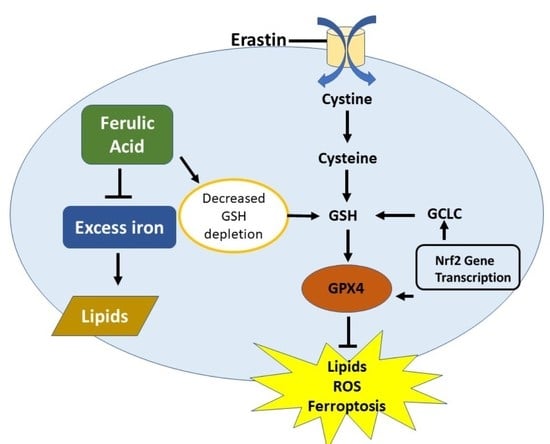
Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells
Abstract
:1. Introduction
2. Results
2.1. FA and FAS Alleviate the Decreased Viability of MIN6 Cells Induced by Erastin
2.2. FA and FAS Suppress Iron Accumulation and Can Act as Iron Chelators
2.3. Treatments of FA and FAS Limit Lipid Peroxidation and ROS Production by Increasing GSH Antioxidant Levels
2.4. Phenolic Acids Activate Nrf2 Pathway in Erastin-Induced MIN6 Cells
2.5. Nrf2 Activation Shows the Protection against Ferroptosis in ML385-Treated MIN6 Cells
2.6. Phenolic Acids Increased Insulin Secretion in Erastin-Induced Ferroptotic MIN6 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. MIN6 Cell Culture
4.3. Cell Viability Assay
4.4. Cellular Iron Levels
4.5. Measurement of Intracellular Reactive Oxygen Species
4.6. Lipid Peroxidation Assay
4.7. GLUTATHIONE ASSAY
4.8. Iron Chelation Assay
4.9. Insulin Secretion Assay
4.10. Western Blot
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fleming, R.E.; Ponka, P. Iron Overload in Human Disease. N. Engl. J Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are oxidative stress—Activated signaling pathways mediators of insulin resistance and β-cell dysfuntion? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzen, S. Oxidative stress: The vulnerable β-cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. In Apoptotic and Non-Apoptotic Cell Death; Springer: Cham, Switzerland, 2016; pp. 143–170. [Google Scholar]
- Li, C.; Deng, X.; Zhang, W.; Xie, X.; Conrad, M.; Liu, Y.; Angeli, J.P.; Lai, L. Novel Allosteric Activators for Ferroptosis Regulator Glutathione Peroxidase 4. J. Med. Chem. 2019, 62, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Song, X.; Sun, X.; Huang, J.; Zhong, M.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox. Biol. 2019, 23, 101–107. [Google Scholar] [CrossRef]
- Song, X.; Long, D. Nrf2 and ferroptosis: A new research direction for neurodegenerative diseases. Front. Neurosci. 2020, 14, 267. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation, and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Ma, Z.; Hong, Q.; Wang, Y.; Liang, Q.; Tan, H.; Xiao, C.; Tang, X.; Shao, S.; Zhou, S.; Gao, Y. Ferulic acid induces heme oxygenase-1 via activation of ERK and Nrf2. Drug Discov. Ther. 2011, 5, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019, 23, 4900–4912. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, Q.; Shi, C.; Jiao, F.; Gong, Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol. Med. Rep. 2019, 20, 4081–4090. [Google Scholar] [CrossRef] [Green Version]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A review on iron chelators in treatment of iron overload syndromes. Int. J. Hematol. Oncol. 2016, 10, 239. [Google Scholar]
- Ishihara, H.; Asano, T.; Tsukuda, K.; Katagiri, H.; Inukai, K.; Anai, M.; Kikuchi, M.; Yazaki, Y.; Miyazaki, J.I.; Oka, Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993, 36, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Liu, X.; Zu, Y.; Fu, Y.; Zhang, S.; Yao, L.; Efferth, T. Cajanol, a novel anticancer agent from Pigeonpea roots, induces apoptosis in human breast cancer cells through a ROS-mediated mitochondrial pathway. Chem. Biol. Interact. 2010, 188, 151–160. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Aust, S.D. The role of iron in the initiation of lipid peroxidation. Chem. Phys. Lipids 1987, 44, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Visalli, G.; Cirmi, S.; Lombardo, G.E.; Laganà, P.; Di Pietro, A.; Navarra, M. Natural iron chelators: Protective role in A549 cells of flavonoids-rich extracts of Citrus juices in Fe3+-induced oxidative stress. Environ. Toxicol. Pharmacol. 2016, 43, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Qi, K.; Gong, Y.; Long, X.; Zhu, S.; Lu, F.; Lin, K.; Xu, J. Ferulic acid alleviates myocardial ischemia reperfusion injury via upregulating AMPKα2 expressionmediated ferroptosis depression. J. Cardiovasc. Pharmacol. 2022, 79, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, N.; Liu, N.; Dong, H. Lipid peroxidation and GPX4 inhibition are common causes for myofibroblast differentiation and ferroptosis. DNA Cell Biol. 2019, 38, 725–733. [Google Scholar] [CrossRef]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent-and AIF-mediated cell death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)-epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.C. The Nrf2–ARE pathway: An indicator and modulator of oxidative stress in neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Valls-Belles, V.; Gonzalez, P.; Muniz, P. Epicatechin effect on oxidative damage induced by tert-BOOH in isolated hepatocytes of fasted rats. Process Biochem. 2004, 39, 1525–1531. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Yao, S.; Zheng, J.; Gong, X.; Xiao, B. Ferulic acid alleviates alveolar epithelial barrier dysfunction in sepsis-induced acute lung injury by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis. Pharm. Biol. 2022, 60, 2286–2294. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bas, M.; Gumruk, F.; Gonc, N.; Cetin, M.; Tuncer, M.; Hazırolan, T.; Yildirim, G.; Karabulut, E.; Unal, S. Biochemical markers of glucose metabolism may be used to estimate the degree and progression of iron overload in the liver and pancreas of patients with β-thalassemia major. Ann. Hematol. 2015, 94, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Saji, N.; Francis, N.; Blanchard, C.L.; Schwarz, L.J.; Santhakumar, A.B. Rice bran phenolic compounds regulate genes associated with antioxidant and anti-inflammatory activity in human umbilical vein endothelial cells with induced oxidative stress. Int. J. Mol. Sci. 2019, 20, 4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.K.; Takii, M.; Kojima, Y.; Yokosawa, H.; Ishikawa, T. Structure-dependent inhibitory effects of green tea catechins on insulin secretion from pancreatic β-cells. Biol. Pharm. Bull. 2015, 38, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.U.; Niederau, C. Oxidative stress-induced changes in pancreatic acinar cells: Insights from in vitro studies. Hepatogastroenterology 1994, 41, 309–312. [Google Scholar] [PubMed]
- Bourne, L.; Paganga, G.; Baxter, D.; Hughes, P.; Rice-Evans, C. Absorption of ferulic acid from low-alcohol beer. Free Radic. Res. 2000, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.M.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.T. Absorption of hydroxycinnamates in humans after high-bran cereal consumption. J. Agric. Food Chem. 2003, 51, 6050–6055. [Google Scholar] [CrossRef]
- Adam, A.; Crespy, V.; Levrat-Verny, M.A.; Leenhardt, F.; Leuillet, M.; Demigné, C.; Rémésy, C. The bioavailability of ferulic acid is governed primarily by the food matrix rather than its metabolism in intestine and liver in rats. J. Nutr. 2002, 132, 1962–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Egashira, Y.; Sanada, H. Phenolic antioxidants richly contained in corn bran are slightly bioavailable in rats. J. Agric. Food Chem. 2005, 53, 5030–5035. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.C.; Rice-Evans, C. Bioavailability of ferulic acid. Biochem. Biophys. Res. Commun. 1998, 253, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Harder, H.; Tetens, I.; Let, M.B.; Meyer, A.S. Rye bran bread intake elevates urinary excretion of ferulic acid in humans but does not affect the susceptibility of LDL to oxidation ex vivo. Eur. J. Nutr. 2004, 43, 230–236. [Google Scholar] [CrossRef]
- Yan, N.; Tang, Z.; Xu, Y.; Li, X.; Wang, Q. Pharmacokinetic study of ferulic acid following transdermal or intragastric administration in rats. AAPS Pharmscitech 2020, 21, 169. [Google Scholar] [CrossRef]
- Miyazaki, J.I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K.I. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocr. J. 1990, 127, 126–132. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red: Blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Farhan, H.; Rammal, H.; Hijazi, A.; Annan, H.; Daher, A.; Reda, M.; Badran, B. Chemical composition, in vitro cytotoxicity, and anti-free radical properties of six extracts from Lebanese Trigonella berythea Boiss. Pak. J. Pharm. Sci. 2013, 26, 1157–1163. [Google Scholar] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, T.; Sharp, P.A.; Latunde-Dada, G.O. Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells. Int. J. Mol. Sci. 2022, 23, 15886. https://doi.org/10.3390/ijms232415886
Kose T, Sharp PA, Latunde-Dada GO. Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells. International Journal of Molecular Sciences. 2022; 23(24):15886. https://doi.org/10.3390/ijms232415886
Chicago/Turabian StyleKose, Tugba, Paul A. Sharp, and Gladys O. Latunde-Dada. 2022. "Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells" International Journal of Molecular Sciences 23, no. 24: 15886. https://doi.org/10.3390/ijms232415886
APA StyleKose, T., Sharp, P. A., & Latunde-Dada, G. O. (2022). Upregulation of Nrf2 Signalling and the Inhibition of Erastin-Induced Ferroptosis by Ferulic Acid in MIN6 Cells. International Journal of Molecular Sciences, 23(24), 15886. https://doi.org/10.3390/ijms232415886