In-Silico Functional Metabolic Pathways Associated to Chlamydia trachomatis Genital Infection
Abstract
:1. Introduction
2. Results
2.1. Metagenomic and Functional Metabolomic Profiling of 16S rDNA Dataset
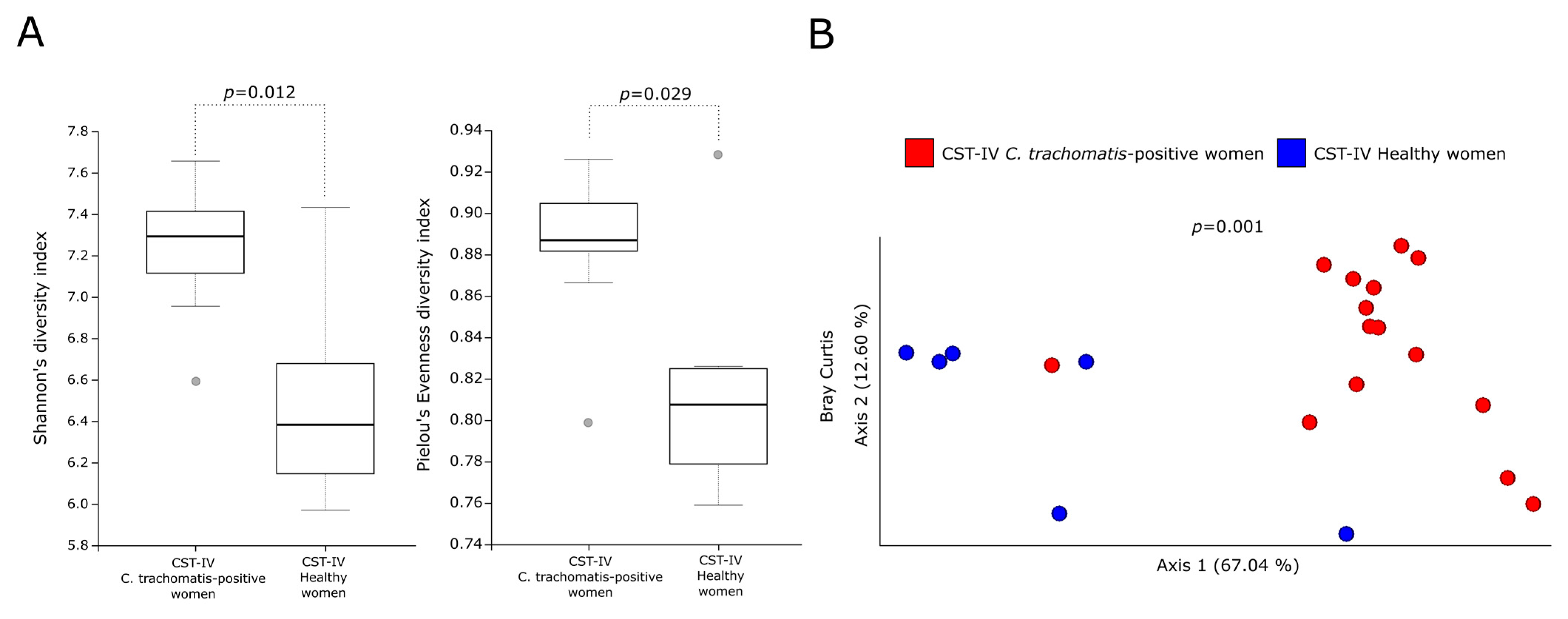
2.2. Alpha- and Beta- Diversity Analysis of MetaCyc Pathways
2.3. Composition of Predicted MetaCyc Pathways
3. Discussion
4. Materials and Methods
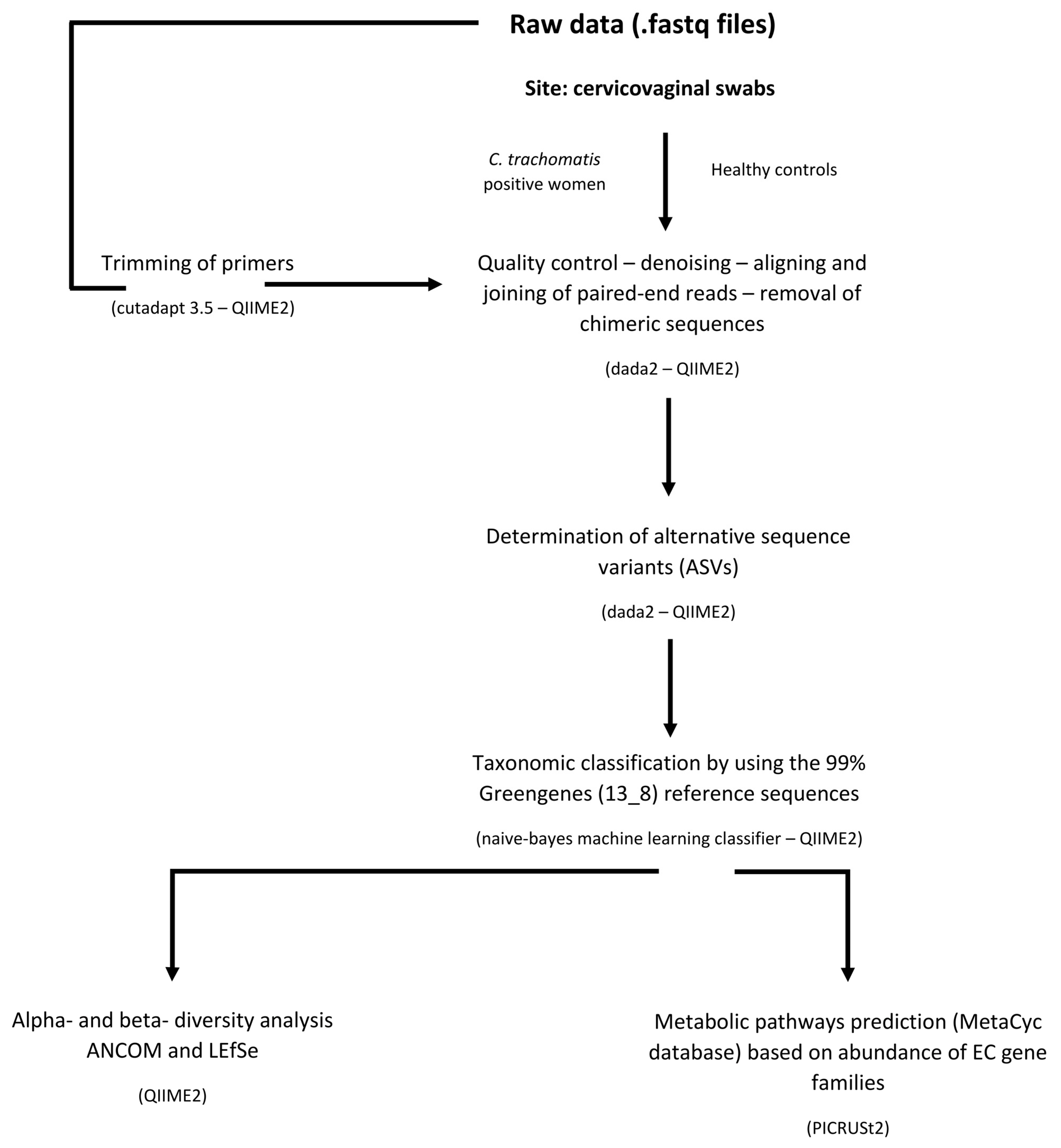
4.1. 16s rDNA Dataset
4.2. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt)
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filardo, S.; Scalese, G.; Virili, C.; Pontone, S.; di Pietro, M.; Covelli, A.; Bedetti, G.; Marinelli, P.; Bruno, G.; Stramazzo, I.; et al. The Potential Role of Hypochlorhydria in the Development of Duodenal Dysbiosis: A Preliminary Report. Front. Cell. Infect. Microbiol. 2022, 12, 854904. [Google Scholar] [CrossRef]
- Filardo, S.; Pietro, M.D.; Tranquilli, G.; Latino, M.A.; Recine, N.; Porpora, M.G.; Sessa, R. Selected Immunological Mediators and Cervical Microbial Signatures in Women with Chlamydia trachomatis Infection. mSystems 2019, 4, e00094-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, A.F.; Lindberg, M.; Jakobsson, H.; Bäckhed, F.; Nyrén, P.; Engstrand, L. Comparative Analysis of Human Gut Microbiota by Barcoded Pyrosequencing. PLoS ONE 2008, 3, e2836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a Deeper Understanding of the Vaginal Microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Sessa, R.; di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli-Lactoferrin Interplay in Chlamydia trachomatis Infection. Pathog. Dis. 2017, 75, ftx054. [Google Scholar] [CrossRef] [Green Version]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus Species as Biomarkers and Agents That Can Promote Various Aspects of Vaginal Health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Sessa, R.; di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of Bovine Lactoferrin on Chlamydia trachomatis Infection and Inflammation. Biochem. Cell Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef] [Green Version]
- van der Veer, C.; Bruisten, S.M.; van der Helm, J.J.; de Vries, H.J.C.; van Houdt, R. The Cervicovaginal Microbiota in Women Notified for Chlamydia trachomatis Infection: A Case-Control Study at the Sexually Transmitted Infection Outpatient Clinic in Amsterdam, The Netherlands. Clin. Infect. Dis. 2017, 64, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- di Pietro, M.; Filardo, S.; Romano, S.; Sessa, R. Chlamydia trachomatis and Chlamydia Pneumoniae Interaction with the Host: Latest Advances and Future Prospective. Microorganisms 2019, 7, 140. [Google Scholar] [CrossRef] [Green Version]
- Pinto, C.N.; Niles, J.K.; Kaufman, H.W.; Marlowe, E.M.; Alagia, D.P.; Chi, G.; van der Pol, B. Impact of the COVID-19 Pandemic on Chlamydia and Gonorrhea Screening in the U.S. Am. J. Prev. Med. 2021, 61, 386–393. [Google Scholar] [CrossRef]
- Lamont, R.; Sobel, J.; Akins, R.; Hassan, S.; Chaiworapongsa, T.; Kusanovic, J.; Romero, R. The Vaginal Microbiome: New Information about Genital Tract Flora Using Molecular Based Techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, F.M.T.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef] [PubMed]
- di Pietro, M.; Filardo, S.; Porpora, M.G.; Recine, N.; Latino, M.A.; Sessa, R. HPV/Chlamydia trachomatis Co-Infection: Metagenomic Analysis of Cervical Microbiota in Asymptomatic Women. New Microbiol. 2018, 41, 34–41. [Google Scholar] [PubMed]
- Filardo, S.; di Pietro, M.; Porpora, M.G.; Recine, N.; Farcomeni, A.; Latino, M.A.; Sessa, R. Diversity of Cervical Microbiota in Asymptomatic Chlamydia trachomatis Genital Infection: A Pilot Study. Front. Cell. Infect. Microbiol. 2017, 7, 321. [Google Scholar] [CrossRef] [Green Version]
- Borgogna, J.-L.C.; Shardell, M.D.; Yeoman, C.J.; Ghanem, K.G.; Kadriu, H.; Ulanov, A.V.; Gaydos, C.A.; Hardick, J.; Robinson, C.K.; Bavoil, P.M.; et al. The Association of Chlamydia trachomatis and Mycoplasma Genitalium Infection with the Vaginal Metabolome. Sci. Rep. 2020, 10, 3420. [Google Scholar] [CrossRef] [Green Version]
- Parolin, C.; Foschi, C.; Laghi, L.; Zhu, C.; Banzola, N.; Gaspari, V.; D’Antuono, A.; Giordani, B.; Severgnini, M.; Consolandi, C.; et al. Insights Into Vaginal Bacterial Communities and Metabolic Profiles of Chlamydia trachomatis Infection: Positioning Between Eubiosis and Dysbiosis. Front. Microbiol. 2018, 9, 600. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef] [Green Version]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial Properties of Lactobacillus Cell-free Supernatants against Multidrug-resistant Urogenital Pathogens. Microbiologyopen 2021, 10, e1173. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Lipson, K.S.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-Level Functional Profiling of Metagenomes and Metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Esvap, E.; Ulgen, K.O. Advances in Genome-Scale Metabolic Modeling toward Microbial Community Analysis of the Human Microbiome. ACS Synth. Biol. 2021, 10, 2121–2137. [Google Scholar] [CrossRef]
- Rother, M.; Teixeira da Costa, A.R.; Zietlow, R.; Meyer, T.F.; Rudel, T. Modulation of Host Cell Metabolism by Chlamydia trachomatis. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Bommana, S.; Richards, G.; Kama, M.; Kodimerla, R.; Jijakli, K.; Read, T.D.; Dean, D. Metagenomic Shotgun Sequencing of Endocervical, Vaginal, and Rectal Samples among Fijian Women with and without Chlamydia trachomatis Reveals Disparate Microbial Populations and Function across Anatomic Sites: A Pilot Study. Microbiol. Spectr. 2022, 10, e00105-22. [Google Scholar] [CrossRef] [PubMed]
- Baldewijns, S.; Sillen, M.; Palmans, I.; Vandecruys, P.; van Dijck, P.; Demuyser, L. The Role of Fatty Acid Metabolites in Vaginal Health and Disease: Application to Candidiasis. Front. Microbiol. 2021, 12, 1656. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.E.; Thiaville, J.J.; Proestos, J.; Juárez-Vázquez, A.L.; McCoy, A.J.; Barona-Gómez, F.; Iwata-Reuyl, D.; de Crécy-Lagard, V.; Maurelli, A.T. Promiscuous and Adaptable Enzymes Fill “Holes” in the Tetrahydrofolate Pathway in Chlamydia Species. mBio 2014, 5, e01378-14. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences From High Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Barnes, C.J.; Rasmussen, L.; Asplund, M.; Knudsen, S.W.; Clausen, M.L.; Agner, T.; Hansen, A.J. Comparing DADA2 and OTU clustering approaches in studying the bacterial communities of atopic dermatitis. J. Med. Microbiol. 2020, 69, 1293–1302. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences With QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc Database of Metabolic Pathways and Enzymes and the BioCyc Collection of Pathway/Genome Databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filardo, S.; Di Pietro, M.; De Angelis, M.; Brandolino, G.; Porpora, M.G.; Sessa, R. In-Silico Functional Metabolic Pathways Associated to Chlamydia trachomatis Genital Infection. Int. J. Mol. Sci. 2022, 23, 15847. https://doi.org/10.3390/ijms232415847
Filardo S, Di Pietro M, De Angelis M, Brandolino G, Porpora MG, Sessa R. In-Silico Functional Metabolic Pathways Associated to Chlamydia trachomatis Genital Infection. International Journal of Molecular Sciences. 2022; 23(24):15847. https://doi.org/10.3390/ijms232415847
Chicago/Turabian StyleFilardo, Simone, Marisa Di Pietro, Marta De Angelis, Gabriella Brandolino, Maria Grazia Porpora, and Rosa Sessa. 2022. "In-Silico Functional Metabolic Pathways Associated to Chlamydia trachomatis Genital Infection" International Journal of Molecular Sciences 23, no. 24: 15847. https://doi.org/10.3390/ijms232415847





