Efficacy of Vaporized Hydrogen Peroxide Combined with Silver Ions against Multidrug-Resistant Gram-Negative and Gram-Positive Clinical Isolates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Resistance and Susceptibility of Clinical Bacterial Isolates
2.2. In Vitro Antibacterial Activity of HP + Ag against MDR Clinical Isolates
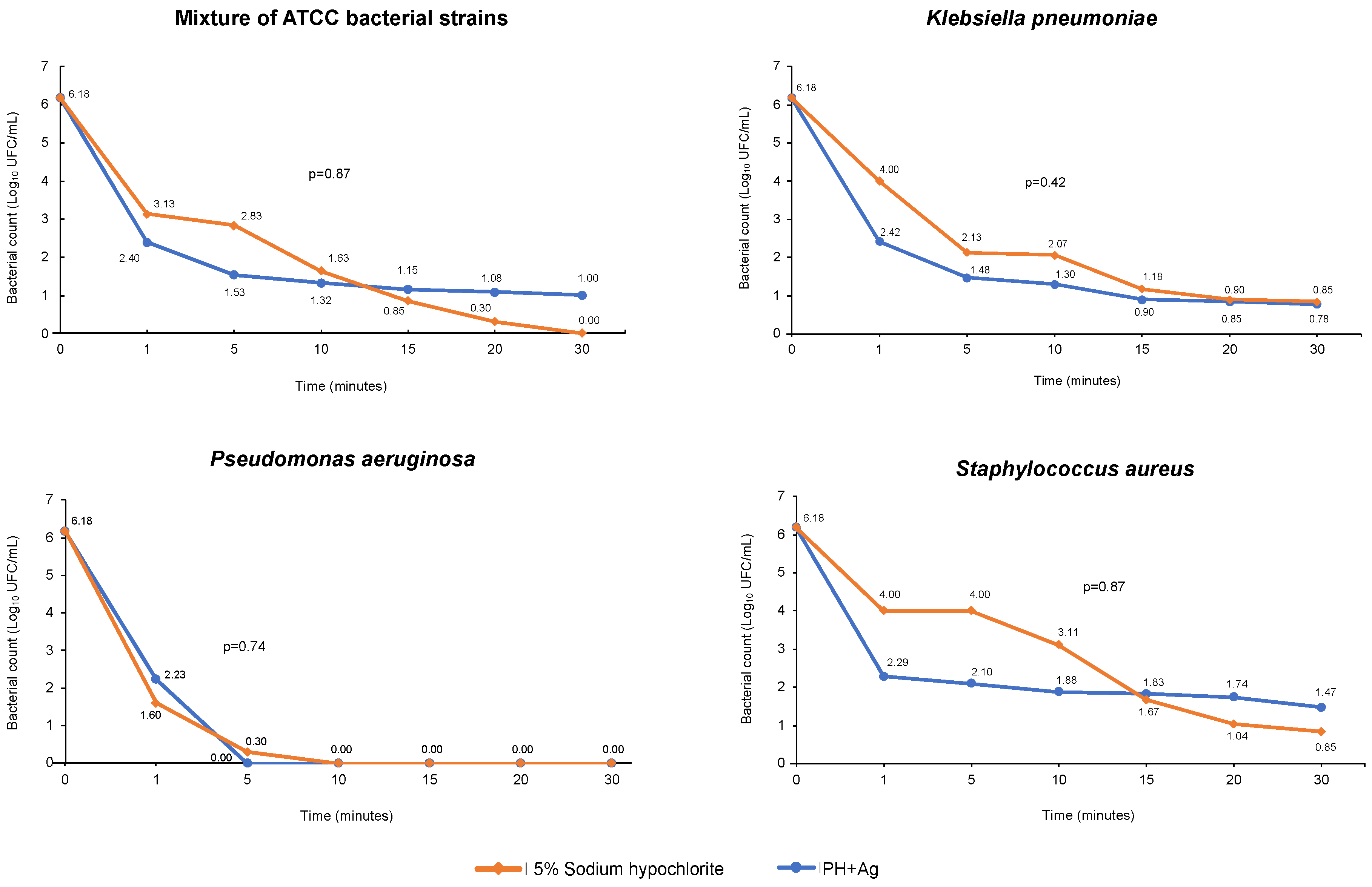
2.3. Antimicrobial Activity of HP + Ag on Bacteria in Suspension
2.4. Antimicrobial Activity of HP + Ag on Surfaces
3. Materials and Methods
3.1. Selection, Identification, and Preparation of Clinical Isolates
3.2. Characterization of Clinical Isolates: Resistance Profiles
3.3. Disinfection Efficacy Test
3.4. Disinfectant Activity on Bacteria in Suspension
3.5. Disinfectant Activity on Surfaces
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iredell, J. Antimicrobial resistance. Microbiol. Aust. 2019, 40, 55–56. [Google Scholar]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 August 2021).
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar]
- Aloush, V.; Navon-Venezia, S.; Seigman-Igra, Y.; Cabili, S.; Carmeli, Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob. Agents Chemother. 2006, 50, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Geitani, R.; Ayoub Moubareck, C.; Touqui, L.; Karam Sarkis, D. Cationic antimicrobial peptides: Alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol. 2019, 19, 54. [Google Scholar] [CrossRef] [Green Version]
- Giske, C.G.; Fröding, I.; Hasan, C.M.; Turlej-Rogacka, A.; Toleman, M.; Livermore, D.; Woodford, N.; Walsh, T.R. Diverse sequence types of Klebsiella pneumoniae contribute to the dissemination of bla NDM-1 in India, Sweden, and the nited Kingdom. Antimicrob. Agents Chemother. 2012, 56, 2735–2738. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.P.; Burns, K.; Rodríguez Baño, J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control 2017, 6, 113. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Biggest Threats and Data. 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html#carp (accessed on 21 August 2021).
- Centers for Disease Control and Prevention Patients: Information about CRE. Available online: https://www.cdc.gov/hai/organisms/cre/cre-patients.html (accessed on 21 August 2021).
- Vanegas, J.M.; Cienfuegos, A.V.; Ocampo, A.M.; Lopez, L.; del Corral, H.; Roncancio, G.; Sierra, P.; Echeverri-Toro, L.; Ospina, S.; Maldonado, N.; et al. Similar frequencies of Pseudomonas aeruginosa isolates producing KPC and VIM carbapenemases in diverse genetic clones at tertiary-care hospitals in Medellin, Colombia. J. Clin. Microbiol. 2014, 52, 3978–3986. [Google Scholar]
- Correa, A.; Del Campo, R.; Perenguez, M.; Blanco, V.M.; Rodríguez-Baños, M.; Perez, F.; Maya, J.J.; Rojas, L.; Cantón, R.; Arias, C.A.; et al. Dissemination of high-risk clones of extensively drug-resistant pseudomonas aeruginosa in Colombia. Antimicrob. Agents Chemother. 2015, 59, 2421–2425. [Google Scholar] [CrossRef] [Green Version]
- Ovalle, M.V.; Saavedra, S.Y.; González, M.N.; Hidalgo, A.M.; Duarte, C.; Beltrán, M. Resultados de la vigilancia nacional de resistencia antimicrobiana en infecciones asociadas a la atención en salud en enterobacterias y Gram negativos no fermentadores, Colombia 2012–2014. Biomedica 2017, 37, 473–485. [Google Scholar] [CrossRef]
- Ocampo, A.; Chen, L.; Cienfuegos, A.; Roncancio, G.; Chavda, K.; Kreiswirth, B.; Jiménez, J. A Two-Year Surveillance in Five Colombian Tertiary Care Hospitals Reveals High Frequency of Non-CG258 Clones of Carbapenem-Resistant Klebsiella pneumoniae with Distinct Clinical Characteristics. Antimicrob. Agents Chemother. 2016, 60, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Ovalle, M.V.; Duarte, C.; Leal, A.L.; Zambrano, C. Resistencia antimicrobiana en infecciones asociadas a la atención en salud, Colombia. Biomedica 2020, 2020, 3. [Google Scholar]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention Chemical Disinfectants. Guideline for Disinfection and Sterilization in Healthcare Facilities 2008. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html (accessed on 23 August 2021).
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Control of Hospital Waste. Mand. Douglas Bennett’s Princ. Pract. Infect. Dis. 2015, 2015, 3294–3309.e4. [Google Scholar] [CrossRef]
- Totaro, M.; Casini, B.; Profeti, S.; Tuvo, B.; Privitera, G.; Baggiani, A. Role of hydrogen peroxide vapor (HPV) for the disinfection of hospital surfaces contaminated by multiresistant bacteria. Pathogens 2020, 9, 408. [Google Scholar] [CrossRef]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–34. [Google Scholar]
- Andersen, B.M.; Rasch, M.; Hochlin, K.; Jensen, F.H.; Wismar, P.; Fredriksen, J.E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 2006, 62, 149–155. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Schouten, M.A.; Van Zanten, A.R.H.; Houmes-Zielman, G.; Nohlmans-Paulssen, M.K.E. Hydrogen peroxide vapor decontamination of an intensive care unit to remove environmental reservoirs of multidrug-resistant gram-negative rods during an outbreak. Am. J. Infect. Control 2010, 38, 754–756. [Google Scholar] [CrossRef]
- Ray, A.; Perez, F.; Beltramini, A.M.; Jakubowycz, M.; Dimick, P.; Jacobs, M.R.; Roman, K.; Bonomo, R.A.; Salata, R.A. Use of Vaporized Hydrogen Peroxide Decontamination during an Outbreak of Multidrug-Resistant Acinetobacter baumannii Infection at a Long-Term Acute Care Hospital. Infect. Control Hosp. Epidemiol. 2010, 31, 1236–1241. [Google Scholar] [CrossRef] [Green Version]
- De Giglio, O.; Coretti, C.; Lovero, G.; Barbuti, G.; Caggiano, G. Pilot study on the antibacterial activity of hydrogen peroxide and silver ions in the hospital environment. Ann. Ig. 2014, 26, 181–185. [Google Scholar] [CrossRef]
- Rada, A.M.; De La Cadena, E.; Agudelo, C.A.; Pallares, C.; Restrepo, E.; Correa, A.; Villegas, M.V.; Capataz, C. Genetic Diversity of Multidrug-Resistant Pseudomonas aeruginosa Isolates Carrying bla VIM − 2 and bla KPC − 2 Genes That Spread on Different Genetic Environment in Colombia Bacterial Isolates and Clinical Data. Front. Microbiol. 2021, 12, 663020. [Google Scholar] [CrossRef]
- Rada, A.M.; Hernández-Gómez, C.; Restrepo, E.; Villegas, M.V. Distribution and molecular characterization of beta-lactamases in Gram negative bacteria in Colombia (2001–2016). Biomedica 2019, 39, 199–220. [Google Scholar] [CrossRef]
- Gordon, A.E.K.; Mathers, A.J.; Cheong, E.Y.L.; Gottlieb, T.; Kotay, S.; Walker, A.S.; Peto, T.E.A.; Crook, D.W.; Stoesser, N. The Hospital Water Environment as a Reservoir for Carbapenem-Resistant Organisms Causing Hospital-Acquired Infections—A Systematic Review of the Literature. Clin. Infect. Dis. 2017, 64, 1435–1444. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, Y.; Du, M.; Bai, Y.; Liu, B.; Gong, M.; Song, H.; Tong, Y.; Liu, Y. Prospective investigation of carbapenem-resistant Klebsiella pneumonia transmission among the staff, environment and patients in five major intensive care units, Beijing. J. Hosp. Infect. 2019, 101, 150–157. [Google Scholar] [CrossRef]
- Heidari, R.; Sheikh, A.F.; Hashemzadeh, M.; Farshadzadeh, Z.; Salmanzadeh, S.; Saki, M. Antibiotic resistance, biofilm production ability and genetic diversity of carbapenem-resistant Pseudomonas aeruginosa strains isolated from nosocomial infections in southwestern Iran. Mol. Biol. Rep. 2022, 49, 3811–3822. [Google Scholar] [CrossRef]
- Camacho-Cruz, J.; Gutiérrez, I.F.; Brand-López, K.; Sosa-Rodríguez, Y.A.; Vásquez-Hoyos, P.; Gómez-Cortés, L.C.; Beltrán-Higuera, S.J.; Romero-Higuera, L.N.; Rojas-Rojas, D.P.; Ortiz-Mendez, C.A.; et al. Differences Between Methicillin-susceptible Versus Methicillin-resistant Staphylococcus aureus Infections in Pediatrics. Pediatr. Infect. Dis. J. 2022, 41, 12–19. [Google Scholar] [CrossRef]
- Valderrama-Beltrán, S.; Gualtero, S.; Álvarez-moreno, C.; Gil, F.; Ruiz, A.J.; Rodríguez, J.Y.; Osorio, J.; Tenorio, I.; Gómez, C.; Mackenzie, S.; et al. Risk factors associated with methicillin-resistant Staphylococcus aureus skin and soft tissue infections in hospitalized patients in Colombia. Int. J. Infect. Dis. 2019, 87, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, J.M.; Salazar-ospina, L.; Gallego, M.A.; Jim, J.N. A longitudinal study shows intermittent colonization by Staphylococcus aureus with a high genetic diversity in hemodialysis patients. Int. J. Med. Microbiol. 2021, 311, 151471. [Google Scholar] [CrossRef]
- Arias-ortiz, P.M.; Calderón, P.; Castillo, J.S.; Moreno, J.; Leal, A.L.; Cortés, J.A.; Álvarez, C.A. Risk factors for methicillin-resistant Staphylococcus aureus bacteremia: A multicenter matched case-control study. Biomedica 2016, 36, 612–618. [Google Scholar]
- Gregory, T.V.; Ellis, K.; Valeriani, R.; Khan, F.; Wu, X.; Murin, L.; Alibayov, B.; Vidal, A.G.J.; Zhao, T.; Vidal, J.E. MoWa: A Disinfectant for Hospital Surfaces Contaminated With Methicillin-Resistant Staphylococcus aureus (MRSA) and Other Nosocomial Pathogens. Front. Cell. Infect. Microbiol. Receiv. 2021, 11, 676638. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Andrade, D.D.; Rigotti, M.A.; Gottardo De Almeida, M. Methicillin-resistant Staphylococcus aureus on surfaces of an Intensive Care Unit. Acta Paul. Enferm. 2011, 24, 453–458. [Google Scholar]
- Shi, L.; Xu, C.; Jia, H.; Chen, W.; Zhou, X.; Li, X. Spread of Staphylococcus aureus between medical staff and high-frequency contact surfaces in a large metropolitan hospital. Int. J. Nurs. Sci. 2015, 2, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Lee, C.; Cho, M.; Yoon, J. Enhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiation. Water Res. 2008, 42, 356–362. [Google Scholar] [CrossRef]
- Davoudi, M.; Ehrampoush, M.H.; Vakili, T.; Absalan, A.; Ebrahimi, A. Antibacterial effects of hydrogen peroxide and silver composition on selected pathogenic enterobacteriaceae. Int. J. Environ. Health Eng. 2012, 1, 408. [Google Scholar] [CrossRef]
- Herruzo, R.; Vizcaíno, M.J.; Herruzo, I. Quantifying GlosairTM 400 efficacy for surface disinfection of American Type Culture Collection strains and micro-organisms recently isolated from intensive care unit patients. J. Hosp. Infect. 2014, 87, 175–178. [Google Scholar] [CrossRef]
- Alkawareek, M.; Bahlool, A.; Abulateefeh, S.; Alaaldin, A. Synergistic antibacterial activity of silver nanoparticles and hydrogen peroxide. PLoS ONE 2019, 14, e0220575. [Google Scholar]
- Watson, F.; Keevil, C.W.; Wilks, S.A.; Chewins, J. Modelling vaporised hydrogen peroxide efficacy against mono-species biofilms. Sci. Rep. 2018, 8, 408. [Google Scholar] [CrossRef] [Green Version]
- Lemmen, S.; Scheithauer, S.; Häfner, H.; Yezli, S.; Mohr, M.; Otter, J.A. Evaluation of hydrogen peroxide vapor for the inactivation of nosocomial pathogens on porous and nonporous surfaces. Am. J. Infect. Control 2015, 43, 82–85. [Google Scholar] [CrossRef]
- Perumal, P.K.; Wand, M.E.; Sutton, J.M.; Bock, L.J. Evaluation of the effectiveness of hydrogen-peroxide-based disinfectants on biofilms formed by Gram-negative pathogens. J. Hosp. Infect. 2014, 87, 227–233. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Disinfection and Sterilization. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html (accessed on 1 September 2022).
- Ferreira, R.L.; Da Silva, B.C.M.; Rezende, G.S.; Nakamura-Silva, R.; Pitondo-Silva, A.; Campanini, E.B.; Brito, M.C.A.; Da Silva, E.M.L.; De Melo Freire, C.C.; Da Cunha, A.F.; et al. High prevalence of multidrug-resistant klebsiella pneumoniae harboring several virulence and β-lactamase encoding genes in a brazilian intensive care unit. Front. Microbiol. 2019, 10, 3198. [Google Scholar] [CrossRef] [Green Version]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, P.P.; Wang, L.H.; Wei, D.D.; Wan, L.G.; Zhang, W. Capsular Polysaccharide Types and Virulence-Related Traits of Epidemic KPC-Producing Klebsiella pneumoniae Isolates in a Chinese University Hospital. Microb. Drug Resist. 2017, 23, 901–907. [Google Scholar] [CrossRef]
- Rutala, W.A.; Gergen, M.F.; Ascp, M.T.; Sickbert-bennett, E.E.; Williams, D.A.; Weber, D.J. Effectiveness of improved hydrogen peroxide in decontaminating privacy curtains contaminated with multidrug-resistant pathogens. Am. J. Infect. Control 2014, 42, 426–428. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standadrs Institute: Wayne, PA, USA, 2019; ISBN 1562388045. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI supplement M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- The United States Pharmacopeial Convention. United States Pharmacopeia USP 39 <1072> Disinfectants and Antiseptic; United States Pharmacopeial Convention: North Bethesda, MD, USA, 2016. [Google Scholar]
- AOAC International. Official Method 960.09 Germicidal and Detergent Sanitizing Action of Disinfectants. In Official Methods of Analysis of AOAC International; AOAC International, Ed.; AOAC International: Arlington, VA, USA, 2019. [Google Scholar]
- ICONTEC Norma Técnica Colombiana. (NTC 2455), Desinfectantes Para uso Doméstico; ICONTEC Norma Técnica Colombiana: Bogota, Colombia, 2000. [Google Scholar]
- AOAC International. Official Method 961.02 Germicidal spray products as disinfectan. In Official Methods of Analysis of AOAC International; AOAC International, Ed.; AOAC International: Arlington, VA, USA, 2019. [Google Scholar]
- ISO 18593:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for Sampling Techniques from Surfaces Using Contact Plates and Swabs. International Organization for Standardization: Geneva, Switzerland, 2004.
| Antibiotics, and Resistance Genes and Mechanisms | Strains/MIC (μg/mL)/Interpretative Categories | |||||||
|---|---|---|---|---|---|---|---|---|
| MDRKp1 | MDRKp2 | MDRKp3 | MDRPa1 | MDRPa2 | MDRPa3 | Ec ATCC 25922 | Pa ATCC 27853 | |
| Ampicillin/sulbactam 1 | ≥32/R | ≥32/R | ≥32/R | – | – | – | ≤2/S | – |
| Piperacillin/tazobactam 1 | ≥128/R | ≥128/R | ≥128/R | ≥128/R | ≥128/R | ≥128/R | ≤4/S | ≤4/S |
| Cefoxitin 2 | 16/R | ≥64/R | 32/R | – | – | – | ≤4/S | – |
| Ceftazidime 3 | ≥64/R | ≥64/R | ≥64/R | 32/R | ≥64/R | ≥64/R | ≤1/S | ≤1/S |
| Ceftriaxone 3 | ≥64/R | 32/R | ≥64/R | – | – | – | ≤1/S | – |
| Cefepime 3 | ≥64/R | ≥64/R | 32/R | 32/R | ≥64/R | 16/I | ≤1/S | ≤1/S |
| Doripenem 4 | ≥8/R | ≥8/R | ≥8/R | ≥8/R | 4/I | ≥8/R | ≤0.12/S | 0.25/S |
| Ertapenem 4 | ≥64/R | 4/R | ≥8/R | – | – | – | ≤0.5/S | – |
| Imipenem 4 | 8/R | 8/R | ≥16/R | ≥16/R | 1/S | ≥16/R | ≤0.25/S | 2/S |
| Meropenem 4 | 16/R | ≥16/R | ≥16/R | 8/R | 4/I | 8/R | ≤0.25/S | ≤0.25/S |
| Amikacin 5 | ≥64/R | 16/S | ≥64/R | ≥64/R | ≥64/R | ≥64/R | ≤2/S | ≤2/S |
| Gentamicin 5 | 4/S | 16/R | 4/S | 4/S | ≥16/R | 4/S | ≤1/S | ≤1/S |
| Ciprofloxacin 6 | ≥4/R | ≥4/R | ≥4/R | ≥4/R | ≥4/R | 2/R | ≤0.25/S | ≤0.25/S |
| Colistin 7 | – | – | – | ≥16/R | ≤0.5/S | ≥16/R | – | ≤0.5/S |
| Carba NP result | POS | POS | POS | POS | POS | POS | NEG | NEG |
| EDTA/SMA synergy test | NEG | NEG | NEG | POS | POS | POS | NEG | NEG |
| Boronic acid synergy test | POS | POS | POS | – | – | – | NEG | NEG |
| Carbapenemase genes | ||||||||
| blaKPC | POS | POS | POS | NEG | NEG | POS | NEG | NEG |
| blaVIM and/or blaIMP | NEG | NEG | NEG | POS | POS | POS | NEG | NEG |
| blaNDM | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG |
| blaOXA-48 | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| Strain | MIC (μg/mL) for Antibiotic/Interpretative Category | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP 1 | CIP 2 | CLI 3 | ERY 4 | GEN 5 | LVX 2 | LZD 6 | MIN 7 | MXF 2 | NIT 8 | OXA 9 | Q-D 10 | RIF 11 | TEC 12 | TET 7 | SXT 13 | VAN 12 | |
| MRSA1 | 0.5/S | ≤0.5/S | ≤0.25/S | ≥8/R | ≤0.5/S | ≤0.12/S | 2/S | ≤0.5/S | ≤0.25/S | ≤16/S | ≥4/R | ≤0.25/S | ≤0.5/S | ≤0.5/S | ≤1/S | ≤10/S | 1/S |
| MRSA2 | 0.5/S | ≤0.5/S | ≤0.25/S | ≤0.25/S | ≤0.5/S | ≤0.12/S | 2/S | ≤0.5/S | ≤0.25/S | ≤16/S | ≥4/R | ≤0.25/S | ≤0.5/S | ≤0.5/S | ≤1/S | ≤10/S | 1/S |
| MRSA3 | 0.5/S | ≤0.5/S | ≤0.25/S | ≤0.25/S | ≤0.5/S | ≤0.12/S | 2/S | ≤0.5/S | ≤0.25/S | ≤16/S | ≥4/R | ≤0.25/S | ≤0.5/S | ≤0.5/S | ≤1/S | ≤10/S | 1/S |
| Sa ATCC 29213 | 0.5/S | ≤0.5/S | ≤0.25/S | ≤0.25/S | ≤0.5/S | ≤0.12/S | 2/S | ≤0.5/S | ≤0.25/S | ≤16/S | ≤0.25/S | ≤0.25/S | ≤0.5/S | ≤0.5/S | ≤1/S | ≤10/S | ≤0.5/S |
| Strains (n) | PH+Ag | Control (Hypochlorite 5%) | p-Value | ||
|---|---|---|---|---|---|
| MIC (mg/L) | MBC (mg/L) | MIC (mg/L) | MBC (mg/L) | ||
| MDRKp (3) | 362.5–725 | 362.5–725 | 2500–5000 | 2500–5000 | 0.04 |
| MDRPa (3) | 1450–5800 | 1450–5800 | 2500–5000 | 2500–5000 | 0.51 |
| MRSA (3) | 725–1450 | 725–1450 | 5000 | 5000 | 0.04 |
| Ec ATCC 25922 | 725–1450 | 725–1450 | 1250 | 1250 | 0.99 |
| Pa ATCC 27853 | 1450–2900 | 1450–2900 | 5000 | 5000 | 0.02 |
| Sa ATCC 25923 | 362.5 | 362.5 | 3750–5000 | 3750–5000 | 0.02 |
| Strains | Bacterial Count Prior to HPV Exposure | Bacterial Count after HPV Exposure | Efficacy (%) | ||
|---|---|---|---|---|---|
| CFU | Log10 Reduction | CFU | Log10 Reduction | ||
| Set of ATCC strains | 9.39 × 102 | 2.97 | 0.00 | 0.48 | 100 |
| Set of resistant clinical isolates | 1.65 × 103 | 3.22 | 3.00 | 0.00 | 99.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Sánchez, S.P.; Rojas-Abadía, J.M.; Ríos-Acevedo, J.J.; Mejía-Hurtado, A.F.; Espinosa-Moya, L.N.; Ocampo-Ibáñez, I.D. Efficacy of Vaporized Hydrogen Peroxide Combined with Silver Ions against Multidrug-Resistant Gram-Negative and Gram-Positive Clinical Isolates. Int. J. Mol. Sci. 2022, 23, 15826. https://doi.org/10.3390/ijms232415826
Rivera-Sánchez SP, Rojas-Abadía JM, Ríos-Acevedo JJ, Mejía-Hurtado AF, Espinosa-Moya LN, Ocampo-Ibáñez ID. Efficacy of Vaporized Hydrogen Peroxide Combined with Silver Ions against Multidrug-Resistant Gram-Negative and Gram-Positive Clinical Isolates. International Journal of Molecular Sciences. 2022; 23(24):15826. https://doi.org/10.3390/ijms232415826
Chicago/Turabian StyleRivera-Sánchez, Sandra Patricia, José María Rojas-Abadía, John Jairo Ríos-Acevedo, Ana Fernanda Mejía-Hurtado, Luz Natalia Espinosa-Moya, and Iván Darío Ocampo-Ibáñez. 2022. "Efficacy of Vaporized Hydrogen Peroxide Combined with Silver Ions against Multidrug-Resistant Gram-Negative and Gram-Positive Clinical Isolates" International Journal of Molecular Sciences 23, no. 24: 15826. https://doi.org/10.3390/ijms232415826
APA StyleRivera-Sánchez, S. P., Rojas-Abadía, J. M., Ríos-Acevedo, J. J., Mejía-Hurtado, A. F., Espinosa-Moya, L. N., & Ocampo-Ibáñez, I. D. (2022). Efficacy of Vaporized Hydrogen Peroxide Combined with Silver Ions against Multidrug-Resistant Gram-Negative and Gram-Positive Clinical Isolates. International Journal of Molecular Sciences, 23(24), 15826. https://doi.org/10.3390/ijms232415826







