Key Characteristics and Development of Psychoceuticals: A Review
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biochemical Mechanisms of Drugs
2.1.1. Serotonin Receptor-Targeted Drugs
2.1.2. NMDA Receptor-Targeted Drugs
2.1.3. Opioid Receptor Targeted Drugs
2.1.4. Multi-Receptor Targeted Drugs
2.1.5. Other Receptor Targeted Drugs
2.2. Pre-Clinical/Clinical Benefits and Risks of Drugs
- LSD includes delusions, visual hallucinations, distortion of one’s sense of time and identity, impaired depth and time perception, artificial sense of euphoria or certainty, distorted perception of the size and shape of objects, movements, colors, sounds, touch, and the user’s body image, severe, terrifying thoughts and feelings, fear of losing control, fear of death, and panic attacks [52].
- Reports of 5-MeO-DMT show agitation and tachycardia with periodic reports of hyperthermia, seizures, coma, increased serum creatinine, and life-threatening experiences, such as cardiac arrest and possible death [53].
- Ibogaine has side effects such as nausea, headache, and visual changes; the most common side effects are reported in [46].
- Noribogaine’s most common adverse events are headache and epistaxis [56].
- Peyote is classified as possessing the highest potential for use disorder and misuse [73].
- Salvinorin A has adverse outcomes, including fear, panic, paranoia, agitated delirium, sadness, irritability, augmented perspiration, and chills [30]. In addition, consumers can feel a lack of insight, making them susceptible to harm, which can occur during the performance of complex tasks, such as driving [30]. Gastrointestinal malaises (e.g., nausea and vomiting) have also been reported [30].
- Ketamine’s side effects include nausea/vomiting and epigastric pain. A rapid ketamine IV injection can cause transient apnea, cystitis, and contracted bladder [67].
- N, N-dimethyltryptamine’s side effects include increased levels of corticotropin, cortisol, prolactin, and growth hormone when administered to human volunteers [11].
- Kratom has shown mental health effects, primarily withdrawal symptoms [71].
2.3. Clinical Trials
2.4. Limitations
2.5. Future Directions
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ban, T.A. Academic psychiatry and the pharmaceutical industry. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L. Fundamental and clinical neuropharmacology, a terra incognita with constantly expanding frontiers. Fundam. Clin. Pharmacol. 2021, 35, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.E.; Seeman, M.V.; Greig, N.H.; Lahiri, D.K. What can triumphs and tribulations from drug research in Alzheimer’s disease tell us about the development of psychotropic drugs in general? Lancet Psychiatry 2015, 2, 756–764. [Google Scholar] [CrossRef] [Green Version]
- Spedding, M. New directions for drug discovery. Dialogues Clin. Neurosci. 2006, 8, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Huskamp, H.A. Managing psychotropic drug costs: Will formularies work? Health Aff. 2003, 22, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, L.; Vannoy, S.; Mintzes, B.; Shaughnessy, A.F. Under the Influence: The Interplay among Industry, Publishing, and Drug Regulation. Account. Res. 2016, 23, 257–279. [Google Scholar] [CrossRef]
- Carrier, F.; Banayan, D.; Boley, R.; Karnik, N. Ethical challenges in developing drugs for psychiatric disorders. Prog. Neurobiol. 2017, 152, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Hirshbein, L. Looking back to the future of psychopharmacology. J. Nerv. Ment. Dis. 2012, 200, 1109–1112. [Google Scholar] [CrossRef]
- Shen, H.W.; Jiang, X.L.; CWinter, J.; Yu, A.M. Psychedelic 5-Methoxy-N,N-Dimethyltryptamine: Metabolism, Pharmacokinetics, Drug Interactions, and Pharmacological Actions. Curr. Drug Metab. 2010, 11, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.M. Indolealkylamines: Biotransformations and Potential Drug–Drug Interactions. AAPS J. 2008, 10, 242–253. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Gatch, M.B. Neuropharmacology of N,N-dimethyltryptamine. Brain Res. Bull. 2016, 126, 74–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute on Drug Abuse. What Are MDMA’s Effects on the Brain? National Institute on Drug Abuse: Rockville, MD, USA, 2017. Available online: https://nida.nih.gov/publications/research-reports/mdma-ecstasy-abuse/what-are-mdmas-effects-on-brain (accessed on 16 September 2022).
- Andén, N.E.; Corrodi, H.; Fuxe, K.; Hökfelt, T. Evidence for a central 5-hydroxytryptamine receptor stimulation by lysergic acid diethylamide. Br. J. Pharmacol. 1968, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Oliveira, R.J.; Pereira, C.L.; da Silva, D.D. Pharmacokinetic and Pharmacodynamic Aspects of Peyote and Mescaline: Clinical and Forensic Repercussions. Curr. Mol. Pharmacol. 2019, 12, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Cybin. CYB003. Cybin. Available online: https://cybin.com/cyb003/ (accessed on 20 September 2022).
- Schmidt, C.J.; Sorensen, S.M.; Kenne, J.H.; Carr, A.A.; Palfreyman, M.G. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Cybin. CYB004. Cybin. Available online: https://cybin.com/cyb004/ (accessed on 20 September 2022).
- Barker, S.A. N, N-Dimethyltryptamine (DMT), an Endogenous Hallucinogen: Past, Present, and Future Research to Determine Its Role and Function. Front. Neurosci. 2018, 12, 536. [Google Scholar] [CrossRef] [Green Version]
- Cybin. Development Pipeline. Cybin. Available online: https://cybin.com/development-pipeline/#:~:text=CYB005%3A%20Discovery%2DPhase%20Phenethylamine%20Derivative (accessed on 20 September 2022).
- Phenethylamine. RxList. Published 17 September 2019. Available online: https://www.rxlist.com/phenethylamine/supplements.htm (accessed on 24 September 2022).
- Irsfeld, M.; Spadafore, M.; Prüß, B.M. β-phenylethylamine, a small molecule with a large impact. Webmedcentral 2013, 4, 4409. [Google Scholar]
- About Psilocybin Therapy: Compass Pathways. Available online: https://compasspathways.com/our-work/about-psilocybin-therapy/ (accessed on 27 September 2022).
- Zanos, P.; Highland, J.N.; Liu, X.; Troppoli, T.A.; Georgiou, P.; Lovett, J.; Gould, T.D. (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br. J. Pharmacol. 2019, 176, 2573–2592. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, L.; Hashimoto, K. Molecular Mechanisms Underlying the Antidepressant Actions of Arketamine: Beyond the NMDA Receptor. Mol. Psychiatry 2021, 27, 559–573. [Google Scholar] [CrossRef]
- Matveychuk, D.; Thomas, R.K.; Swainson, J.; Khullar, A.; MacKay, M.A.; Baker, G.B.; Dursun, S.M. Ketamine as an antidepressant: Overview of its mechanisms of action and potential predictive biomarkers. Adv. Psychopharmacol. 2020, 10, 2045125320916657. [Google Scholar] [CrossRef]
- Derakhshanian, S.; Zhou, M.; Rath, A.; Barlow, R.; Bertrand, S.; DeGraw, C.; Kaye, A.D. Role of Ketamine in the Treatment of Psychiatric Disorders. Health Psychol. Res. 2021, 9, 25091. [Google Scholar] [CrossRef] [PubMed]
- Atai Life Sciences. Deu-Mitragynine. Atai Life Sciences. Available online: https://www.atai.life/programs/deu-mitragynine/ (accessed on 28 September 2022).
- Shukla, A.K. Dwivedi-Agnihotri, H. Structure and function of β-arrestins, their emerging role in breast cancer, and potential opportunities for therapeutic manipulation. Adv. Cancer Res. 2020, 145, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Brito-da-Costa, A.M.; Dias-da-Silva, D.; Gomes, N.G.M.; Dinis-Oliveira, R.J.; Madureira-Carvalho, Á. Pharmacokinetics and Pharmacodynamics of Salvinorin A and Salvia divinorum: Clinical and Forensic Aspects. Pharmaceuticals 2021, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.J.; Shenvi, R.A. A review of salvinorin analogs and their kappa-opioid receptor activity. Bioorg. Med. Chem. Lett. 2018, 28, 1436–1445. [Google Scholar] [CrossRef]
- Glick, S.D.; Maisonneuve, I.S. Mechanisms of antiaddictive actions of ibogaine. Ann. N. Y. Acad. Sci. 1998, 844, 214–226. [Google Scholar] [CrossRef]
- Healthtown. Pharmacodynamics, Pharmacokinetics and Chemistry of Ibogaine in Ibogaine Treatment: Health Town; (Canada) 2021. Available online: https://healthtown.ca/pharmacodynamics-pharmacokinetics-and-chemistry-of-ibogaine-in-ibogaine-treatment/#:~:text=The%20mechanism%20of%20Ibogaine%20includes%20action%20like%20opioid (accessed on 28 September 2022).
- Atai Life Sciences. Ibogaine & Noribogaine. Atai Life Sciences. Available online: https://atai.life/programs/ibogaine/ (accessed on 28 September 2022).
- Maillet, E.L.; Milon, N.; Heghinian, M.D.; Fishback, J.; Schürer, S.C.; Garamszegi, N.; Mash, D.C. Noribogaine is a G-protein biased κ-opioid receptor agonist. Neuropharmacology 2015, 99, 675–688. [Google Scholar] [CrossRef] [Green Version]
- Seelos Therapeutics. SLS-005 (Trehalose). Available online: https://seelostherapeutics.com/sls-005-trehalose/ (accessed on 30 September 2022).
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Poletti, A. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, Y.S.; Lee, S.J. Mechanism of neuroprotection by trehalose: Controversy surrounding autophagy induction. Cell Death Dis. 2018, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Davies, J.E.; Huang, Z.; Tunnacliffe, A.; Rubinsztein, D.C. Trehalose, a Novel mTOR-independent Autophagy Enhancer, Accelerates the Clearance of Mutant Huntingtin and α-Synuclein *. J. Biol. Chem. 2007, 282, 5641–5652. [Google Scholar] [CrossRef] [Green Version]
- Seelos Therapeutics, Inc. Seelos Therapeutics Receives a Notice of Allowance in the U.S. for an Additional Patent for SLS-007. Available online: https://www.prnewswire.com/news-releases/seelos-therapeutics-receives-a-notice-of-allowance-in-the-us-for-an-additional-patent-for-sls-007-301549493.html (accessed on 19 September 2022).
- SLS-007 (Peptidic Inhibitor). Seelos Therapeutics. Available online: https://seelostherapeutics.com/sls-007/ (accessed on 19 September 2022).
- Dos Tenório, M.C.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D. MindMed Reports Topline Data from Phase 1 Trial of MM-110 in Development for the Treatment of Opioid Withdrawal. MindMed: New York, NY, USA, 2022; Available online: https://mindmed.co/news/press-release/mindmed-reports-topline-data-from-phase-1-trial-of-mm-110-in-development-for-the-treatment-of-opioid-withdrawal/ (accessed on 22 September 2022).
- Deu-Etifoxine. Atai Life Sciences. Available online: https://atai.life/programs/deu-etifoxine/#:~:text=Enter%20GABA%20Therapeutics%E2%80%99%20flagship%20compound%2C%20deuterated%20etifoxine.%20History (accessed on 24 September 2022).
- Corkery, J.M. Ibogaine as a treatment for substance misuse: Potential benefits and practical dangers. Prog. Brain Res. 2018, 242, 217–257. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Cape, G.; Tunnicliff, D.; Lockhart, M.; Lam, F.; Hung, N.; Friedhoff, L. Ascending Single-Dose, Double-Blind, Placebo-Controlled Safety Study of Noribogaine in Opioid-Dependent Patients. Clin. Pharmacol. Drug Dev. 2016, 5, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T.; Zippel, R.; Levy, R.; Saya, D.; Ezra, V.; Barg, J.; Vogel, Z. κ-Opioid receptor-transfected cell lines: Modulation of adenylyl cyclase activity following acute and chronic opioid treatments. FEBS Lett. 1995, 361, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brundin, P.; Dave, K.D.; Kordower, J.H. Therapeutic approaches to target alpha-synuclein pathology. Exp. Neurol. 2017, 298, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Zolunicant (MM-110) | Nicotinic α3β4 Receptor Inhibitor | MedChemExpress. MedchemExpress.com. Available online: https://www.medchemexpress.com/zolunicant.html (accessed on 22 September 2022).
- Programs & Research. MindMed. Available online: https://mindmed.co/programs-research/#scroll_section-2 (accessed on 22 September 2022).
- Bodnár, K.J.; Kakuk, P. Research ethics aspects of experimentation with LSD on human subjects: A historical and ethical review. Med. Health Care Philos. 2019, 22, 327–337. [Google Scholar] [CrossRef]
- Lancelotta, R.L.; Davis, A.K. Use of Benefit Enhancement Strategies among 5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT) Users: Associations with Mystical, Challenging, and Enduring Effects. J. Psychoact. Drugs 2020, 53, 237–281. [Google Scholar] [CrossRef]
- Davis, A.K.; Barsuglia, J.P.; Lancelotta, R.; Grant, R.M.; Renn, E. The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J. Psychopharmacol. 2018, 32, 779–792. [Google Scholar] [CrossRef]
- Cybin. Psychedelics to TherapeuticsTM. 2022. Available online: https://s28.q4cdn.com/259445127/files/doc_presentation/2022/Jan/Cybin-Psychedelics-to-Therapeutics2.0.pdf (accessed on 10 October 2022).
- Glue, P.; Lockhart, M.; Lam, F.; Hung, N.; Hung, C.-T.; Friedhoff, L. Ascending-dose study of noribogaine in healthy volunteers: Pharmacokinetics, pharmacodynamics, safety, and tolerability. J. Clin. Pharmacol. 2014, 55, 189–194. [Google Scholar] [CrossRef]
- Mash, D.C. Breaking the cycle of opioid use disorder with Ibogaine. Am. J. Drug Alcohol Abus. 2017, 44, 1–3. [Google Scholar] [CrossRef]
- Uthaug, M.V.; Davis, A.K.; Haas, T.F.; Davis, D.; Dolan, S.B.; Lancelotta, R.; Ramaekers, J.G. The epidemiology of mescaline use: Pattern of use, motivations for consumption, and perceived consequences, benefits, and acute and enduring subjective effects. J. Psychopharmacol. 2022, 36, 309–320. [Google Scholar] [CrossRef]
- Golani, L.K.; Divović, B.; Sharmin, D.; Pandey, K.P.; Mian, M.Y.; Cerne, R.; Witkin, J.M. Metabolism, pharmacokinetics, and anticonvulsant activity of a deuterated analog of the α2/3-selective GABAkine KRM-II-81. Biopharm. Drug Dispos. 2022, 43, 66–75. [Google Scholar] [CrossRef]
- Witkin, J.M.; Lippa, A.; Smith, J.L.; Jin, X.; Ping, X.; Biggerstaff, A.; Cook, J.M. The imidazodiazepine, KRM-II-81: An example of a newly emerging generation of GABAkines for neurological and psychiatric disorders. Pharmacol. Biochem. Behav. 2022, 213, 173321. [Google Scholar] [CrossRef] [PubMed]
- Listos, J.; Merska, A.; Fidecka, S. Pharmacological activity of salvinorin A, the major component of Salvia divinorum. Pharmacol. Rep. 2011, 63, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Portbury, S.D.; Hare, D.J.; Finkelstein, D.I.; Adlard, P.A. Trehalose improves traumatic brain injury-induced cognitive impairment. PLoS ONE 2017, 12, e0183683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, G.C.; Bandeira, I.D.; Correia-Melo, F.S.; Telles, M.; Mello, R.P.; Vieira, F.; Quarantini, L.C. Intravenous arketamine for treatment-resistant depression: Open-label pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Johannesdottir, A.; Sigurdsson, E. The use of psilocybin for treatment-resistant depression. Laeknabladid 2022, 108, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Holland, J. Ecstasy: The Complete Guide: A Comprehensive Look at the Risks and Benefits of MDMA; Inner Traditions/Bear & Co.: Rochester, VT, USA, 2001; Available online: https://books.google.com/books?hl=en&lr=&id=CUCcyklcO00C&oi=fnd&pg=PP12&dq=ecstasy+risks&ots=2Fr82FHvpe&sig=OwI7oLniItoG4ZsNbyCl4zytI9k#v=onepage&q=ecstasy%20risks&f=false (accessed on 5 October 2022).
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Doblin, R. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Gao, M.; Rejaei, D.; Liu, H. Ketamine use in current clinical practice. Acta Pharmacol. Sin. 2016, 37, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Ershad, M.; Vearrier, D. N Acetylcysteine. Nih.gov, 19 March 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537183/ (accessed on 13 October 2022).
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef] [PubMed]
- Swogger, M.T.; Walsh, Z. Kratom use and mental health: A systematic review. Drug Alcohol. Depend. 2018, 183, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Neuroscience Trials Australia, Seelos Therapeutics, Inc. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Treatment Effects and Safety of SLS-005 (Trehalose Injection, 90.5 mg/mL for Intravenous Infusion) in Participants with Alzheimer’s Disease (AD); clinicaltrials.gov; Neuroscience Trials Australia: North Melbourne, Australia, 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05332678 (accessed on 6 October 2022).
- Preuss, C.V.; Kalava, A.; King, K.C. Prescription of Controlled Substances: Benefits and Risks. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Boot, B.P.; McGregor, I.S.; Hall, W. MDMA (Ecstasy) neurotoxicity: Assessing and communicating the risks. Lancet 2000, 355, 1818–1821. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.A.; Ribeiro, D.M.; Tapadas, M.; Cotrim, M.D. Ecstasy (3,4-methylenedioxymethamphetamine): Cardiovascular effects and mechanisms. Eur. J. Pharmacol. 2021, 903, 174156. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Osório, F.L.; Crippa, J.A.; Riba, J.; Zuardi, A.W.; Hallak, J.E. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): A systematic review of clinical trials published in the last 25 years. Ther. Adv. Psychopharmacol. 2016, 6, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Multidisciplinary Association for Psychedelic Studies. LSD-assisted Psychotherapy in Persons Suffering from Anxiety Associated with Advanced-Stage Life Threatening Diseases; A Phase-II, Double-Blind, Placebo-Controlled Dose-Response Pilot Study. clinicaltrials.gov; Multidisciplinary Association for Psychedelic Studies: San Jose, CA, USA, 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT00920387 (accessed on 8 October 2022).
- Davis, A.K.; So, S.; Lancelotta, R.; Barsuglia, J.P.; Griffiths, R.R. 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. Am. J. Drug Alcohol. Abuse 2019, 45, 161–169. [Google Scholar] [CrossRef]
- Reckweg, J.; Mason, N.L.; van Leeuwen, C.; Toennes, S.W.; Terwey, T.H.; Ramaekers, J.G. A Phase 1, Dose-Ranging Study to Assess Safety and Psychoactive Effects of a Vaporized 5-Methoxy-N, N-Dimethyltryptamine Formulation (GH001) in Healthy Volunteers. Front. Pharmacol. 2021, 12, 760671. [Google Scholar] [CrossRef]
- Globenewswire. COMPASS Pathways Presents Largest Ever Study of Psilocybin Therapy at American Psychiatric. Market Wire News. 23 May 2022. Available online: https://marketwirenews.com/news-releases/compass-pathways-presents-largest-ever-study-of-psil-7816213443619056.html (accessed on 12 October 2022).
- Check, E. Psychedelic drugs: The ups and downs of ecstasy. Nature 2004, 429, 126–128. [Google Scholar] [CrossRef]
- Cole, J.C.; Sumnall, H.R. Altered states: The clinical effects of Ecstasy. Pharmacol. Ther. 2003, 98, 35–58. [Google Scholar] [CrossRef]
- De La Torre, R.; Farre, M.; Ortuno, J.; Mas, M.; Brenneisen, R.; Roset, P.N.; Cami, J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br. J. Clin. Pharmacol. 2000, 49, 104–109. [Google Scholar] [CrossRef]
- Mash, D.C.; Duque, L.; Page, B.; Allen-Ferdinand, K. Ibogaine Detoxification Transitions Opioid and Cocaine Abusers Between Dependence and Abstinence: Clinical Observations and Treatment Outcomes. Front. Pharmacol. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.K.; Alper, K. Treatment of opioid use disorder with ibogaine: Detoxification and drug use outcomes. Am. J. Drug Alcohol. Abus. 2018, 44, 24–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappendijk, S.L.; Dzoljic, M.R. Inhibitory effects of ibogaine on cocaine self-administration in rats. Eur. J. Pharmacol. 1993, 241, 261–265. [Google Scholar] [CrossRef]
- Schenberg, E.E.; de Castro Comis, M.A.; Alexandre, J.F.M.; Chaves, B.D.R.; Tófoli, L.F.; da Silveira, D.X. Treating drug dependence with the aid of ibogaine: A qualitative study. J. Psychedelic Stud. 2017, 1, 10–19. [Google Scholar] [CrossRef]
- Köck, P.; Froelich, K.; Walter, M.; Lang, U.; Dürsteler, K.M. A systematic literature review of clinical trials and therapeutic applications of ibogaine. J. Subst. Abuse Treat. 2022, 138, 108717. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Bouso, J.C.; Hallak, J.E.C. The antiaddictive effects of ibogaine: A systematic literature review of human studies. J. Psychedelic Stud. 2017, 1, 20–28. [Google Scholar] [CrossRef]
- Brown, T.K. Ibogaine in the treatment of substance dependence. Curr. Drug Abuse Rev. 2013, 6, 3–16. [Google Scholar] [CrossRef] [PubMed]
- MacLean, K.A.; Johnson, M.W.; Reissig, C.J.; Prisinzano, T.E.; Griffiths, R.R. Dose-related effects of salvinorin A in humans: Dissociative, hallucinogenic, and memory effects. Psychopharmacology 2013, 226, 381–392. [Google Scholar] [CrossRef]
- Halpern, J.H.; Sherwood, A.R.; Hudson, J.I.; Yurgelun-Todd, D.; Pope, H.G., Jr. Psychological and cognitive effects of long-term peyote use among Native Americans. Biol. Psychiatry 2005, 58, 624–631. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, B.; Sun, W.; Wang, J.; Fu, Y.; Wang, A.; Han, R. Effect of esketamine on perioperative depressive symptoms in major surgery patients (PASSION II): Study protocol for a randomized controlled trial. BMJ Open 2022, 12, e056713. [Google Scholar] [CrossRef]

| Drug Name | Chemical Structure | Target Type | Method of Action | Citations |
|---|---|---|---|---|
| 5-Methoxy-N,N-dimenthyltryptamine (5-MeO-DMT) |  | Serotonin Receptor | 5-MeO-DMT acts as a 5-HT analog in the serotonin system with a high affinity for the 5-HT1A receptor. | Shen et al. [9] Yu [10] |
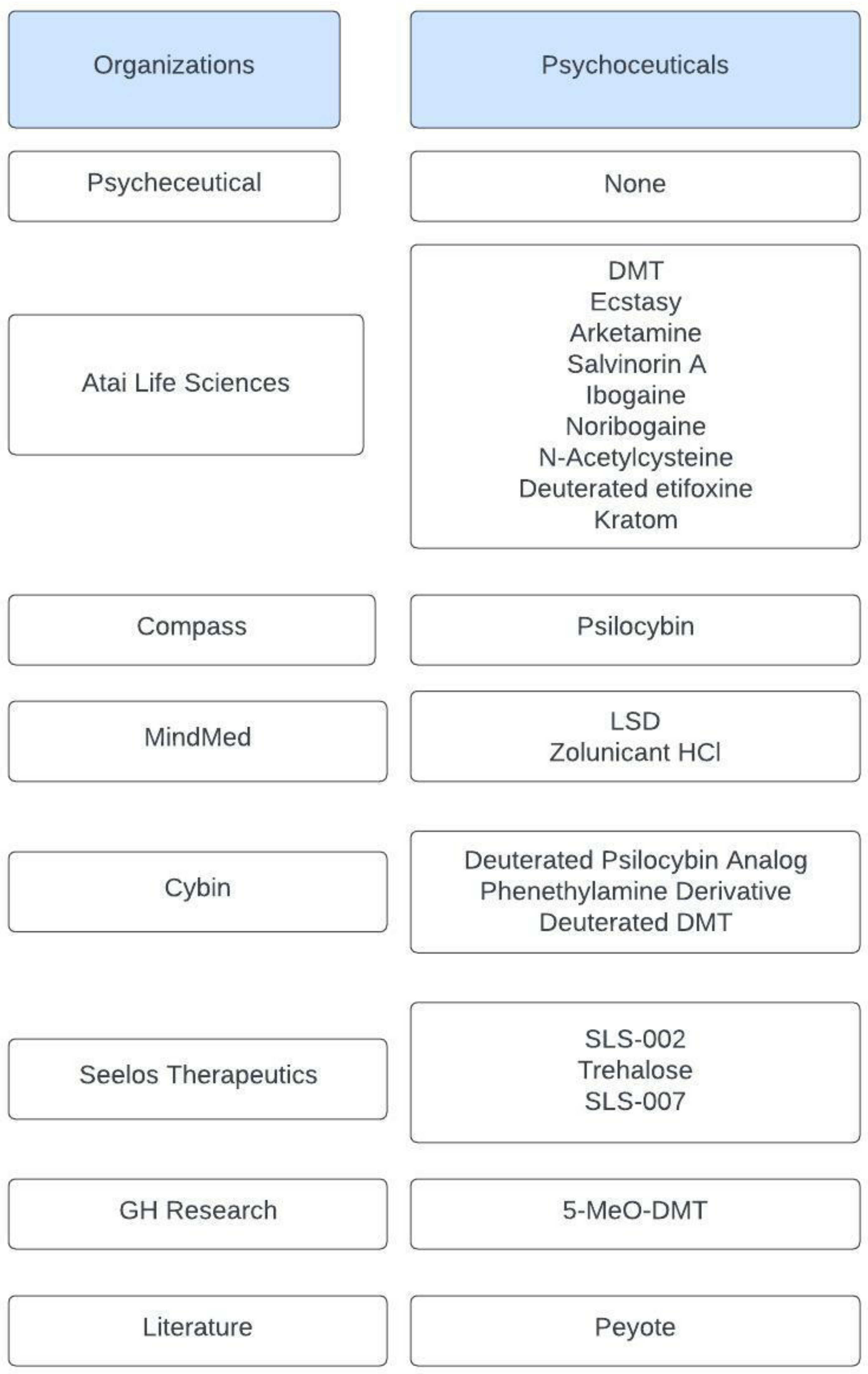
| N,N-dimethyltryptamine (DMT) | 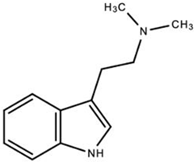 | DMT activates pharmacological receptors, such as serotonin receptors, within the synaptic cleft after its secretion as a neurotransmitter. Although the exact molecular pathway of DMT remains unclear, the effects of DMT as a modulator for serotonergic systems are currently being tested. | Carbonaro, Gatch [11] | |
| MDMA-Derivative (Ecstasy) | 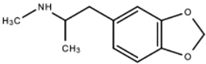 | MDMA and derivatives increase serotonin and norepinephrine release while blocking their reuptake within the synaptic cleft. | National Institute on Drug Abuse [12] | |
| Lysergic Acid Diethylamide (LSD) | 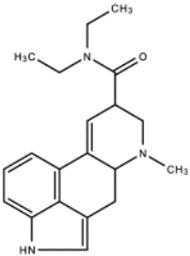 | LSD functions to increase serotonin concentration and acts as a 5-HT1A and 5-HT2 receptor agonist. LSD has inhibitory effects acting upon 5-HT1A receptors and stimulating effects acting upon 5-HT2 receptors. | Andén et al. [13] Passie et al. [14] | |
| Peyote | 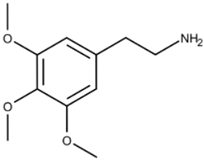 * Mescaline, the active component of Peyote | Mescaline, the active component of Peyote, functions as a serotonin receptor agonist, especially 5-HT2 receptors, with a higher affinity for 5-HT2C receptors than for 5-HT2A receptors and 5-HT2B receptors. Peyote increases cytosolic levels of IP3 through Gq coupling of G-proteins to activate phospholipase C, stimulating calcium channel opening. | Dinis-Oliveira et al. [15] | |
| Deuterated Psilocybin Analog (CYB003) | * | Once converted to psilocin, CYB003 functions as a partial agonist of 5-HT2A receptors, a subtype of 5-HT2 receptors that act as serotonin and G protein-coupled (GCP) receptors. | Cybin [16] Schmidt et al. [17] | |
| Deuterated DMT (CYB004) | * | CYB004 acts as an agonist of 5-HT2A serotonin receptors. Uptake of DMT occurs through serotonin uptake transporters (SERT) and is sequestered into synaptic vesicles via monoamine transporters. | Cybin [18] Barker [19] Carbonaro, Gatch [11] | |
| Phenethylamine derivative (CYB005) | * | CYB005 inhibits serotonin and dopamine transporters within the synaptic cleft. | Cybin [20] RxList [21] Irsfeld et al. [22] | |
| Psilocybin (COMP360) | 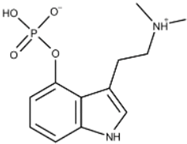 | COMP360 acts as an agonist of 5-HT2A receptors, a subtype of 5-HT2 receptors that act on serotonin and G protein-coupled (GCP) receptors. | Compass [23] Schmidt et al. [17] | |
| (R)-Ketamine (Arketamine) |  | NMDA Receptor | Arketamine acts as an NMDA receptor inhibitor with long lasting effects. Although the metabolism of Arketamine to 2R,6Rhydroxynorketamine (HNK) is known to have antidepressant effects, the exact molecular mechanisms of arketamine remain unclear. | Zanos et al. [24] Wei et al. [25] |
| SLS-002 (Ketamine) | * | SLS-002 functions as an NMDAr antagonist that binds to GABAergic interneurons, resulting in the disinhibition of glutamatergic neurons. A glutamate surge follows, stimulating AMPA receptors to potentiate BDNF and mTORC1 signaling pathways. | Matveychuk et al. [26] Derakhshanian et al. [27] | |
| Deu-mitragynine (Kratom) | * | Opioid Receptor | Deu-mitragynine acts as a mu-opioid (MOR) agonist with the ability to interact with G-protein coupled receptors, resulting in its analgesic effects. Deu-mitragynine has been shown to exhibit opioid-receptor-dependent analgesic effects and G-protein-based agonists of MOR. | Atai Life Sciences [28] Shukla et al. [29] |
| Salvinorin A | 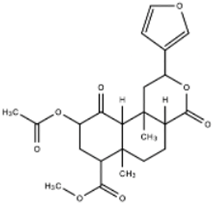 | Salvinorin A acts as an agonist of k-opioid receptors (KOPr) and is coupled to Gi/o proteins. Salvinorin A inhibits adenylyl cyclase and activates beta-arrestin mediated pathways and ERK/MAPK pathways. | Brito-da-Costa et al. [30] Roach, Shenvi [31] | |
| 12-methoxyibogamine (Ibogaine) | 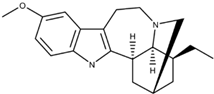 | Serotonin Receptor NMDA Receptor Opioid Receptor | Ibogaine functions as a kappa opioid receptor agonist, NMDA receptor antagonist, serotonin uptake receptor antagonist, and nicotinic receptor antagonist. Ibogaine’s underlying mechanism remains unclear. | Glick et al. [32] Healthtown [33] Atai Life Sciences [34] |
| 12-hydroxyibogamine (Noribogaine) |  | Noribogaine is the main metabolite of Ibogaine and has a higher affinity for opioid receptors than its parent compound. Noribogaine acts as an NMDA receptor antagonist, k-opioid receptor agonist, serotonin reuptake inhibitor, and µ-opioid receptor antagonist. The exact mechanism behind its effects on k-opioid receptors remains unclear. | Maillet et al. [35] Atai Life Sciences [34] | |
| Trehalose (SLS-005) | 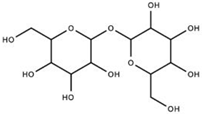 | GLUT8 Receptor | Trehalose stabilizes proteins, enhances autophagy, and enhances lysosomal pathways through mTOR-independent pathways. By increasing cytosolic levels of light chain 3-II, the inner and outer membranes of autophagosomes prepare for lysosome fusion. SLS-005 must act upon the mammalian trehalose transporter (GLUT8) for trehalose-induced autophagy in hepatocytes, but is unknown in neuronal cells. | Seelos Therapeutics [36] Rusmini et al. [37] Lee et al. [38] Sarkar et al. [39] |
| SLS-007 | * | Peptide | SLS-007 inhibits protein aggregation by targeting alpha-synuclein’s non-amyloid component cores (NAcore). | Inc ST [40] Seelos Therapeutics [41] |
| N-acetylcysteine (NAC) | 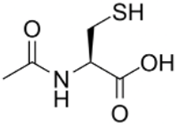 | NF-κB | NAC increases intracellular glutathione (GSH) concentration, modulates glutamatergic, neurotrophic, and inflammatory pathways, and suppresses NF-κB activity. | Tenório et al. [42] Bavarsad [43] |
| Zolunicant HCL (MM-110) | * | α3β4 receptors | MM-110 functions as an inhibitor for nicotinic α3β4 receptors and regulates dopamine levels during withdrawal. | O’Brien [44] |
| Deuterated Etifoxine (Etifoxine-d3) | 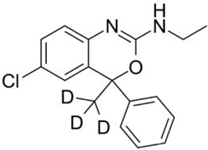 | GABA receptors | Deuterated etifoxine targets GABA channel subunits and projects GABAergic transmission via GABA receptors. Deuterated etifoxine can increase mitochondrial translocator protein (TSPO) to stimulate neurosteroid synthesis. | Atai Life Sciences [45] |
| Drug | Benefit | Citations | Drug | Benefit | Citations |
|---|---|---|---|---|---|
| LSD | Treats chronic alcoholism and anxiety | Bodnár, Kakuk [52] | MM-110 (zolunicant HCl) | Potential clinical utility to safely mitigate symptoms of opioid withdrawal | O’Brien [44] |
| 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) | Fewer suicidal thoughts, plans, attempts, and psychological distress | Lancelota, Davis [53] Davis et al. [54] | CYB005 (Phenethylamine derivative) | Treats neuroinflammation and psychiatric conditions | Cybin [20] Cybin [55] |
| Ibogaine | Humans: reduces cravings for heroin and withdrawal symptoms Rats: reduces opioid withdrawal, as well as cocaine and heroin self-administration | Corkery [46] | CYB004 (Deuterated DMT) | Anxiety disorders, including generalized anxiety disorder, GAD, and social anxiety disorder, SAD. | Cybin [18] |
| Noribogaine | Blocks opioid cravings Mice: fewer stress effects and less acute toxicity | Glue et al. [56] Mash [57] | CYB003 (Deuterated Psilocybin Analog) | Treats major depressive disorder (MDD) and alcohol use disorder (AUD). | Cybin [16] |
| Peyote (mescaline) | Improves depression, anxiety, and PTSD | Uthaug et al. [58] | Deuterated etifoxine | Treatment of anxiety | Golani et al. [59] Witkin et al. [60] |
| Salvinorin A | Feelings of calm and relaxation | Listos et al. [61] Brito et al. [30] | SLS-005 (Trehalose) | Improving functional outcomes following TBI | Portbury et al. [62] |
| Arketamine | Antidepressant | Zanos et al. [24] Leal et al. [63] | Psilocybin (COMP360) | Psilocybin significantly reduces depressive symptoms | Johannesdottir, Sigurdsson [64] |
| Ecstasy | Mice: extinction of fear memories Humans: attenuated amygdala response when shown negative stimuli | Holland [65] Mitchell et al. [66] | Ketamine | Anti-depressant, pain management, anti-inflammatory | Gao et al. [67] |
| N-acetylcysteine | Improves cognition in schizophrenics | Schwalfenberg [68] Muhammed, Vearrier [69] | SLS 007 | Treatment for PD | Inc ST [40] Vidović, Rikalovic [70] |
| N,N-dimethyltryptamine | Anti-anxiety/anti-psychotic via actions at the trace amino acid receptor | Carbonaro, Gatch [11] | Kratom | Substitute for opioids among people who are addicted. Kratom also enhances mood and relieves anxiety | Swogger, Walsh [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, G.H.; Dean, J.; Abreu, S.P.; Rodríguez, A.H.; Abbasi, C.; Hinson, M.; Lucke-Wold, B. Key Characteristics and Development of Psychoceuticals: A Review. Int. J. Mol. Sci. 2022, 23, 15777. https://doi.org/10.3390/ijms232415777
Cano GH, Dean J, Abreu SP, Rodríguez AH, Abbasi C, Hinson M, Lucke-Wold B. Key Characteristics and Development of Psychoceuticals: A Review. International Journal of Molecular Sciences. 2022; 23(24):15777. https://doi.org/10.3390/ijms232415777
Chicago/Turabian StyleCano, Genaro Herrera, Jordan Dean, Samuel Padilla Abreu, Amanda Hernández Rodríguez, Cyrena Abbasi, Madison Hinson, and Brandon Lucke-Wold. 2022. "Key Characteristics and Development of Psychoceuticals: A Review" International Journal of Molecular Sciences 23, no. 24: 15777. https://doi.org/10.3390/ijms232415777





