A Golgi-Located Transmembrane Nine Protein Gene TMN11 Functions in Manganese/Cadmium Homeostasis and Regulates Growth and Seed Development in Rice
Abstract
:1. Introduction
2. Results
2.1. Structure and Sequence of OsTMN11 Gene and Protein
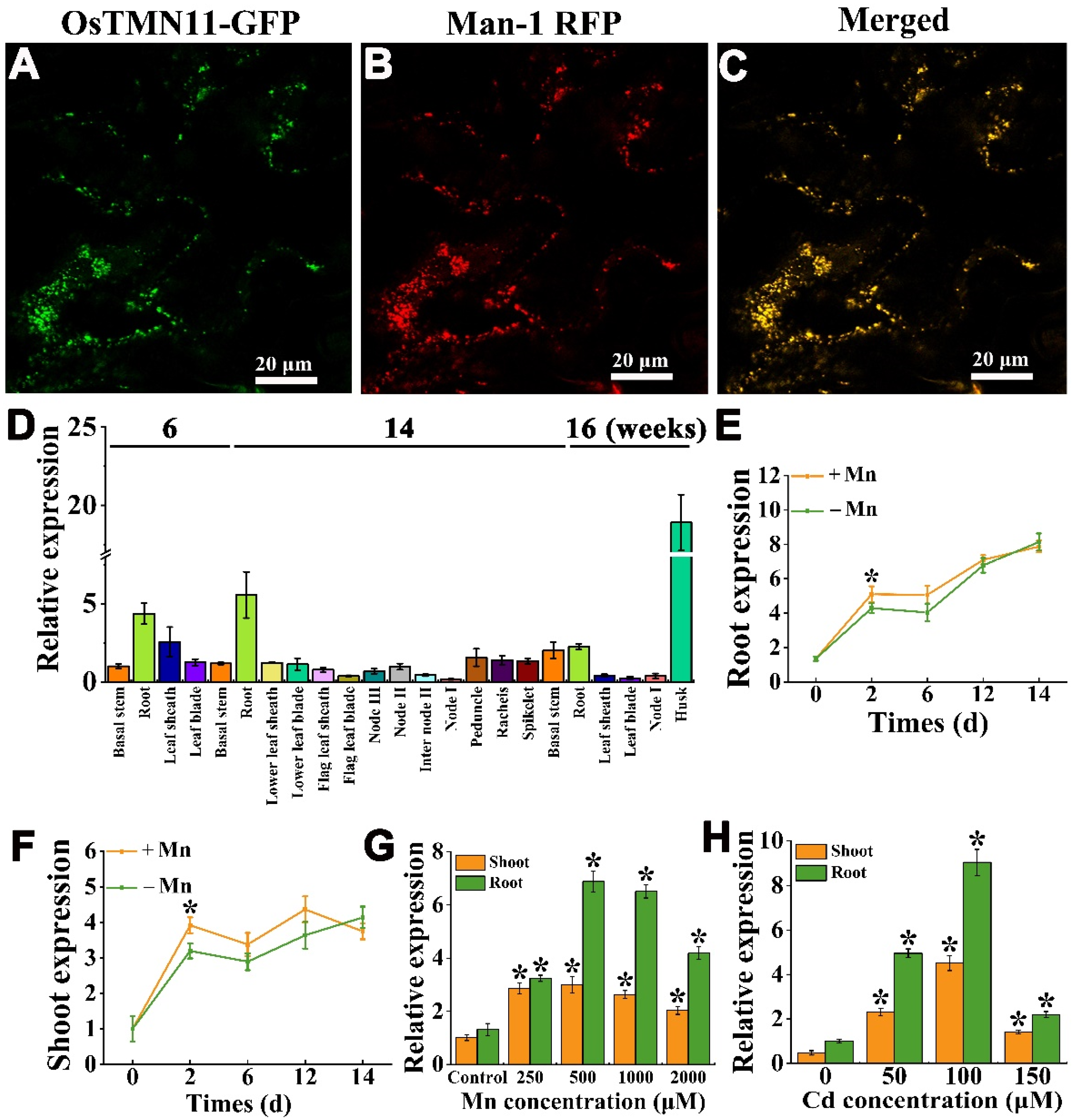
2.2. OsTMN11 Is Localized to the Cis-Golgi Apparatus and Induced under Mn and Cd Stress
2.3. OsTMN11 Knockdown RNAi Lines Are Sensitive to Excessive Mn and Cd Ions
2.4. OsTMN11 Knockdown Results in Overaccumulation of Mn and Cd in Rice Plants
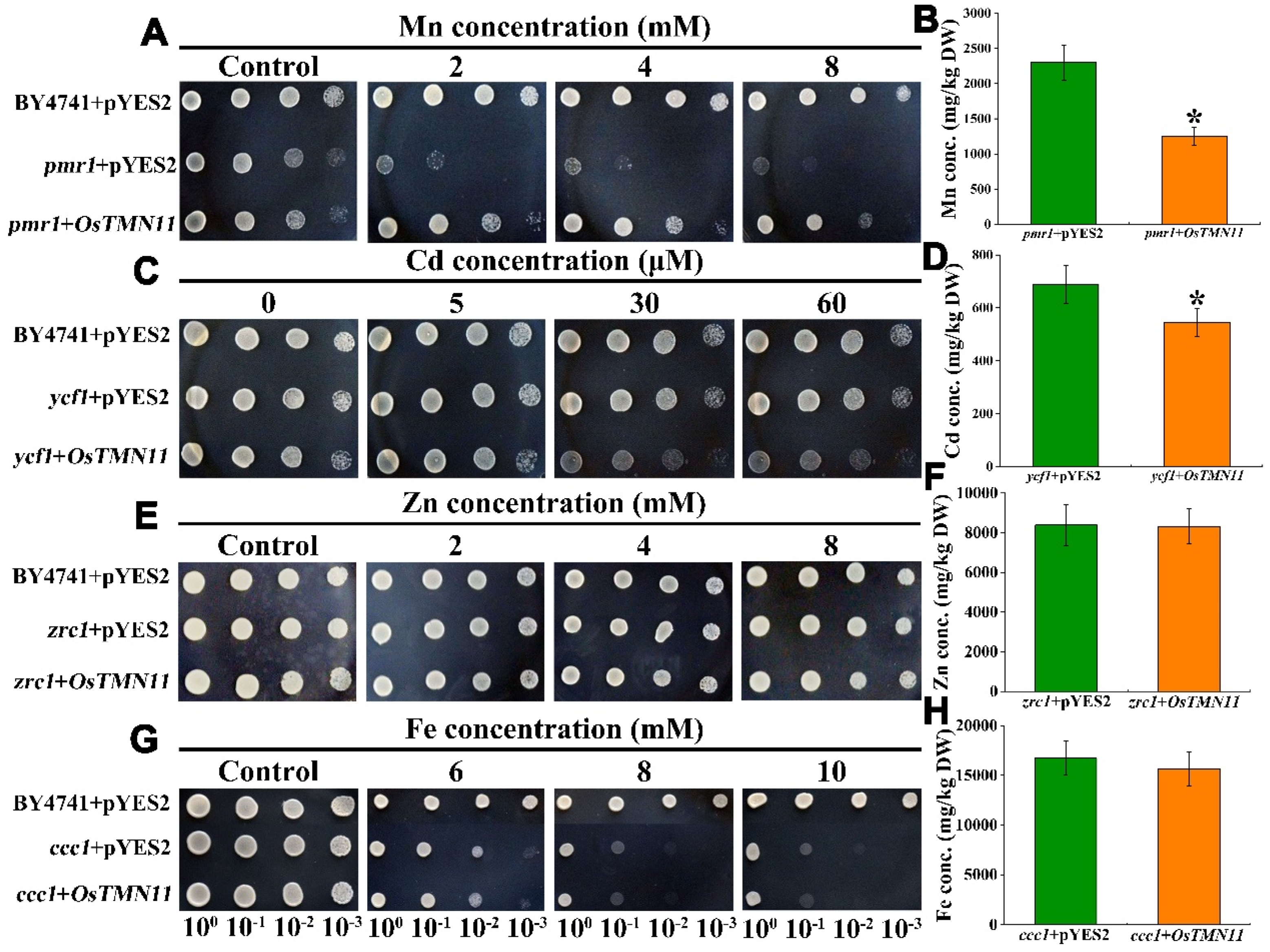
2.5. OsTMN11 Can Complement a Mn-Sensitive Phenotype of Golgi-Resident pmr1 Mutant Yeast and Regulate Cellular Mn Efflux
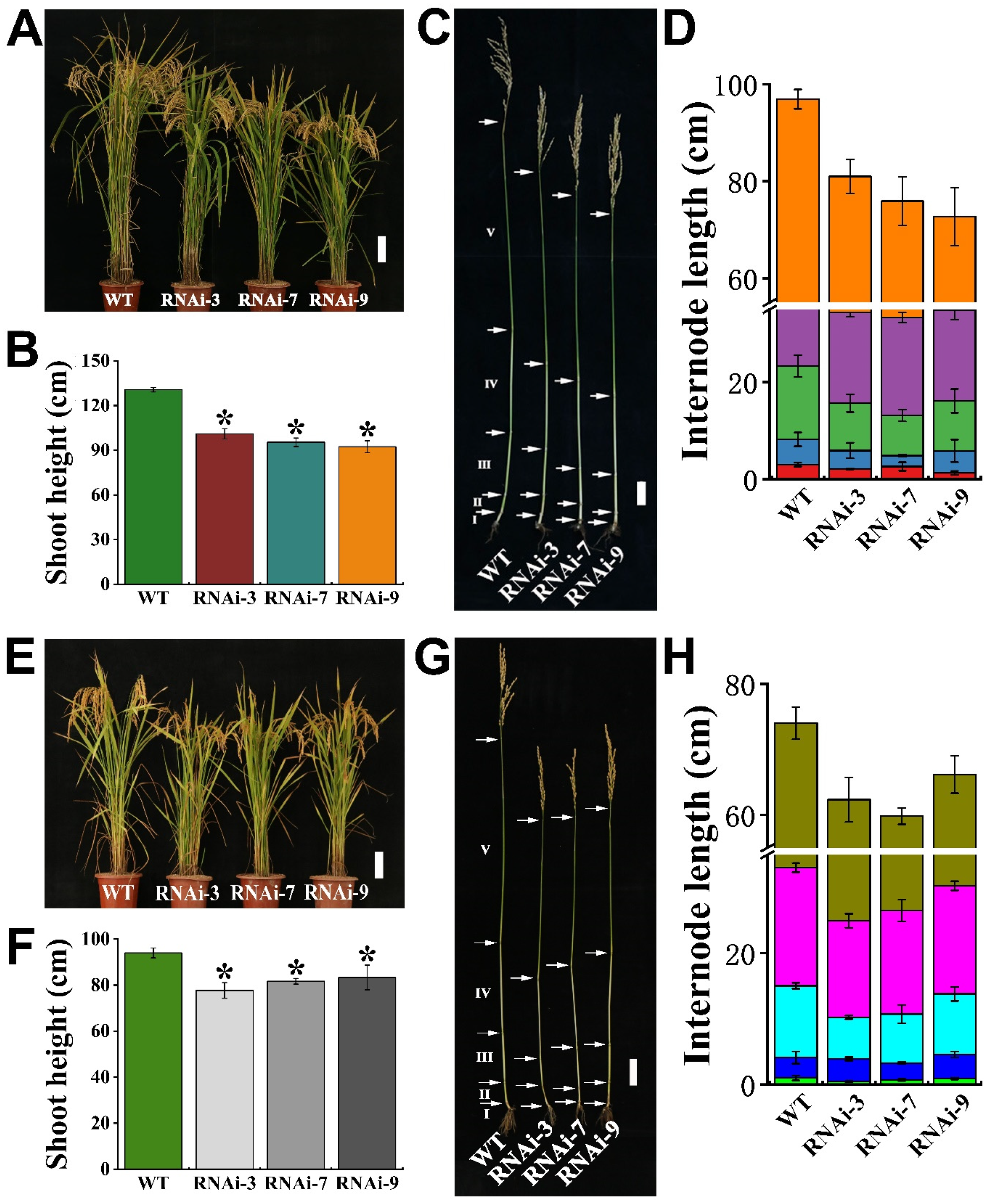
2.6. OsTMN11 Suppression Dampens Rice Growth and Development under Natural Conditions
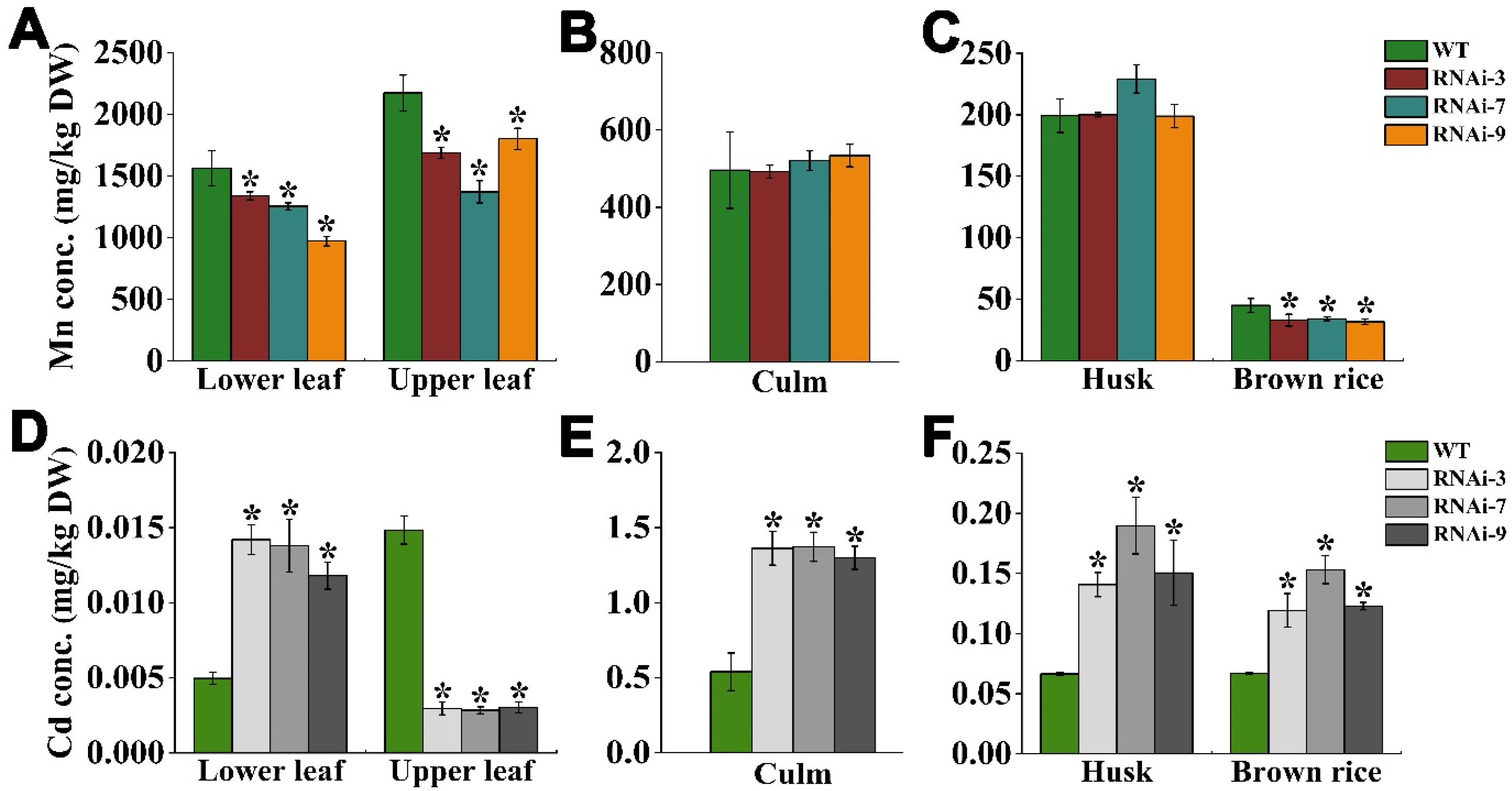
2.7. OsTMN11 Disruption Impaired Mn and Cd Accumulation in Maturity Rice Plants
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Treatment
5.2. Analysis of Bioinformatics Related to OsTMN11 Gene and Protein Sequences
5.3. Transcriptional Expression of OsTMN11
5.4. Generation of Rice RNAi Lines
5.5. Analysis of Subcellular Localization of OsTMN11
5.6. Transformation OsTMN11 into Yeast Cells
5.7. Measurement of Metal Concentration
5.8. Measurement of Chlorophyll and Malondialdehyde
5.9. Analysis of Morphology and Phenotypes Relevant to Seed Development
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2011; pp. 135–178. [Google Scholar]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Kellokumpu, S. Golgi pH, Ion and Redox Homeostasis: How Much Do They Really Matter? Front. Cell Dev. Biol. 2019, 7, 93. [Google Scholar] [CrossRef] [Green Version]
- Rono, J.K.; Sun, D.; Yang, Z.M. Metallochaperones: A critical regulator of metal homeostasis and beyond. Gene 2022, 822, 146352. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Rono, J.K.; Wang, M.Q.; Yang, Z.M. A Metal Chaperone Gene Regulates Rice Growth and Seed Development by Manganese Acquisition and Homeostasis. Agronomy 2022, 12, 1676. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ishikawa, S.; Abe, T.; Baba, K.; Arao, T.; Terada, T. Role of the node in controlling traffic of cadmium, zinc, and manganese in rice. J. Exp. Bot. 2012, 63, 2729–2737. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yang, F.; Liu, J.L.; Wu, H.T.; Yang, H.; Shi, Y.; Liu, J.; Zhang, Y.F.; Luo, Y.R.; Chen, K.M. Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 2022, 809, 151099. [Google Scholar] [CrossRef]
- Benghezal, M.; Cornillon, S.; Gebbie, L.; Alibaud, L.; Brückert, F.; Letourneur, F.; Cosson, P. Synergistic control of cellular adhesion by transmembrane 9 proteins. Mol. Biol. Cell 2003, 14, 2890–2899. [Google Scholar] [CrossRef]
- Perrin, J.; Mortier, M.; Jacomin, A.C.; Viargues, P.; Thevenon, D.; Fauvarque, M.O. The nonaspanins TM9SF2 and TM9SF4 regulate the plasma membrane localization and signalling activity of the peptidoglycan recognition protein PGRP-LC in Drosophila. J. Innate. Immun. 2015, 7, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Froque, R.; Coadic, M.L.; Perrin, J.; Cherix, N.; Cornillon, S.; Cosson, P. TM9/Phg1 and SadA proteins control surface expression and stability of SibA adhesion molecules in Dictyostelium. Mol. Biol. Cell 2012, 23, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, R.; Spinello, I.; Quaranta, M.T.; Pasquini, L.; Pelosi, E.; Coco, F.L.; Testa, U.; Labbaye, C. Human TM9SF4 Is a New Gene Down-Regulated by Hypoxia and Involved in Cell Adhesion of Leukemic Cells. PLoS ONE 2015, 10, e0126968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegelund, J.N.; Jahn, T.P.; Baekgaard, L.; Palmgren, M.G.; Schjoerring, J.K. Transmembrane nine proteins in yeast and Arabidopsis affect cellular metal contents without changing vacuolar morphology. Physiol. Plant 2010, 140, 355–367. [Google Scholar] [CrossRef]
- Yamaji, T.; Sekizuka, T.; Tachida, Y.; Sakuma, C.; Morimoto, K.; Kuroda, M.; Hanada, K. A CRISPR Screen Identifies LAPTM4A and TM9SF Proteins as Glycolipid-Regulating Factors. iScience 2019, 11, 409–424. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Meng, Z.; Zhu, Y.; Lu, J.; Li, Z.; Zhao, Q.; Huang, Y.; Jiang, L.; Yao, X. TM9SF4 is a novel factor promoting autophagic flux under amino acid starvation. Cell Death Differ. 2018, 25, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Welch, L.G.; Munro, S. A tale of short tails, through thick and thin: Investigating the sorting mechanisms of Golgi enzymes. FEBS Lett. 2019, 593, 2452–2465. [Google Scholar] [CrossRef] [Green Version]
- Hiroguchi, A.; Sakamoto, S.; Mitsuda, N.; Miwa, K. Golgi-localized membrane protein AtTMN1/EMP12 functions in the deposition of rhamnogalacturonan II and I for cell growth in Arabidopsis. J. Exp. Bot. 2021, 72, 3611–3629. [Google Scholar] [CrossRef]
- Gao, C.; Yu, C.K.; Qu, S.; San, M.W.; Li, K.Y.; Lo, S.W.; Jiang, L. The Golgi-localized Arabidopsis endomembrane protein12 contains both endoplasmic reticulum export and Golgi retention signals at its C terminus. Plant Cell 2012, 24, 2086–2104. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.J.; Liu, X.S.; Tao, H.; Tan, S.K.; Chu, S.S.; Oono, Y.; Zhang, X.D.; Chen, J.; Yang, Z.M. Variation of DNA methylation patterns associated with gene expression in rice (Oryza sativa) exposed to cadmium. Plant Cell Environ. 2016, 39, 2629–2649. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Li, C.; Li, H.; Liu, X.S.; Yang, Z.M. OsZIP11 is a trans-Golgi-residing transporter required for rice iron accumulation and development. Gene 2022, 836, 146678. [Google Scholar] [CrossRef] [PubMed]
- Dürr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapinskas, P.J.; Cunningham, K.W.; Liu, X.F.; Fink, G.R.; Culotta, V.C. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell Biol. 1995, 15, 1382–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiter, E.; Montanini, B.; Gobert, A.; Pedas, P.; Husted, S.; Maathuis, F.J.; Blaudez, D.; Chalot, M.; Sanders, D. A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 8532–8537. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Li, J.; Li, J.; Li, Y.; Gu, D.; Chen, C.; Cui, J.; Chen, X.; Zhang, W. OsMTP11, a trans-Golgi network localized transporter, is involved in manganese tolerance in rice. Plant Sci. 2018, 274, 59–69. [Google Scholar] [CrossRef]
- Wemmie, J.A.; Szczypka, M.S.; Thiele, D.J.; Moye-Rowley, W.S. Cadmium tolerance mediated by the yeast AP-1 protein requires the presence of an ATP-binding cassette transporter-encoding gene, YCF1. J. Biol. Chem. 1994, 269, 32592–32597. [Google Scholar] [CrossRef]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The Key to Mn Homeostasis in Plants: Regulation of Mn Transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef]
- Rui, Q.; Tan, X.; Liu, F.; Bao, Y. An Update on the Key Factors Required for Plant Golgi Structure Maintenance. Front. Plant Sci. 2022, 13, 933283. [Google Scholar] [CrossRef]
- Foulquier, F.; Legrand, D. Biometals and glycosylation in humans: Congenital disorders of glycosylation shed lights into the crucial role of Golgi manganese homeostasis. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129674. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y. Golgi Metal Ion Homeostasis in Human Health and Diseases. Cells 2022, 11, 289. [Google Scholar] [CrossRef]
- Lebredonchel, E.; Houdou, M.; Hoffmann, H.H.; Kondratska, K.; Krzewinski, M.A.; Vicogne, D.; Rice, C.M.; Klein, A.; Foulquier, F. Investigating the functional link between TMEM165 and SPCA1. Biochem. J. 2019, 476, 3281–3293. [Google Scholar] [CrossRef] [PubMed]
- Potelle, S.; Morelle, W.; Dulary, E.; Duvet, S.; Vicogne, D.; Spriet, C.; Krzewinski-Recchi, M.A.; Morsomme, P.; Jaeken, J.; Matthijs, G.; et al. Glycosylation abnormalities in Gdt1p/TMEM165 deficient cells result from a defect in Golgi manganese homeostasis. Hum. Mol. Genet. 2016, 25, 1489–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farthing, E.C.; Menguer, P.K.; Fett, J.P.; Williams, L.E. OsMTP11 is localised at the Golgi and contributes to Mn tolerance. Sci. Rep. 2017, 7, 15258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liu, B. Identification of a rice metal tolerance protein OsMTP11 as a manganese transporter. PLoS ONE 2017, 12, e0174987. [Google Scholar] [CrossRef] [Green Version]
- Tsunemitsu, Y.; Genga, M.; Okada, T.; Yamaji, N.; Ma, J.F.; Miyazaki, A.; Kato, S.I.; Iwasaki, K.; Ueno, D. A member of cation diffusion facilitator family, MTP11, is required for manganese tolerance and high fertility in rice. Planta 2018, 248, 231–241. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, C.; Singh, S.; Fan, N.; Liu, S.; Zhao, L.; Cao, H.; Xie, W.; Yang, C.; Huang, C.F. Golgi-localised manganese transporter PML3 regulates Arabidopsis growth through modulating Golgi glycosylation and cell wall biosynthesis. New Phytol. 2021, 231, 2200–2214. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Zhou, X.; Yin, X.; Wu, M.; Chen, W.; Liu, Y.; Gong, C.; Zhang, W.; Chen, X.; Xu, E. Metal tolerance protein family members are involved in Mn homeostasis through internal compartmentation and exocytosis in Brassica napus. Environ. Exp. Bot. 2022, 195, 104785. [Google Scholar] [CrossRef]
- He, J.; Yang, B.; Hause, G.; Rössner, N.; Peiter-Volk, T.; Schattat, M.H.; Voiniciuc, C.; Peiter, E. The trans-Golgi-localized protein BICAT3 regulates manganese allocation and matrix polysaccharide biosynthesis. Plant Physiol. 2022, 190, kiac387. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Makino, T.; Kunhikrishnan, A.; Kim, P.J.; Kirkham, M.B. Cadmium Contamination and Its Risk Management in Rice Ecosystems. Adv. Agron. 2013, 119, 183–273. [Google Scholar]
- Zhang, X.D.; Wang, Y.; Li, H.B.; Yang, Z.M. Isolation and identification of rapeseed (Brassica napus) cultivars for potential higher and lower Cd accumulation. J. Plant Nutr. 2018, 181, 479–487. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Song, J.B.; Zhao, W.T.; Yang, Z.M. AtHO1 is involved in iron homeostasis in an NO-dependent manner. Plant Cell Physiol. 2013, 54, 1105–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.U.; Rono, J.K.; Liu, X.S.; Feng, S.J.; Yang, Z.M. Functional characterization of a new metallochaperone for reducing cadmium concentration in rice crop. J. Clean Prod. 2020, 272, 123152. [Google Scholar] [CrossRef]
- Feng, S.J.; Liu, X.S.; Cao, H.W.; Yang, Z.M. Identification of a rice metallochaperone for cadmium tolerance by an epigenetic mechanism and potential use for clean up in wetland. Environ. Pollut. 2021, 288, 117837. [Google Scholar] [CrossRef]
- Sun, J.Y.; Liu, X.S.; Khan, I.U.; Wu, X.C.; Yang, Z.M. OsPIP2;3 as an aquaporin contributes to rice resistance to water deficit but not to salt stress. Environ. Exp. Bot. 2020, 183, 104342. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, C.; Liu, X.; Yang, Z. A Golgi-Located Transmembrane Nine Protein Gene TMN11 Functions in Manganese/Cadmium Homeostasis and Regulates Growth and Seed Development in Rice. Int. J. Mol. Sci. 2022, 23, 15883. https://doi.org/10.3390/ijms232415883
Li H, Li C, Liu X, Yang Z. A Golgi-Located Transmembrane Nine Protein Gene TMN11 Functions in Manganese/Cadmium Homeostasis and Regulates Growth and Seed Development in Rice. International Journal of Molecular Sciences. 2022; 23(24):15883. https://doi.org/10.3390/ijms232415883
Chicago/Turabian StyleLi, He, Chao Li, Xuesong Liu, and Zhimin Yang. 2022. "A Golgi-Located Transmembrane Nine Protein Gene TMN11 Functions in Manganese/Cadmium Homeostasis and Regulates Growth and Seed Development in Rice" International Journal of Molecular Sciences 23, no. 24: 15883. https://doi.org/10.3390/ijms232415883



