 2021, 22, e13124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, R.; Liu, Y.; Zhao, Y.; Bi, Z.; Yao, Y.; Liu, Q.; Shi, H.; Wang, F.; Wang, Y. m(6)A mRNA methylation controls autophagy and adipogenesis by targeting Atg5 and Atg7. Autophagy 2020, 16, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Liu, Y.; Yao, Y.; Zhao, Y.; Bi, Z.; Jiang, Q.; Liu, Q.; Cai, M.; Wang, F.; Wang, Y.; et al. FTO regulates adipogenesis by controlling cell cycle progression via m(6)A-YTHDF2 dependent mechanism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, Y.; Zhang, L.; Jia, Y.; Cai, D.; Qian, S.B.; Du, M.; Zhao, R. GR-mediated FTO transactivation induces lipid accumulation in hepatocytes via demethylation of m(6)A on lipogenic mRNAs. RNA Biol. 2020, 17, 930–942. [Google Scholar] [CrossRef]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D., Jr. Bilirubin Binding to PPARalpha Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [Green Version]
- Takei, R.; Inoue, T.; Sonoda, N.; Kohjima, M.; Okamoto, M.; Sakamoto, R.; Inoguchi, T.; Ogawa, Y. Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS ONE 2019, 14, e0223302. [Google Scholar] [CrossRef] [Green Version]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.; Colao, A. Complications of Cushing’s syndrome: State of the art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef]
- Campbell, J.E.; Peckett, A.J.; D’Souza, A.M.; Hawke, T.J.; Riddell, M.C. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am. J. Physiol. Cell Physiol. 2011, 300, C198–C209. [Google Scholar] [CrossRef]
- Cai, Y.; Song, Z.; Wang, X.; Jiao, H.; Lin, H. Dexamethasone-induced hepatic lipogenesis is insulin dependent in chickens (Gallus gallus domesticus). Stress 2011, 14, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Guo, Y.; Yuan, F.; Chen, S.; Yin, H.; Jiang, X.; Jiao, F.; Wang, F.; Ji, H.; Hu, G.; et al. Autophagy inhibition prevents glucocorticoid-increased adiposity via suppressing BAT whitening. Autophagy 2020, 16, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Giroud, M.; Tsokanos, F.F.; Caratti, G.; Kotschi, S.; Khani, S.; Jouffe, C.; Vogl, E.S.; Irmler, M.; Glantschnig, C.; Gil-Lozano, M.; et al. HAND2 is a novel obesity-linked adipogenic transcription factor regulated by glucocorticoid signalling. Diabetologia 2021, 64, 1850–1865. [Google Scholar] [CrossRef] [PubMed]
- Galitzky, J.; Bouloumie, A. Human visceral-fat-specific glucocorticoid tuning of adipogenesis. Cell Metab. 2013, 18, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Vienberg, S.G.; Bjornholm, M. Chronic glucocorticoid treatment increases de novo lipogenesis in visceral adipose tissue. Acta Physiol. 2014, 211, 257–259. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Cho, Y.K.; Saha, A.; Kwon, H.J.; Park, J.H.; Kim, M.; Jung, Y.S.; Kim, S.N.; Choi, C.; Seong, J.K.; et al. Adipocyte-specific Beclin1 deletion impairs lipolysis and mitochondrial integrity in adipose tissue. Mol. Metab. 2020, 39, 101005. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, E.; Goldberg, E.B.; Makarova, K.S.; Lin, L.; Brown, W.J.; Jackson, C.L. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006, 7, 106–113. [Google Scholar] [CrossRef]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Zimmermann, R.; Hayn, M.; Theussl, C.; Waeg, G.; Wagner, E.; Sattler, W.; Magin, T.M.; Wagner, E.F.; Zechner, R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J. Biol. Chem. 2002, 277, 4806–4815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, K.; Shen, W.J.; Patel, S.; Natu, V.; Wang, J.; Osuga, J.; Ishibashi, S.; Kraemer, F.B. Resistance to high-fat diet-induced obesity and altered expression of adipose-specific genes in HSL-deficient mice. Am. J. Physiology. Endocrinol. Metab. 2003, 285, E1182–E1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiya, M.; Osuga, J.; Okazaki, H.; Yahagi, N.; Harada, K.; Shen, W.J.; Tamura, Y.; Tomita, S.; Iizuka, Y.; Ohashi, K.; et al. Absence of hormone-sensitive lipase inhibits obesity and adipogenesis in Lep ob/ob mice. J. Biol. Chem. 2004, 279, 15084–15090. [Google Scholar] [CrossRef] [Green Version]
- Anthonsen, M.W.; Degerman, E.; Holm, C. Partial purification and identification of hormone-sensitive lipase from chicken adipose tissue. Biochem. Biophys. Res. Commun. 1997, 236, 94–99. [Google Scholar] [CrossRef]
- Honda, K.; Kurachi, K.; Takagi, S.; Saneyasu, T.; Kamisoyama, H. Role of Corticosterone in Lipid Metabolism in Broiler Chick White Adipose Tissue. J. Poult. Sci. 2022, 59, 152–158. [Google Scholar] [CrossRef]
- Choi, G.E.; Han, H.J. Glucocorticoid impairs mitochondrial quality control in neurons. Neurobiol. Dis. 2021, 152, 105301. [Google Scholar] [CrossRef]
- Picard, M.; Juster, R.P.; McEwen, B.S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 2014, 10, 303–310. [Google Scholar] [CrossRef]
- Luijten, I.H.N.; Brooks, K.; Boulet, N.; Shabalina, I.G.; Jaiprakash, A.; Carlsson, B.; Fischer, A.W.; Cannon, B.; Nedergaard, J. Glucocorticoid-Induced Obesity Develops Independently of UCP1. Cell Rep. 2019, 27, 1686–1698.e5. [Google Scholar] [CrossRef] [Green Version]
- Sotome, R.; Hirasawa, A.; Kikusato, M.; Amo, T.; Furukawa, K.; Kuriyagawa, A.; Watanabe, K.; Collin, A.; Shirakawa, H.; Hirakawa, R.; et al. In vivo emergence of beige-like fat in chickens as physiological adaptation to cold environments. Amino Acids 2021, 53, 381–393. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Lee, J.Y.; Teraminami, A.; Kim, Y.I.; Hirai, S.; Uemura, T.; Inoue, H.; Takahashi, N.; Kawada, T. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J. Lipid Res. 2011, 52, 873–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wake, D.J.; Stimson, R.H.; Tan, G.D.; Homer, N.Z.; Andrew, R.; Karpe, F.; Walker, B.R. Effects of peroxisome proliferator-activated receptor-alpha and -gamma agonists on 11beta-hydroxysteroid dehydrogenase type 1 in subcutaneous adipose tissue in men. J. Clin. Endocrinol. Metab. 2007, 92, 1848–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, T.; Yang, Y.; Wei, H.; Xie, X.; Lu, J.; Zeng, Q.; Peng, J.; Zhou, Y.; Jiang, S.; Peng, J. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 2019, 47, 6130–6144. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Song, C.; Wang, N.; Li, S.; Liu, Q.; Sun, Z.; Wang, K.; Yu, S.C.; Yang, Q. NADP modulates RNA m(6)A methylation and adipogenesis via enhancing FTO activity. Nat. Chem. Biol. 2020, 16, 1394–1402. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Fernandez-Quintela, A.; Garcia-Marzo, C.; Ayo, J.; Dentin, R.; Portillo, M.P. cis-9,trans-11,cis-15 and cis-9,trans-13,cis-15 CLNA mixture activates PPARalpha in HEK293 and reduces triacylglycerols in 3T3-L1 cells. Lipids 2011, 46, 1005–1012. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.; Han, D.; Ma, H.; Weng, X.; Chen, K.; Shi, H.; He, C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.S.; Hao, Y.J.; Sun, B.F.; Sun, H.Y.; Li, A.; Ping, X.L.; Lai, W.Y.; et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef] [Green Version]
- Ke, S.; Alemu, E.A.; Mertens, C.; Gantman, E.C.; Fak, J.J.; Mele, A.; Haripal, B.; Zucker-Scharff, I.; Moore, M.J.; Park, C.Y.; et al. A majority of m6A residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes Dev. 2015, 29, 2037–2053. [Google Scholar] [CrossRef]
- Choi, J.; Ieong, K.W.; Demirci, H.; Chen, J.; Petrov, A.; Prabhakar, A.; O’Leary, S.E.; Dominissini, D.; Rechavi, G.; Soltis, S.M.; et al. N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat. Struct. Mol. Biol. 2016, 23, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Chai, G.; Wu, Y.; Li, J.; Chen, F.; Liu, J.; Luo, G.; Tauler, J.; Du, J.; Lin, S.; et al. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat. Commun. 2019, 10, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Dong, L.; Liu, X.M.; Guo, J.; Ma, H.; Shen, B.; Qian, S.B. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat. Commun. 2019, 10, 5332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Li, A.; Sun, B.; Sun, J.G.; Zhang, J.; Zhang, T.; Chen, Y.; Xiao, Y.; Gao, Y.; Zhang, Q.; et al. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019, 29, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wei, J.; He, C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell 2019, 74, 640–650. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Tang, Q.; Wei, L.; Zhang, X.; Jia, G. An Elongation- and Ligation-Based qPCR Amplification Method for the Radiolabeling-Free Detection of Locus-Specific N(6) -Methyladenosine Modification. Angew. Chem. Int. Ed. Engl. 2018, 57, 15995–16000. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhang, A.; Liu, X.; Yang, Y.; Zhao, R.; Jia, Y. m6A-Mediated PPARA Translational Suppression Contributes to Corticosterone-Induced Visceral Fat Deposition in Chickens. Int. J. Mol. Sci. 2022, 23, 15761. https://doi.org/10.3390/ijms232415761
Zhou Z, Zhang A, Liu X, Yang Y, Zhao R, Jia Y. m6A-Mediated PPARA Translational Suppression Contributes to Corticosterone-Induced Visceral Fat Deposition in Chickens. International Journal of Molecular Sciences. 2022; 23(24):15761. https://doi.org/10.3390/ijms232415761
Chicago/Turabian StyleZhou, Zixuan, Aijia Zhang, Xinyi Liu, Yang Yang, Ruqian Zhao, and Yimin Jia. 2022. "m6A-Mediated PPARA Translational Suppression Contributes to Corticosterone-Induced Visceral Fat Deposition in Chickens" International Journal of Molecular Sciences 23, no. 24: 15761. https://doi.org/10.3390/ijms232415761






