Diosgenin Exerts Analgesic Effects by Antagonizing the Selective Inhibition of Transient Receptor Potential Vanilloid 1 in a Mouse Model of Neuropathic Pain
Abstract
:1. Introduction
2. Results
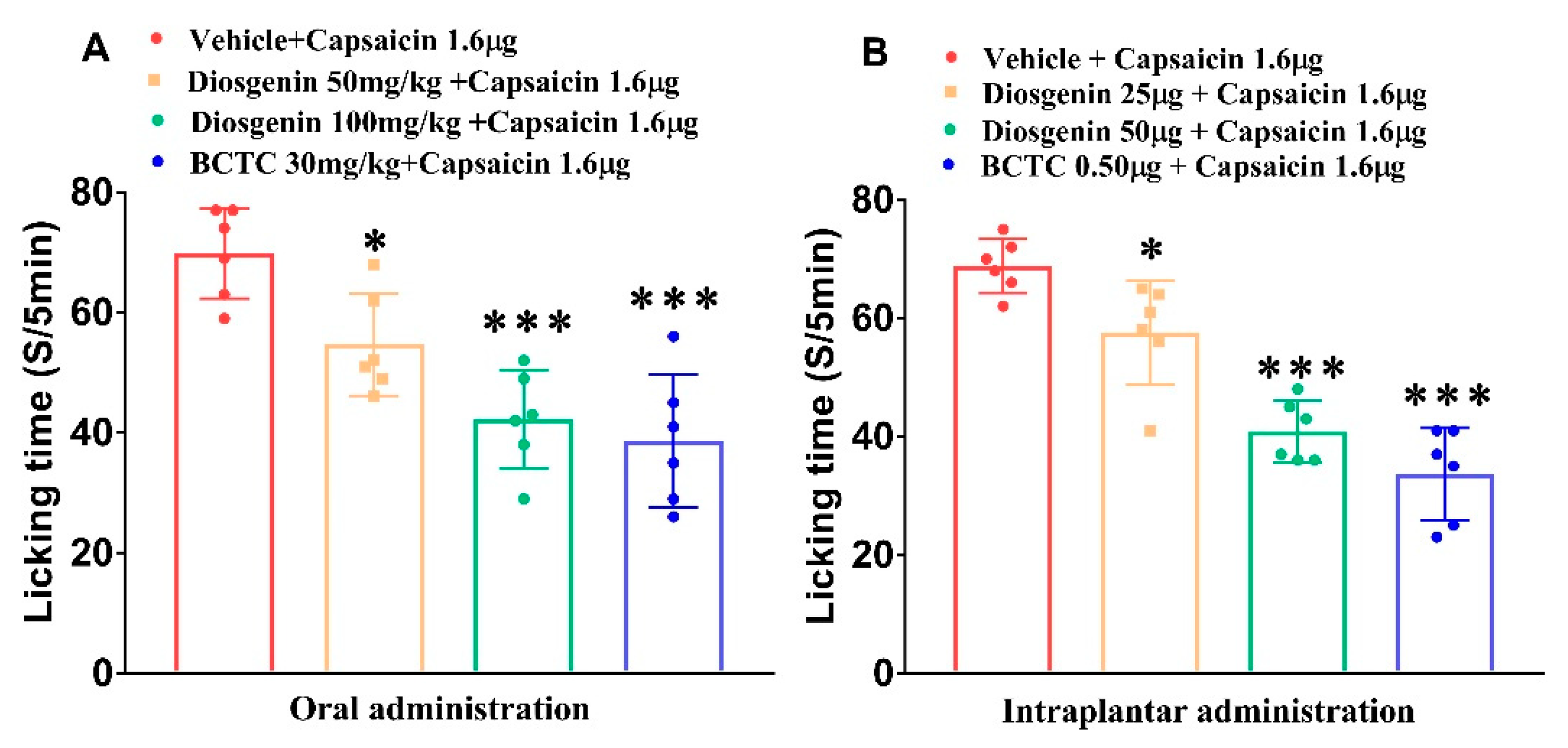
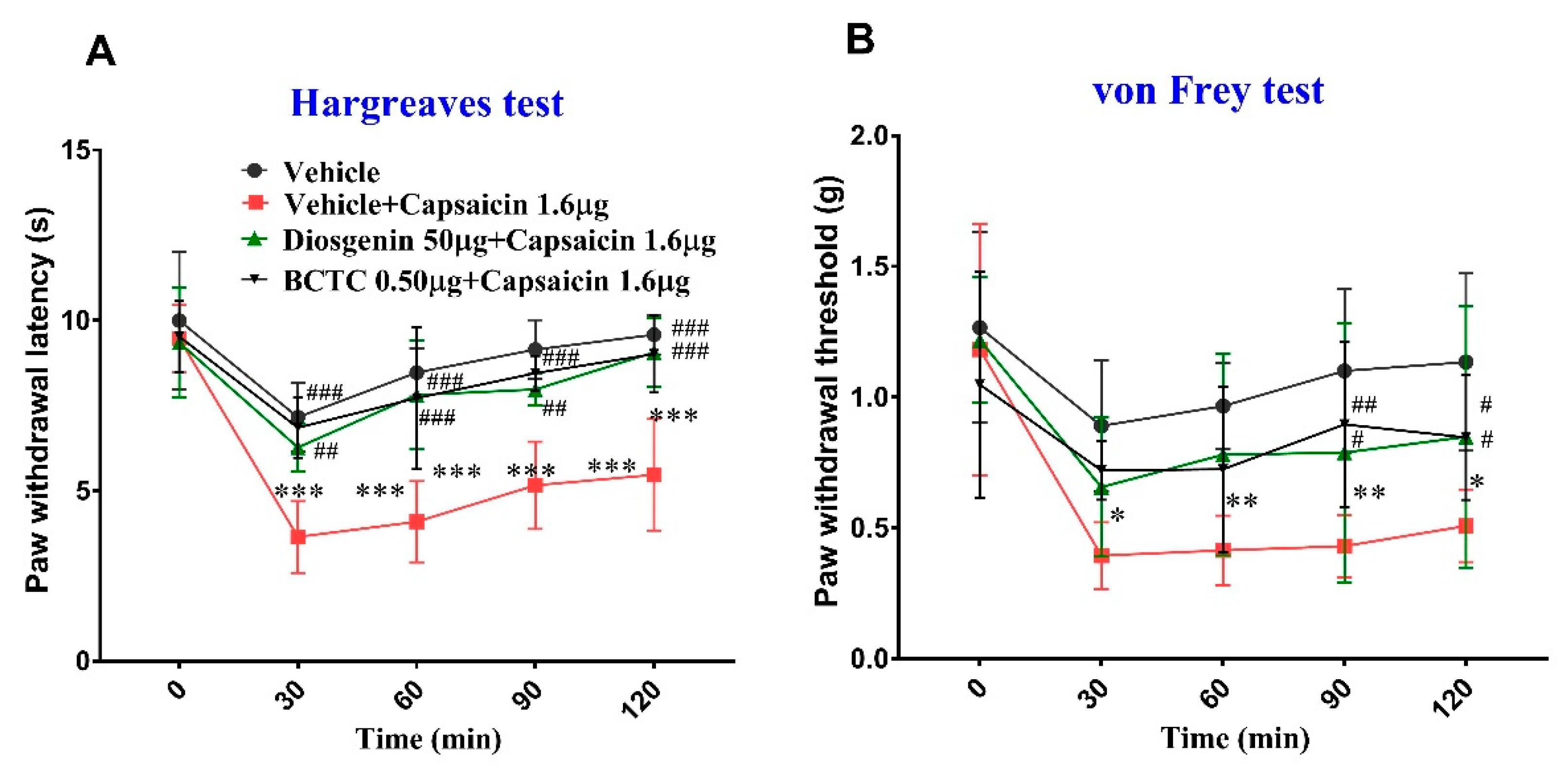
2.1. Effects of Diosgenin on Capsaicin-Induced Acute Nociceptive Behavior
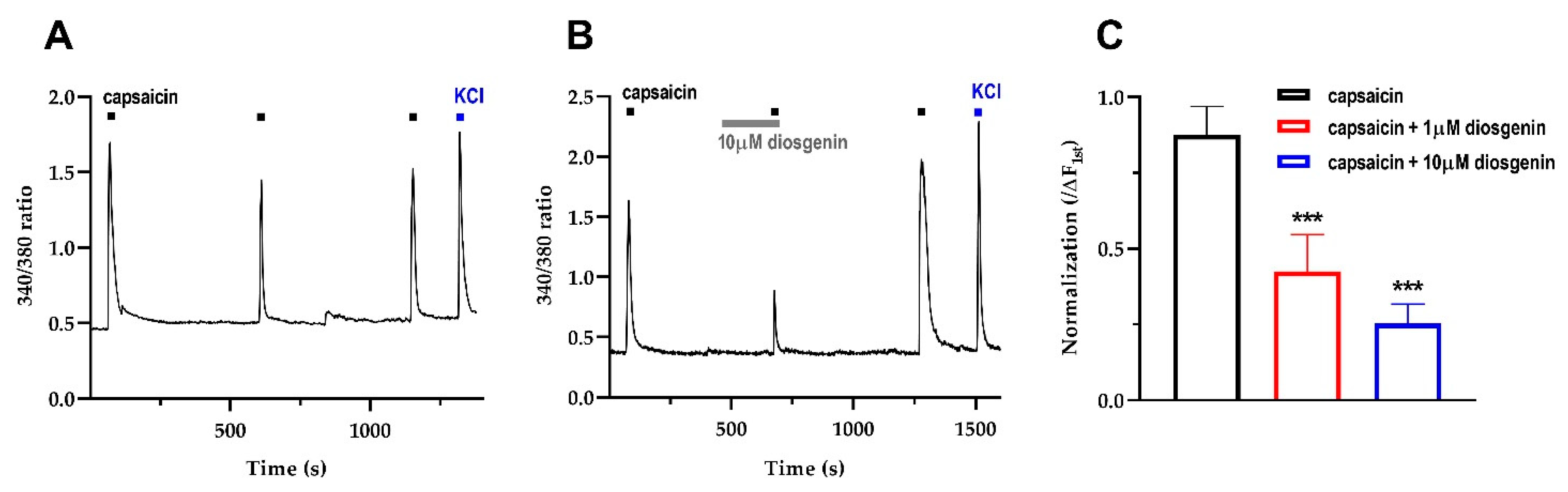
2.2. Diosgenin Reduced Transient Capsaicin-Induced Ca2+ Currents in Mouse DRG
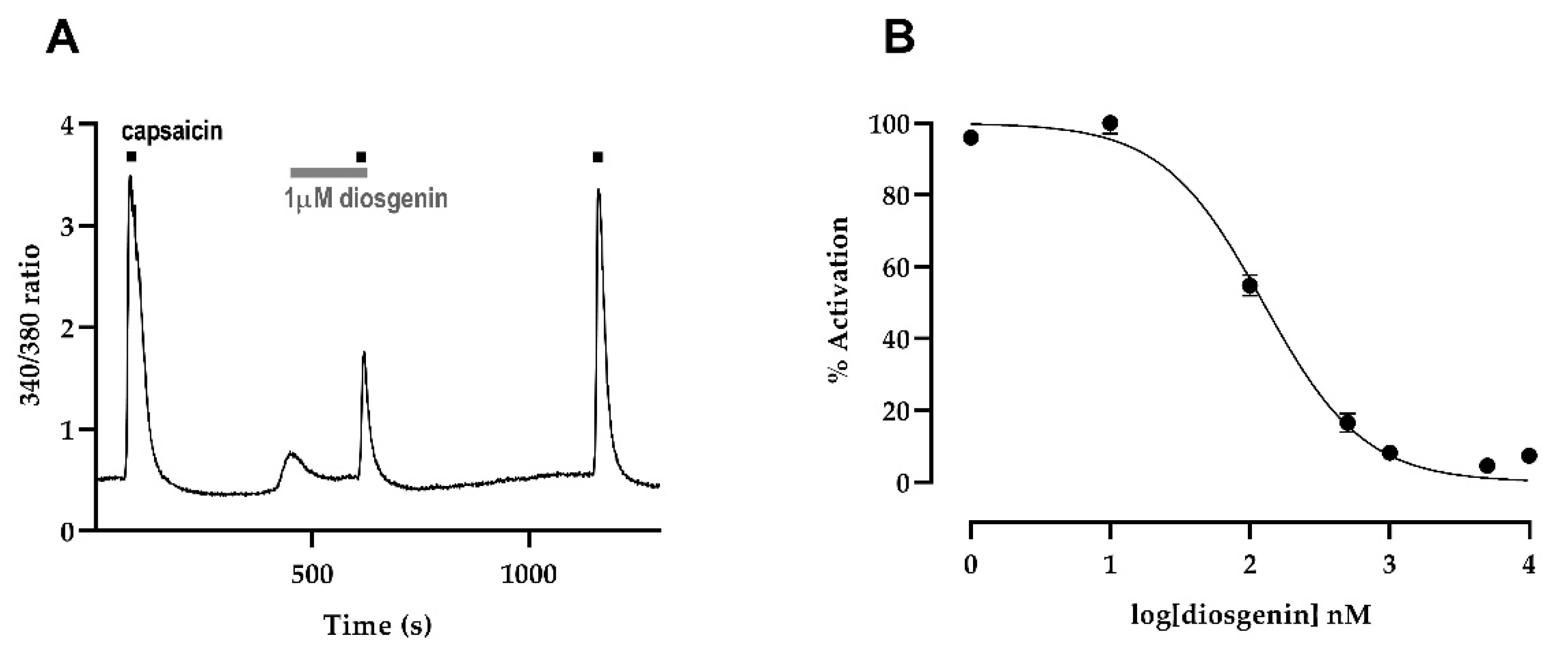
2.3. Intracellular Ca2+ Concentration in Chinese Hamster Ovary Cells Expressing Human TRPV1
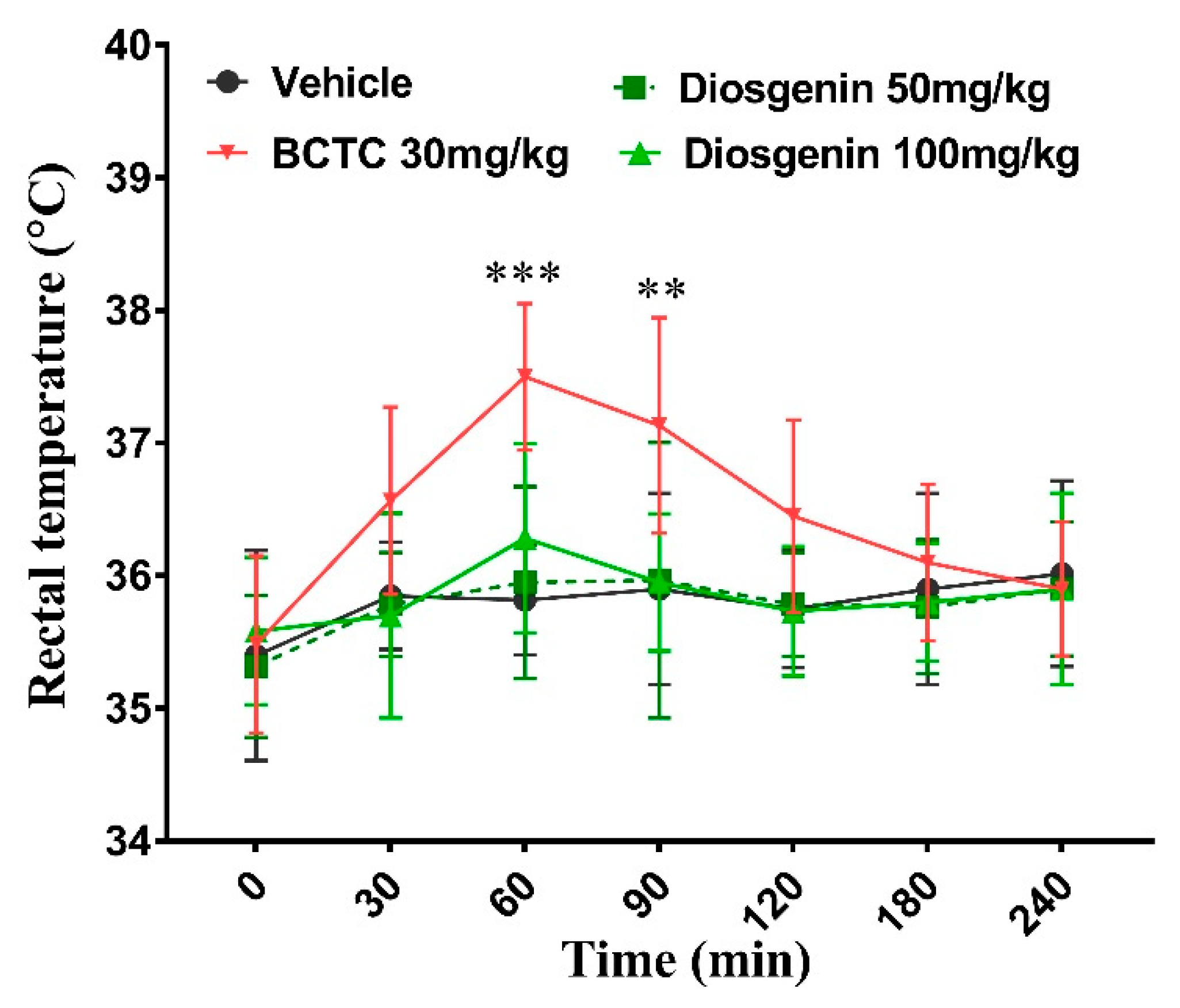
2.4. Effects of Oral Administration Diosgenin on Rectal Temperature
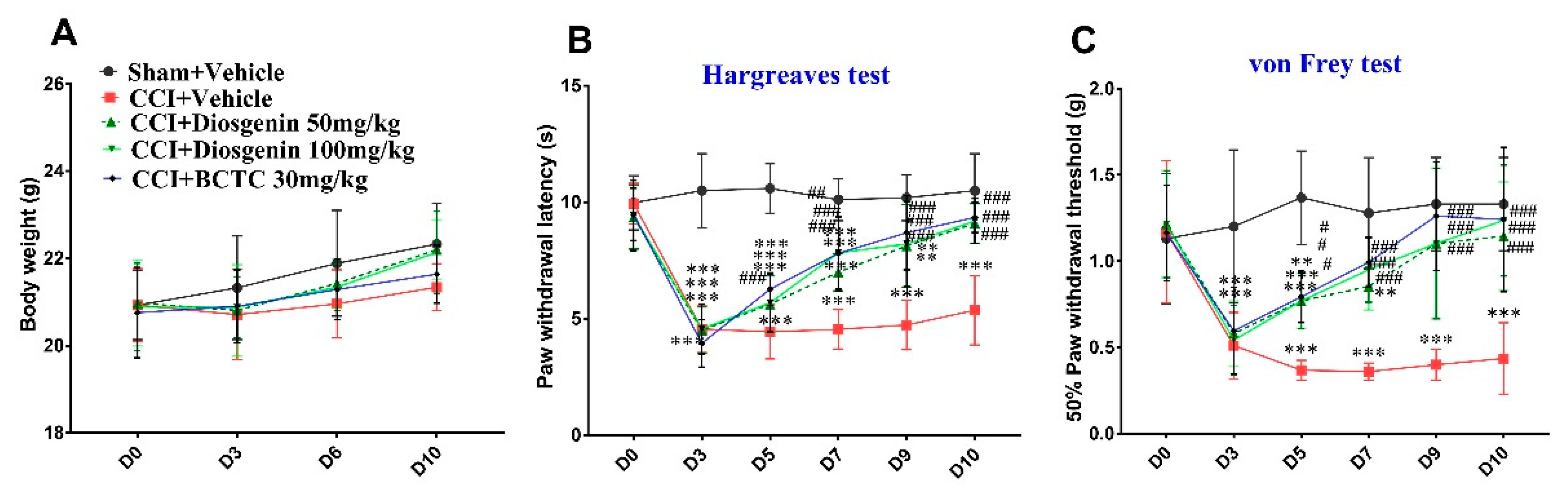
2.5. Effects of Oral Diosgenin Administration on Body Weight and Nociceptive Behavior
2.6. Effects of Diosgenin on Pro-Inflammatory Cytokines and TRPV1 Expression in DRG Tissues
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Capsaicin-Induced Acute Nociception and Behavioral Tests
4.3. Rectal Temperature Recording
4.4. Preparation of Mouse DRG
4.5. Intracellular Calcium Imaging
4.6. CCI Model and Behavioral Tests
4.7. Real Time-Quantitative Polymerase Chain Reaction Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Velzen, M.; Dahan, A.; Niesters, M. Neuropathic Pain: Challenges and Opportunities. Front. Pain Res. 2020, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D. Neurological diseases and pain. Brain 2012, 135, 320–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attal, N.; Martinez, V.; Bouhassira, D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021, 6, e884. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Liu, H.; Wang, X.Q. Top 100 Most-Cited Papers in Neuropathic Pain From 2000 to 2020: A Bibliometric Study. Front. Neurol. 2021, 12, 765193. [Google Scholar] [CrossRef]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Brandt, M.R.; Beyer, C.E.; Stahl, S.M. TRPV1 Antagonists and Chronic Pain: Beyond Thermal Perception. Pharmaceuticals 2012, 5, 114–132. [Google Scholar] [CrossRef]
- Abbas, M.A. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem. Biol. Interact. 2020, 330, 109178. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [Green Version]
- Aremu, A.O.; Pendota, S.C. Medicinal Plants for Mitigating Pain and Inflammatory-Related Conditions: An Appraisal of Ethnobotanical Uses and Patterns in South Africa. Front. Pharmacol. 2021, 12, 758583. [Google Scholar] [CrossRef]
- Dong, M.; Meng, Z.; Kuerban, K.; Qi, F.; Liu, J.; Wei, Y.; Wang, Q.; Jiang, S.; Feng, M.; Ye, L. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018, 9, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, B.; Zhang, Y.; Wang, Z.; Xu, D.; Jia, Y.; Guan, Y.; Liao, A.; Liu, G.; Chun, C.; Li, J. Therapeutic Potential of Diosgenin and Its Major Derivatives against Neurological Diseases: Recent Advances. Oxid. Med. Cell. Longev. 2020, 2020, 3153082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiasalari, Z.; Rahmani, T.; Mahmoudi, N.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin ameliorates development of neuropathic pain in diabetic rats: Involvement of oxidative stress and inflammation. Biomed. Pharmacother. 2017, 86, 654–661. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wang, P.F.; Song, H.G.; Sun, N. Diosgenin attenuates neuropathic pain in a rat model of chronic constriction injury. Mol. Med. Rep. 2017, 16, 1559–1564. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Go, E.J.; Park, J.W.; Kim, Y.H.; Park, C.K. Resolvins: Potent Pain Inhibiting Lipid Mediators via Transient Receptor Potential Regulation. Front. Cell Dev. Biol. 2020, 8, 584206. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarei, M.; Lam, J.T.; Yazdi, S.A.; Kariminia, A.; Dorado, J.N.; Kuzeljevic, B.; Syyong, H.T.; Hu, K.; van Breemen, C. Diosgenin modulates vascular smooth muscle cell function by regulating cell viability, migration, and calcium homeostasis. J. Pharmacol. Exp. Ther. 2011, 336, 925–939. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.J.; Wang, Z.; Ju, Y.; Wong, R.N.; Wu, Q.Y. Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother. Pharmacol. 2005, 55, 79–90. [Google Scholar] [CrossRef]
- Jayachandran, K.S.; Vasanthi, H.R.; Rajamanickam, G.V. Antilipoperoxidative and membrane stabilizing effect of diosgenin, in experimentally induced myocardial infarction. Mol. Cell. Biochem. 2009, 327, 203–210. [Google Scholar] [CrossRef]
- Leng, J.; Li, X.; Tian, H.; Liu, C.; Guo, Y.; Zhang, S.; Chu, Y.; Li, J.; Wang, Y.; Zhang, L. Neuroprotective effect of diosgenin in a mouse model of diabetic peripheral neuropathy involves the Nrf2/HO-1 pathway. BMC Complement. Med. Ther. 2020, 20, 126. [Google Scholar] [CrossRef] [Green Version]
- Groninger, H.; Schisler, R.E. Topical capsaicin for neuropathic pain #255. J. Palliat. Med. 2012, 15, 946–947. [Google Scholar] [CrossRef]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Junior, C.J.; Milano, J.; Souza, A.H.; Silva, J.F.; Rigo, F.K.; Dalmolin, G.; Cordeiro, M.N.; Richardson, M.; Barros, A.G.; Gomez, R.S.; et al. Phalpha1beta toxin prevents capsaicin-induced nociceptive behavior and mechanical hypersensitivity without acting on TRPV1 channels. Neuropharmacology 2013, 71, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Payrits, M.; Horvath, A.; Biro-Suto, T.; Erostyak, J.; Makkai, G.; Saghy, E.; Pohoczky, K.; Kecskes, A.; Kecskes, M.; Szolcsanyi, J.; et al. Resolvin D1 and D2 Inhibit Transient Receptor Potential Vanilloid 1 and Ankyrin 1 Ion Channel Activation on Sensory Neurons via Lipid Raft Modification. Int. J. Mol. Sci. 2020, 21, 5019. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.W.S.; Yuan, L.; Braz, J.M.; Basbaum, A.I.; Julius, D. TRPV1 drugs alter core body temperature via central projections of primary afferent sensory neurons. eLife 2022, 11, e80139. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Back, S.K.; Davies, A.J.; Jeong, H.; Jo, H.J.; Chung, G.; Na, H.S.; Bae, Y.C.; Kim, S.J.; Kim, J.S.; et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 2012, 74, 640–647. [Google Scholar] [CrossRef] [Green Version]
- Cernit, V.; Senecal, J.; Othman, R.; Couture, R. Reciprocal Regulatory Interaction between TRPV1 and Kinin B1 Receptor in a Rat Neuropathic Pain Model. Int. J. Mol. Sci. 2020, 21, 821. [Google Scholar] [CrossRef] [Green Version]
- Malek, N.; Pajak, A.; Kolosowska, N.; Kucharczyk, M.; Starowicz, K. The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol. Cell. Neurosci. 2015, 65, 1–10. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, Q.; Li, S.; Chen, M.; Jin, B.; Wang, M. TRPV1, Targeted by miR-338-3p, Induces Neuropathic Pain by Interacting with NECAB2. J. Mol. Neurosci. 2021, 71, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.L.; Cheng, M.J.; Zhang, X.X.; Wang, L. Elevated expression of transient receptor potential vanilloid type 1 in dorsal root ganglia of rats with endometriosis. Mol. Med. Rep. 2017, 16, 1920–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhe, V.; Plee-Gautier, E.; Carre, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Dastan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An Updated Pharmacological Review and Therapeutic Perspectives. Oxid. Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Shih, Y.W.; Huang, H.C.; Cheng, H.W. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS ONE 2011, 6, e20164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorno, T.B.S.; Moreira, I.; Rezende, C.M.; Fernandes, P.D. New (beta)N-octadecanoyl-5-hydroxytryptamide: Antinociceptive effect and possible mechanism of action in mice. Sci. Rep. 2018, 8, 10027. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Rong, Y.; Liu, M.; Cheng, S.; Liu, X.; Li, X.; Yu, Y.; Yang, G.; Yang, X. Analgesic Effects of Triterpenoid Saponins From Stauntonia chinensis via Selective Increase in Inhibitory Synaptic Response in Mouse Cortical Neurons. Front. Pharmacol. 2018, 9, 1302. [Google Scholar] [CrossRef] [Green Version]
- Son, D.B.; Choi, W.; Kim, M.; Go, E.J.; Jeong, D.; Park, C.K.; Kim, Y.H.; Lee, H.; Suh, J.W. Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells 2021, 10, 547. [Google Scholar] [CrossRef]

| Target GENE (Product Length) | Forward | Reverse |
|---|---|---|
| Mouse TRPV1 (203 bp) | AAGGCTTGCCCCCCTATAA | CACCAGCATGAACAGTGACTGT |
| Mouse TNF-α (206 bp) | CGTCAGCCGATTTGCTATCT | CGGACTCCGCAAAGTCTAAG |
| Mouse IL-1β (140 bp) | TGACGGACCCCAAAAGATGA | TCTCCACAGCCACAATGAGT |
| Mouse IL-6 (228 bp) | AGACTTCCATCCAGTTGCCT | AGTGCATCATCGTTGTTCATACA |
| Mouse GAPDH (150 bp) | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Jo, H.J.; Park, C.-K.; Kim, Y.H. Diosgenin Exerts Analgesic Effects by Antagonizing the Selective Inhibition of Transient Receptor Potential Vanilloid 1 in a Mouse Model of Neuropathic Pain. Int. J. Mol. Sci. 2022, 23, 15854. https://doi.org/10.3390/ijms232415854
Rahman MM, Jo HJ, Park C-K, Kim YH. Diosgenin Exerts Analgesic Effects by Antagonizing the Selective Inhibition of Transient Receptor Potential Vanilloid 1 in a Mouse Model of Neuropathic Pain. International Journal of Molecular Sciences. 2022; 23(24):15854. https://doi.org/10.3390/ijms232415854
Chicago/Turabian StyleRahman, Md. Mahbubur, Hyun Jung Jo, Chul-Kyu Park, and Yong Ho Kim. 2022. "Diosgenin Exerts Analgesic Effects by Antagonizing the Selective Inhibition of Transient Receptor Potential Vanilloid 1 in a Mouse Model of Neuropathic Pain" International Journal of Molecular Sciences 23, no. 24: 15854. https://doi.org/10.3390/ijms232415854
APA StyleRahman, M. M., Jo, H. J., Park, C.-K., & Kim, Y. H. (2022). Diosgenin Exerts Analgesic Effects by Antagonizing the Selective Inhibition of Transient Receptor Potential Vanilloid 1 in a Mouse Model of Neuropathic Pain. International Journal of Molecular Sciences, 23(24), 15854. https://doi.org/10.3390/ijms232415854







