Investigations with Drugs and Pesticides Revealed New Species- and Substrate-Dependent Inhibition by Elacridar and Imazalil in Human and Mouse Organic Cation Transporter OCT2
Abstract
1. Introduction
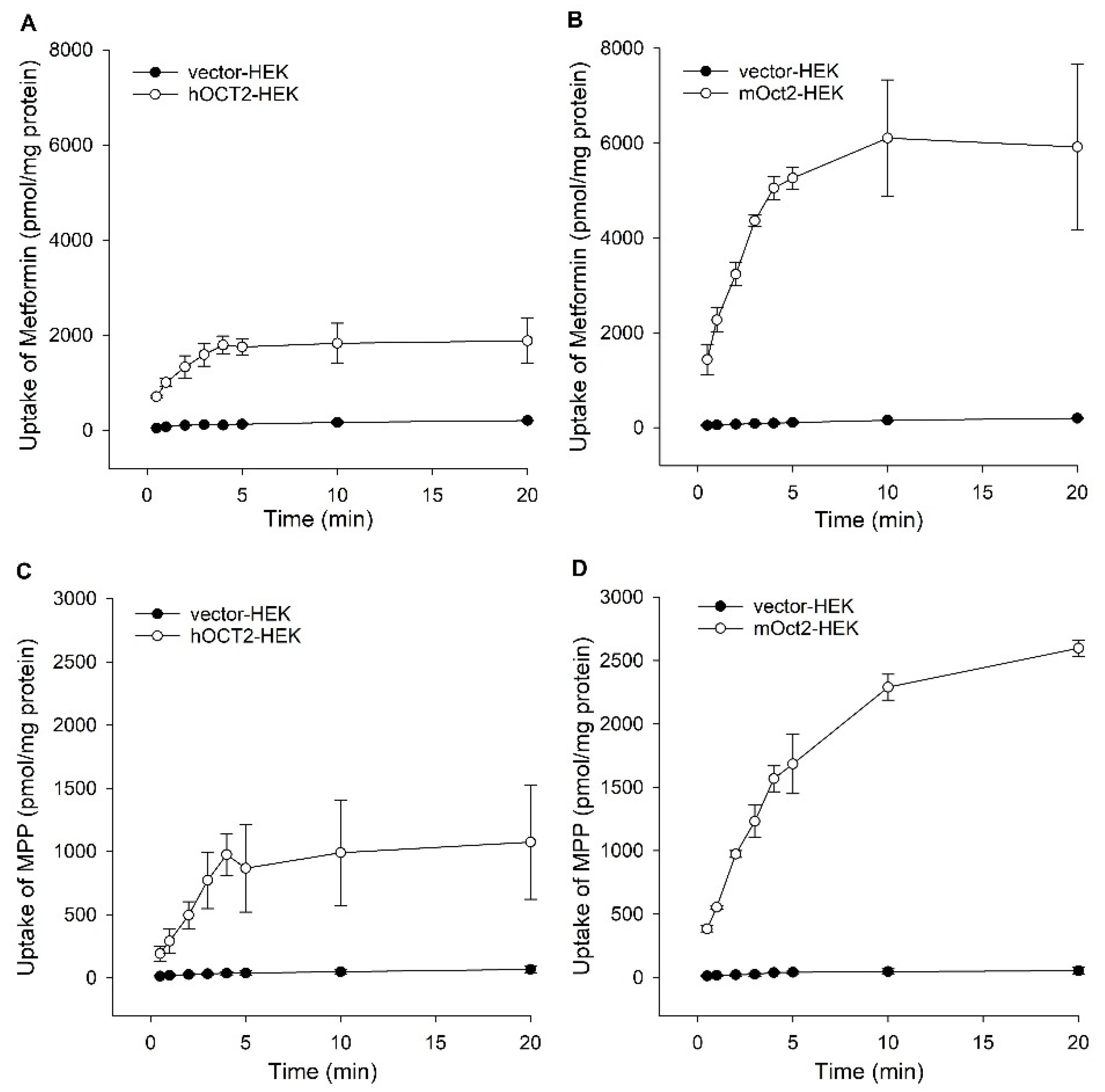
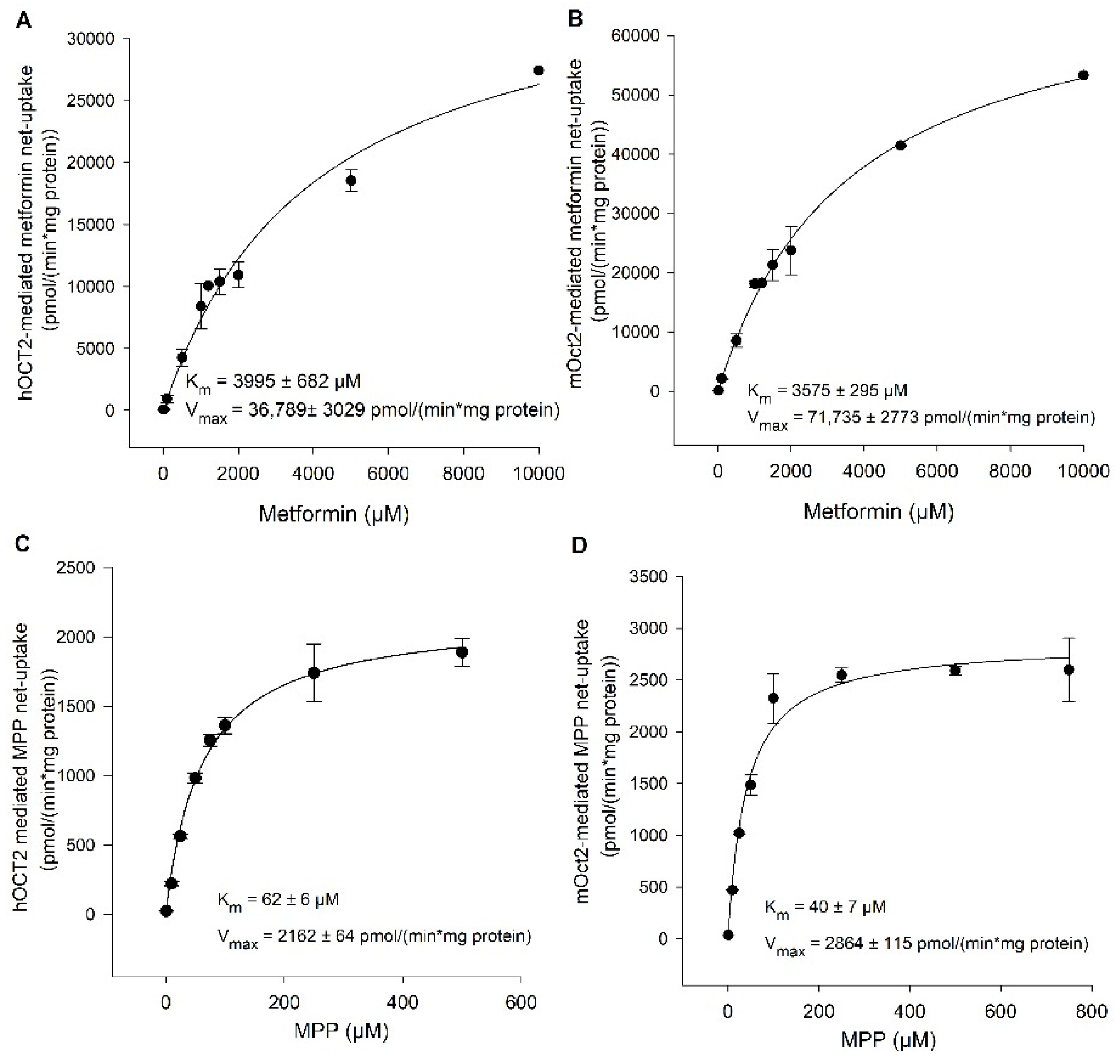
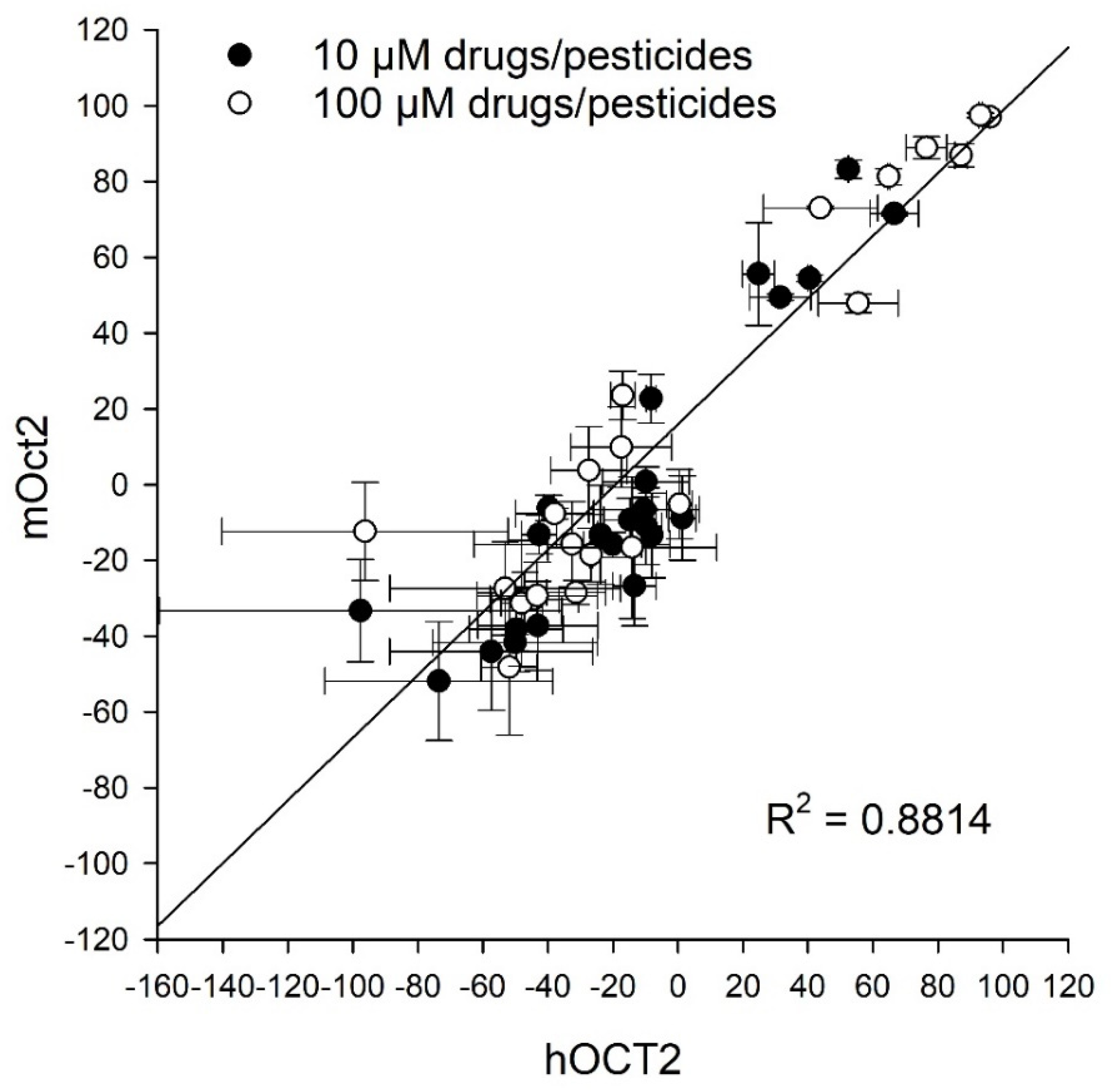
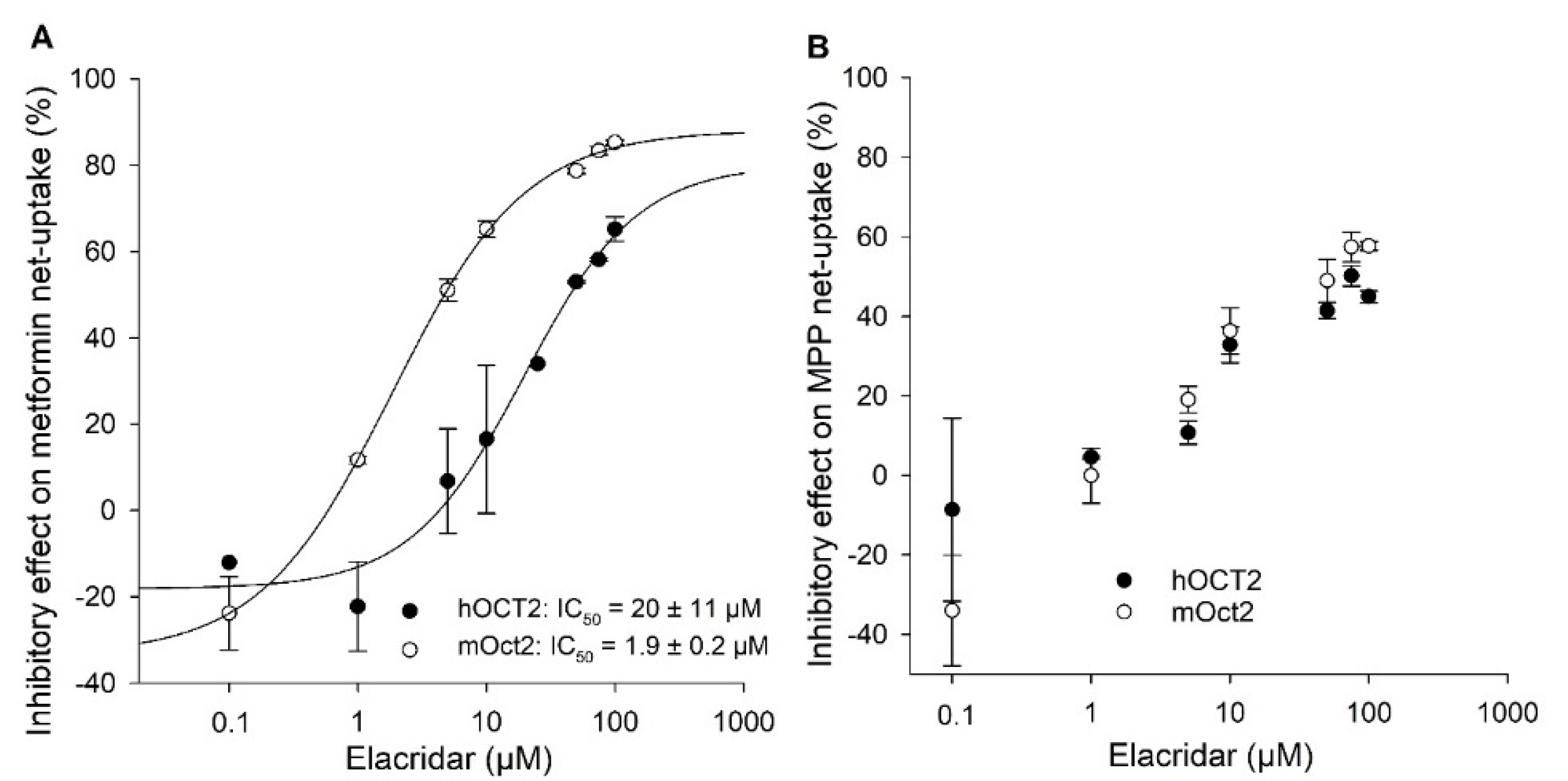
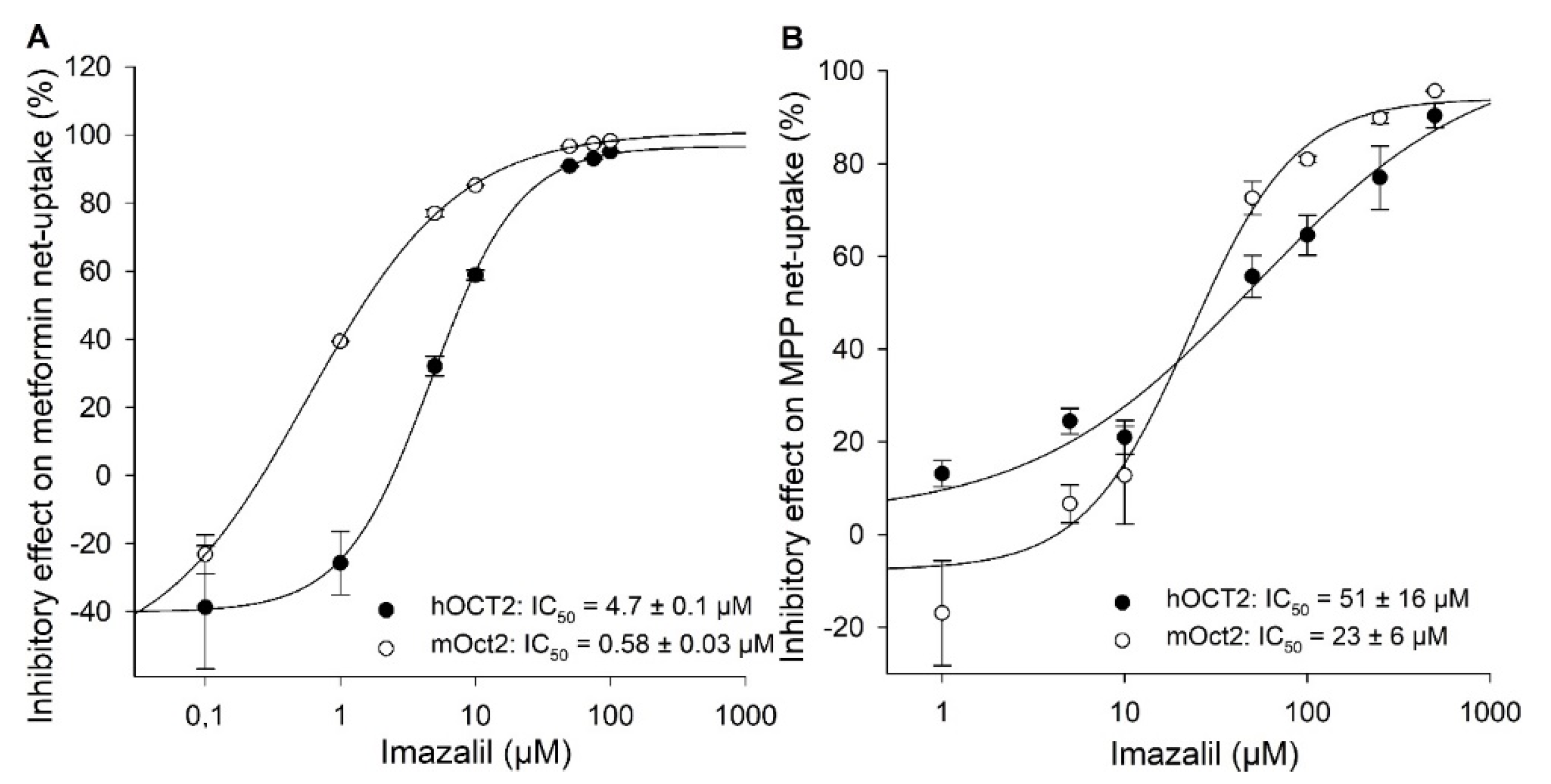
2. Results
Functional Characterization of hOCT2 and mOct2
3. Discussion
4. Materials and Methods
4.1. Material and Cell Lines
4.2. Transporter Mediated Uptake of Radiolabeled Substrates
4.3. Inhibition Experiments
4.4. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Motohashi, H.; Sakurai, Y.; Saito, H.; Masuda, S.; Urakami, Y.; Goto, M.; Fukatsu, A.; Ogawa, O.; Inui, K. Gene Expression Levels and Immunolocalization of Organic Ion Transporters in the Human Kidney. J. Am. Soc. Nephrol. 2002, 13, 866–874. [Google Scholar] [CrossRef]
- Motohashi, H.; Nakao, Y.; Masuda, S.; Katsura, T.; Kamba, T.; Ogawa, O.; Inui, K.I. Precise Comparison of Protein Localization among OCT, OAT, and MATE in Human Kidney. J. Pharm. Sci. 2013, 102, 3302–3308. [Google Scholar] [CrossRef]
- Lips, K.S.; Volk, C.; Schmitt, B.M.; Pfeil, U.; Arndt, P.; Miska, D.; Ermert, L.; Kummer, W.; Koepsell, H. Polyspecific Cation Transporters Mediate Luminal Release of Acetylcholine from Bronchial Epithelium. Am. J. Respir. Cell Mol. Biol. 2005, 33, 79–88. [Google Scholar] [CrossRef]
- Karbach, U.; Kricke, J.; Meyer-Wentrup, F.; Gorboulev, V.; Volk, C.; Loffing-Cueni, D.; Kaissling, B.; Bachmann, S.; Koepsell, H. Localization of Organic Cation Transporters OCT1 and OCT2 in Rat Kidney. Am. J. Physiol. Renal. Physiol. 2000, 279, F679–F687. [Google Scholar] [CrossRef]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef]
- Koepsell, H.; Lips, K.; Volk, C. Polyspecific Organic Cation Transporters: Structure, Function, Physiological Roles, and Biopharmaceutical Implications. Pharm. Res. 2007, 24, 1227–1251. [Google Scholar] [CrossRef]
- Koepsell, H. The SLC22 Family with Transporters of Organic Cations, Anions and Zwitterions. Mol. Aspects. Med. 2013, 34, 413–435. [Google Scholar] [CrossRef]
- Rennick, B.R.; Moe, G.K. Absorption and Renal Excretion of the Tetraethylammonium Ion. J. Pharmacol. Exp. Ther. 1947, 91, 210–217. [Google Scholar] [PubMed]
- Peters, L. Renal Tubular Excretion of Organic Bases. Pharmacol. Rev. 1960, 12, 1–35. [Google Scholar] [PubMed]
- Ullrich, K.J.; Papavassiliou, F.; David, C.; Rumrich, G.; Fritzsch, G. Contraluminal Transport of Organic Cations in the Proximal Tubule of the Rat Kidney. I. Kinetics of N1-Methylnicotinamide and Tetraethylammonium, Influence of K+, HCO3-, PH; Inhibition by Aliphatic Primary, Secondary and Tertiary Amines and Mono- and Bisquaternary Compounds. Pflugers. Arch 1991, 419, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Dantzler, W.H. Organic Acid (or Anion) and Organic Base (or Cation) Transport by Renal Tubules of Nonmammalian Vertebrates. J. Exp. Zool. 1989, 249, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Daunomycin Secretion by Killfish Renal Proximal Tubules. Am. J. Physiol. 1995, 269, R370–R379. [Google Scholar] [CrossRef]
- Miller, D.S.; Holohan, P.D. Organic Cation Secretion in Flounder Renal Tissue. Am. J. Physiol. 1987, 253, R861–R867. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Holliday, C.W. Organic Cation Secretion by Cancer Borealis Urinary Bladder. Am. J. Physiol. 1987, 252, R153–R159. [Google Scholar] [CrossRef]
- Burckhardt, G.; Pritchard, J.B. Organic Anion and Cation Antiporters. In The Kidney: Physiology & Pathophysiology, 3rd ed.; Donald, W.S., Gerhard, G., Eds.; Lippincott Williams&Wilkins: Philadelphia, PA, USA, 2000; Chapter 7; pp. 193–222. [Google Scholar]
- Hacker, K.; Maas, R.; Kornhuber, J.; Fromm, M.F.; Zolk, O. Substrate-Dependent Inhibition of the Human Organic Cation Transporter OCT2: A Comparison of Metformin with Experimental Substrates. PLoS ONE 2015, 10, e0136451. [Google Scholar] [CrossRef]
- Keller, T.; Gorboulev, V.; Mueller, T.D.; Dötsch, V.; Bernhard, F.; Koepsell, H. Rat Organic Cation Transporter 1 Contains Three Binding Sites for Substrate 1-Methyl-4-Phenylpyridinium per Monomer. Mol. Pharmacol. 2019, 95, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Multiple Binding Sites in Organic Cation Transporters Require Sophisticated Procedures to Identify Interactions of Novel Drugs. Biol. Chem. 2019, 400, 195–207. [Google Scholar] [CrossRef]
- Belzer, M.; Morales, M.; Jagadish, B.; Mash, E.A.; Wright, S.H. Substrate-Dependent Ligand Inhibition of the Human Organic Cation Transporter OCT2. J. Pharmacol. Exp. Ther. 2013, 346, 300–310. [Google Scholar] [CrossRef]
- Gründemann, D.; Liebich, G.; Kiefer, N.; Köster, S.; Schömig, E. Selective Substrates for Non-Neuronal Monoamine Transporters. Mol. Pharmacol. 1999, 56, 1–10. [Google Scholar] [CrossRef]
- Zolk, O.; Solbach, T.F.; König, J.; Fromm, M.F. Structural Determinants of Inhibitor Interaction with the Human Organic Cation Transporter OCT2 (SLC22A2). Naunyn. Schmiedebergs. Arch. Pharmacol. 2009, 379, 337–348. [Google Scholar] [CrossRef]
- Goralski, K.B.; Lou, G.; Prowse, M.T.; Gorboulev, V.; Volk, C.; Koepsell, H.; Sitar, D.S. The Cation Transporters ROCT1 and ROCT2 Interact with Bicarbonate but Play Only a Minor Role for Amantadine Uptake into Rat Renal Proximal Tubules. J. Pharmacol. Exp. Ther. 2002, 303, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Lancaster, C.S.; Schlatter, E.; Franke, R.M.; Sprowl, J.A.; Pavenstädt, H.; Massmann, V.; Guckel, D.; Mathijssen, R.H.J.; Yang, W.; et al. Proximal Tubular Secretion of Creatinine by Organic Cation Transporter OCT2 in Cancer Patients. Clin. Cancer Res. 2012, 18, 1101–1108. [Google Scholar] [CrossRef]
- Higgins, J.W.; Bedwell, D.W.; Zamek-Gliszczynski, M.J. Ablation of Both Organic Cation Transporter (OCT)1 and OCT2 Alters Metformin Pharmacokinetics but Has No Effect on Tissue Drug Exposure and Pharmacodynamics. Drug Metab. Dispos. 2012, 40, 1170–1177. [Google Scholar] [CrossRef]
- Jonker, J.W.; Wagenaar, E.; Van Eijl, S.; Schinkel, A.H. Deficiency in the Organic Cation Transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in Mice Abolishes Renal Secretion of Organic Cations. Mol. Cell Biol. 2003, 23, 7902–7908. [Google Scholar] [CrossRef]
- Kölz, C.; Schaeffeler, E.; Schwab, M.; Nies, A.T. Genetic and Epigenetic Regulation of Organic Cation Transporters. Handb. Exp. Pharmacol. 2021, 266, 81–100. [Google Scholar] [CrossRef]
- Horton, R.E.; Apple, D.M.; Owens, W.A.; Baganz, N.L.; Cano, S.; Mitchell, N.C.; Vitela, M.; Gould, G.G.; Koek, W.; Daws, L.C. Decynium-22 Enhances SSRI-Induced Antidepressant-like Effects in Mice: Uncovering Novel Targets to Treat Depression. J. Neurosci. 2013, 33, 10534–10543. [Google Scholar] [CrossRef] [PubMed]
- Hayer-Zillgen, M.; Bruss, M.; Bonisch, H. Expression and Pharmacological Profile of the Human Organic Cation Transporters HOCT1, HOCT2 and HOCT3. Br. J. Pharmacol. 2002, 136, 829–836. [Google Scholar] [CrossRef]
- Gorboulev, V.; Ulzheimer, J.C.; Akhoundova, A.; Ulzheimer-Teuber, I.; Karbach, U.; Quester, S.; Baumann, C.; Lang, F.; Busch, A.E.; Koepsell, H. Cloning and Characterization of Two Human Polyspecific Organic Cation Transporters. DNA Cell Biol. 1997, 16, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Floerl, S.; Kuehne, A.; Hagos, Y. Functional and Pharmacological Comparison of Human, Mouse, and Rat Organic Cation Transporter 1 toward Drug and Pesticide Interaction. Int. J. Mol. Sci. 2020, 21, 6871. [Google Scholar] [CrossRef]
- Song, I.S.; Shin, H.J.; Shin, J.G. Genetic Variants of Organic Cation Transporter 2 (OCT2) Significantly Reduce Metformin Uptake in Oocytes. Xenobiotica 2008, 38, 1252–1262. [Google Scholar] [CrossRef]
- Noskova, V.; Bottalico, B.; Olsson, H.; Ehinger, A.; Pilka, R.; Casslén, B.; Hansson, S.R. Histamine Uptake by Human Endometrial Cells Expressing the Organic Cation Transporter EMT and the Vesicular Monoamine Transporter-2. Mol. Hum. Reprod. 2006, 12, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, M.B.; Zur, A.A.; Khuri, N.; Kido, Y.; Kosaka, A.; Zhang, X.; Morrissey, K.M.; Sali, A.; Huang, Y.; Giacomini, K.M. Discovery of Potent, Selective Multidrug and Toxin Extrusion Transporter 1 (MATE1, SLC47A1) Inhibitors through Prescription Drug Profiling and Computational Modeling 2. J. Med. Chem. 2013, 56, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Severance, A.C.; Sandoval, P.J.; Wright, S.H. Correlation between Apparent Substrate Affinity and OCT2 Transport Turnover. J. Pharmacol. Exp. Ther. 2017, 362, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Minematsu, T.; Iwai, M.; Umehara, K.; Usui, T.; Kamimura, H. Characterization of Human Organic Cation Transporter 1 (OCT1/SLC22A1)- and OCT2 (SLC22A2)-Mediated Transport of 1-(2-Methoxyethyl)-2-Methyl-4,9-Dioxo-3-(Pyrazin-2-Ylmethyl)- 4,9-Dihydro-1H-Naphtho[2,3-d]Imidazolium Bromide (YM155 Monobromide), a Novel Small Molecule Survivin Suppressant. Drug Metab. Dispos. 2010, 38, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tahara, H.; Kusuhara, H.; Endou, H.; Koepsell, H.; Imaoka, T.; Fuse, E.; Sugiyama, Y. A Species Difference in the Transport Activities of H2 Receptor Antagonists by Rat and Human Renal Organic Anion and Cation Transporters. J. Pharmacol. Exp. Ther. 2005, 315, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Humphreys, J.E.; Webster, L.O.; Balakrishnan, A.; Keogh, J.P.; Kunta, J.R.; Serabjit-Singh, C.J.; Polli, J.W. In Vitro P-Glycoprotein Inhibition Assays for Assessment of Clinical Drug Interaction Potential of New Drug Candidates: A Recommendation for Probe Substrates. Drug Metab. Dispos. 2006, 34, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Pozza, A.; Muñoz-Martínez, F.; Bates, S.E.; Castanys, S.; Gamarro, F.; Di Pietro, A.; Pérez-Victoria, J.M. Flavonoid Structure-Activity Studies Identify 6-Prenylchrysin and Tectochrysin as Potent and Specific Inhibitors of Breast Cancer Resistance Protein ABCG2. Cancer Res. 2005, 65, 4852–4860. [Google Scholar] [CrossRef]
- Vermeer, L.M.M.; Isringhausen, C.D.; Ogilvie, B.W.; Buckley, D.B. Evaluation of Ketoconazole and Its Alternative Clinical CYP3A4/5 Inhibitors as Inhibitors of Drug Transporters: The In Vitro Effects of Ketoconazole, Ritonavir, Clarithromycin, and Itraconazole on 13 Clinically-Relevant Drug Transporters. Drug Metab. Dispos. 2016, 44, 453–459. [Google Scholar] [CrossRef]
- Floerl, S.; Kuehne, A.; Geyer, J.; Brockmoeller, J.; Tzvetkov, M.V.; Hagos, Y. Functional and Pharmacological Comparison of Human and Mouse Na+/Taurocholate Cotransporting Polypeptide (NTCP). SLAS Discov. 2021, 26, 1055–1064. [Google Scholar] [CrossRef]
- Floerl, S.; Kuehne, A.; Hagos, Y. Functional Characterization and Comparison of Human and Mouse Organic Anion Transporter 1 as Drugs and Pesticides Uptake Carrier. Eur. J. Pharm. Sci. 2022, 175, 106217. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Sorani, M.; Giacomini, K.M. Transport of Paraquat by Human Organic Cation Transporters and Multidrug and Toxic Compound Extrusion Family. J. Pharmacol. Exp. Ther. 2007, 322, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Guéniche, N.; Bruyere, A.; Ringeval, M.; Jouan, E.; Huguet, A.; Le Hégarat, L.; Fardel, O. Differential Interactions of Carbamate Pesticides with Drug Transporters. Xenobiotica 2020, 50, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]

| Drugs/Pos. Control Inhibitor * | Type of Drug | Charge at pH 7.4 | Inhibitory Effects (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| hOCT2 | mOct2 | |||||||||
| 10 µM | 100 µM | 10 µM | 100 µM | |||||||
| Mean | AD | Mean | AD | Mean | AD | Mean | AD | |||
| Zosuquidar | antineoplastic drug | 37% uncharged 63% cation | −10 | 5 | −47 | 11 | −11 | 10 | −30 | 7 |
| Procainamid | class I antiarrhythmic agent | 100% cation | −20 | 9 | −44 | 14 | −16 | 3 | −30 | 0 |
| Ritonavir | antiretroviral HIV | 100% cation | −10 | 13 | −38 | 12 | 1 | 4 | −8 | 1 |
| Ranitidine | H2 histamine receptor antagonist | 100% cation | −43 | 18 | −33 | 30 | −37 | 12 | −16 | 11 |
| Quinidine | class I antiarrhythmic agent | 100% cation | −50 | 14 | −31 | 9 | −38 | 11 | −28 | 3 |
| Amiodarone | class III antiarrhythmic agent | 100% cation | −10 | 17 | −27 | 12 | −7 | 0 | 4 | 12 |
| Reserpine | hypertension | 70% uncharged 30% cation | −13 | 7 | −27 | 1 | −27 | 11 | −19 | 7 |
| Cimetidine | H2 histamine receptor antagonist | 75% uncharged 25% cation | −50 | 25 | −17 | 4 | −42 | 2 | 24 | 6 |
| CyclosporinA | immunsuppressant | 100% cation | −8 | 1 | 0 | 4 | −13 | 11 | −5 | 9 |
| Verapamil | class IV antiarrhythmic agent | 100% cation | −8 | 2 | 44 | 18 | 23 | 6 | 73 | 0 |
| Elacridar | bioenhancer targeting drug resistance in tumors | 100% cation | 25 | 5 | 65 | 0 | 56 | 14 | 81 | 2 |
| Clonidine | hypertension | 100% cation | 40 | 0 | 76 | 6 | 54 | 1 | 89 | 3 |
| Ketoconazole | antifungal | 82% uncharged 18% cation | 31 | 9 | 87 | 2 | 49 | 1 | 87 | 3 |
| Decynium22 * | inhibitor for cation transporter | 100% cation | 67 | 8 | 96 | 0 | 72 | 1 | 97 | 0 |
| Pesticides | Type of Pesticide | Charge at pH 7.4 | Inhibitory Effects (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| hOCT2 | mOct2 | |||||||||
| 10 µM | 100 µM | 10 µM | 100 µM | |||||||
| Mean | AD | Mean | AD | Mean | AD | Mean | AD | |||
| Amitraz | insecticide | 100% cation | −98 | 62 | −96 | 44 | −33 | 13 | −12 | 13 |
| Atrazin | herbicide | 100% uncharged | −57 | 31 | −53 | 35 | −44 | 15 | −27 | 12 |
| Paraquat | herbicide | 100% cation | −15 | 8 | −52 | 9 | −9 | 6 | −48 | 18 |
| Glyphosat | herbicide | 73% anion 27% ± charge | −24 | 1 | −48 | 6 | −13 | 13 | −31 | 17 |
| Imidacloprid | insecticide | 100% ± charge | −74 | 35 | −43 | 18 | −52 | 16 | −29 | 9 |
| Prochloraz | fungicide | 100% cation | −43 | 5 | −17 | 15 | −13 | 5 | 10 | 11 |
| Azoxystrobin | fungicide | 100% uncharged | −40 | 2 | −14 | 26 | −6 | 3 | −17 | 19 |
| Propamocarb | fungicide | 100% uncharged | 1 | 4 | 55 | 12 | −9 | 11 | 48 | 2 |
| Imazalil | fungicide | 81% uncharged 19% cation | 52 | 0 | 93 | 0 | 83 | 2 | 97 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuehne, A.; Floerl, S.; Hagos, Y. Investigations with Drugs and Pesticides Revealed New Species- and Substrate-Dependent Inhibition by Elacridar and Imazalil in Human and Mouse Organic Cation Transporter OCT2. Int. J. Mol. Sci. 2022, 23, 15795. https://doi.org/10.3390/ijms232415795
Kuehne A, Floerl S, Hagos Y. Investigations with Drugs and Pesticides Revealed New Species- and Substrate-Dependent Inhibition by Elacridar and Imazalil in Human and Mouse Organic Cation Transporter OCT2. International Journal of Molecular Sciences. 2022; 23(24):15795. https://doi.org/10.3390/ijms232415795
Chicago/Turabian StyleKuehne, Annett, Saskia Floerl, and Yohannes Hagos. 2022. "Investigations with Drugs and Pesticides Revealed New Species- and Substrate-Dependent Inhibition by Elacridar and Imazalil in Human and Mouse Organic Cation Transporter OCT2" International Journal of Molecular Sciences 23, no. 24: 15795. https://doi.org/10.3390/ijms232415795
APA StyleKuehne, A., Floerl, S., & Hagos, Y. (2022). Investigations with Drugs and Pesticides Revealed New Species- and Substrate-Dependent Inhibition by Elacridar and Imazalil in Human and Mouse Organic Cation Transporter OCT2. International Journal of Molecular Sciences, 23(24), 15795. https://doi.org/10.3390/ijms232415795




