EBF1 Negatively Regulates Brassinosteroid-Induced Apical Hook Development and Cell Elongation through Promoting BZR1 Degradation
Abstract
1. Introduction
2. Results
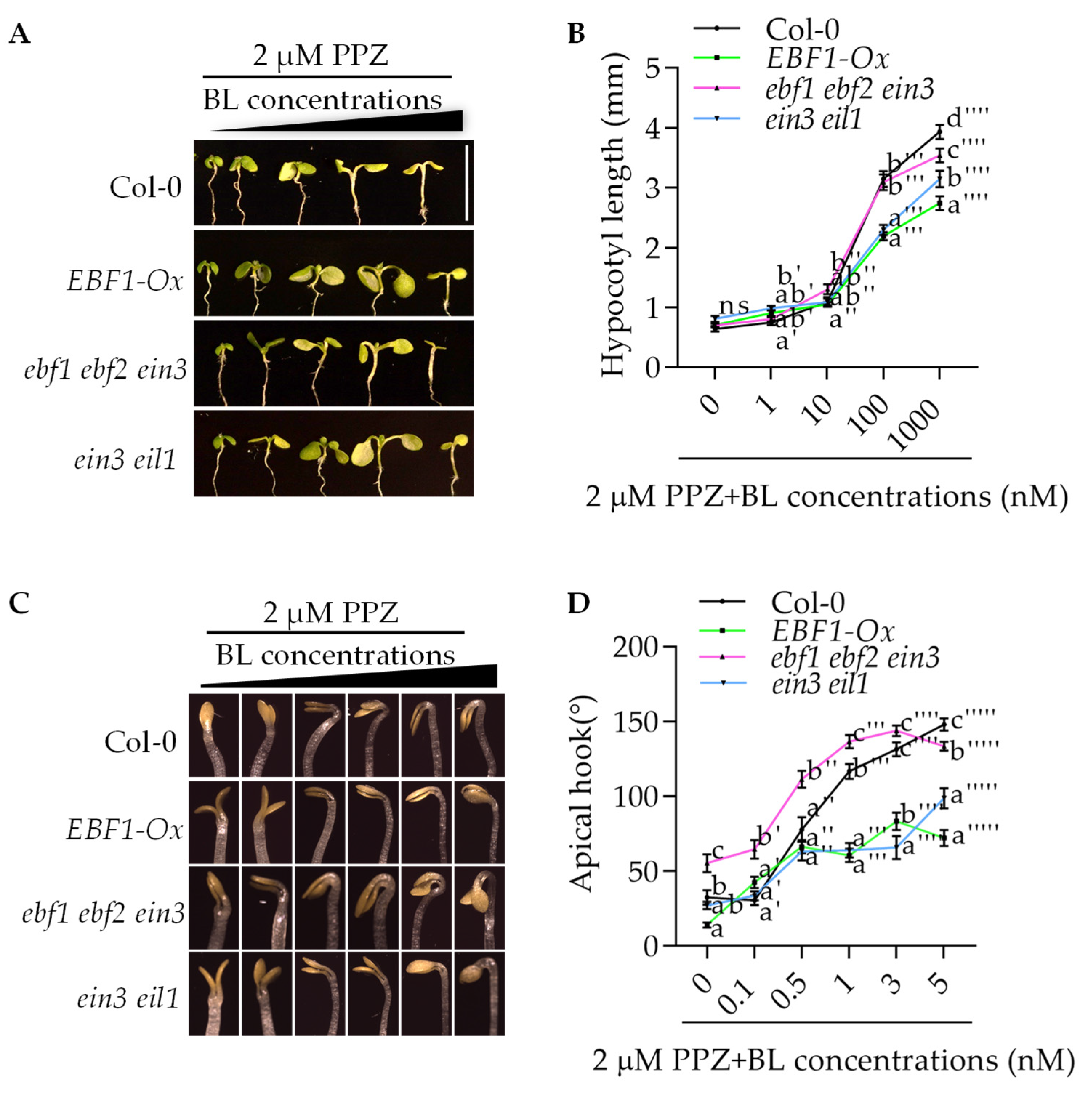
2.1. EBF1 Inhibits BR-Induced Apical Hook Development and Hypocotyl Elongation
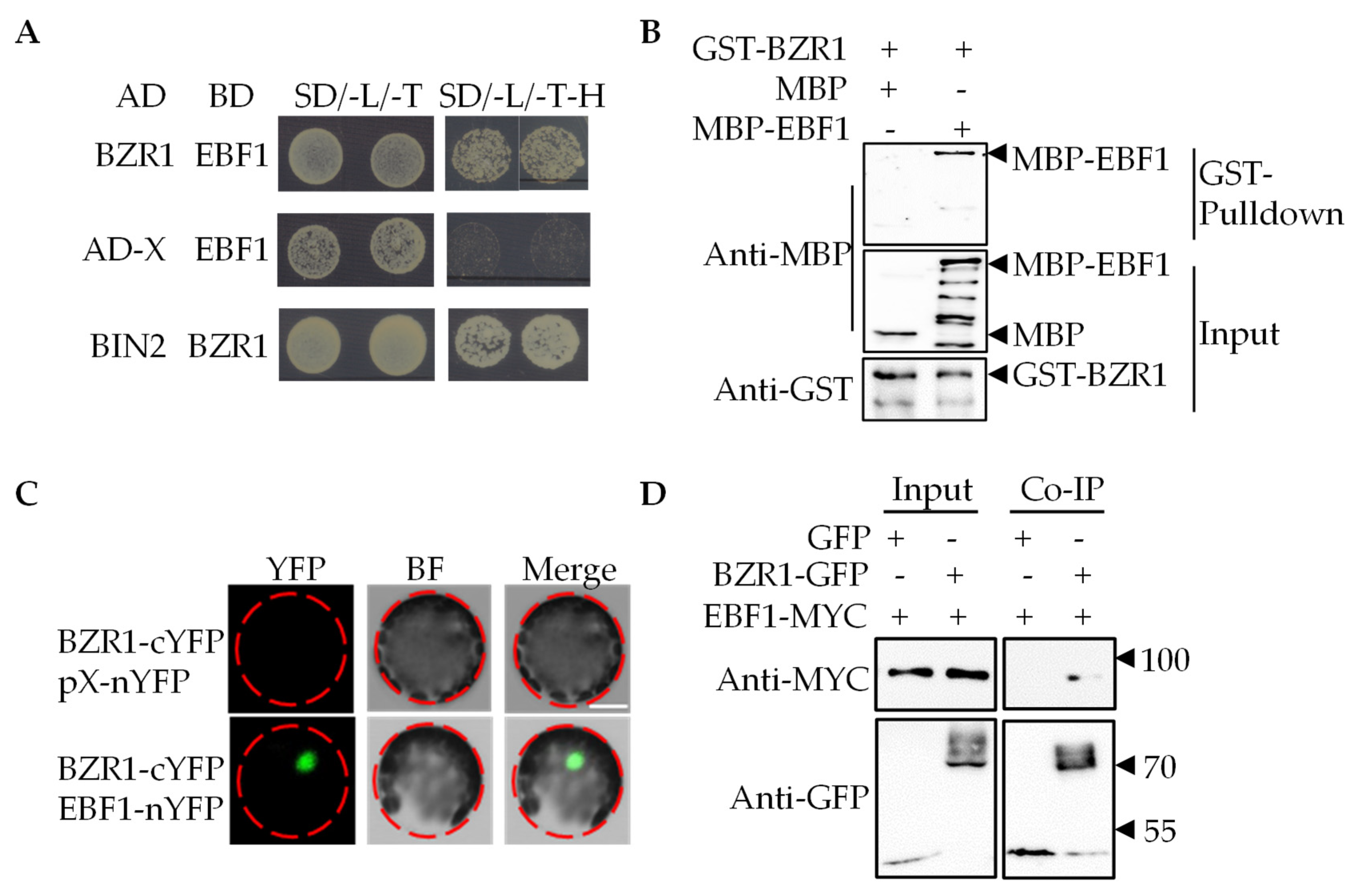
2.2. EBF1 Interacts with BZR1 In Vivo and In Vitro
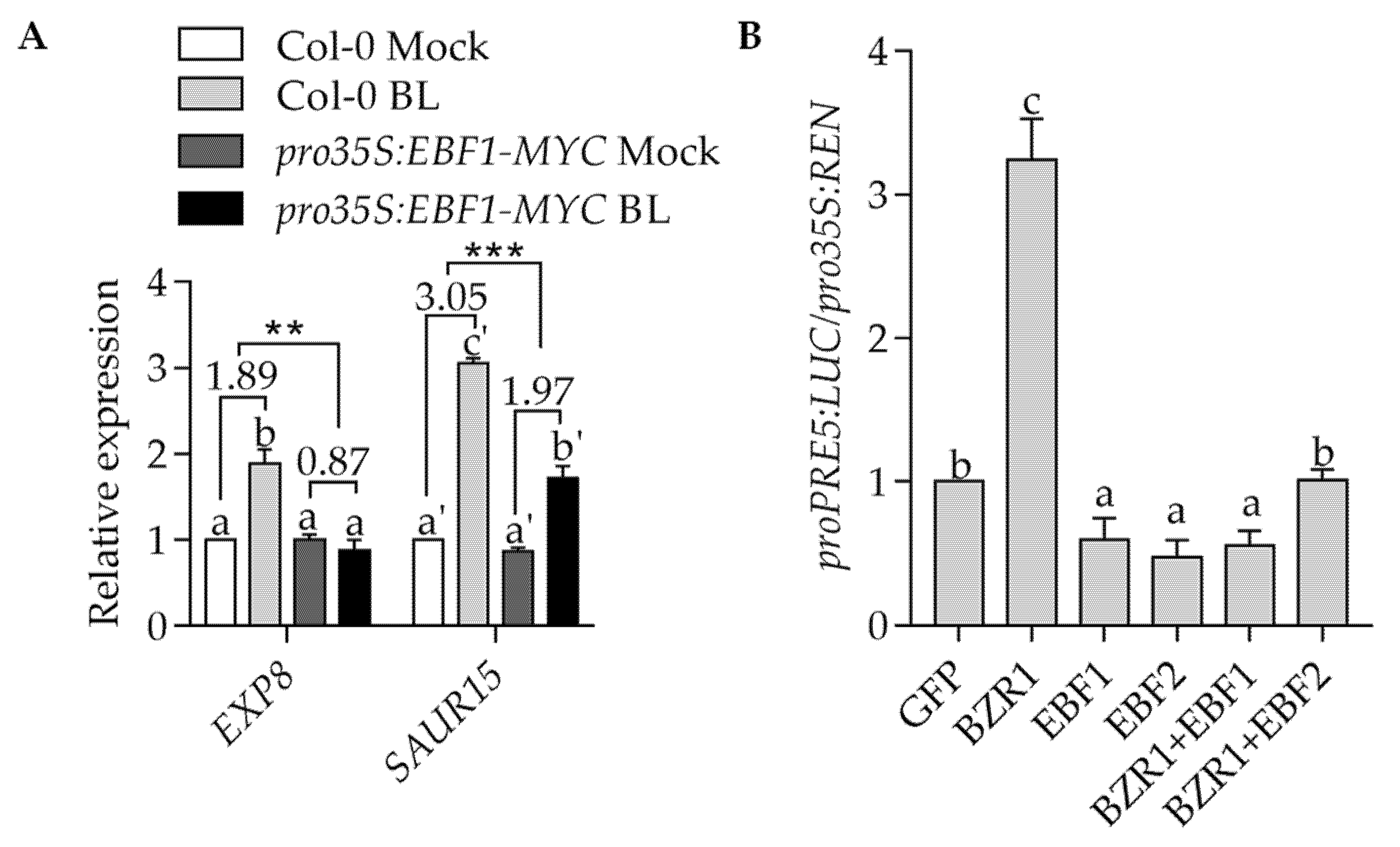
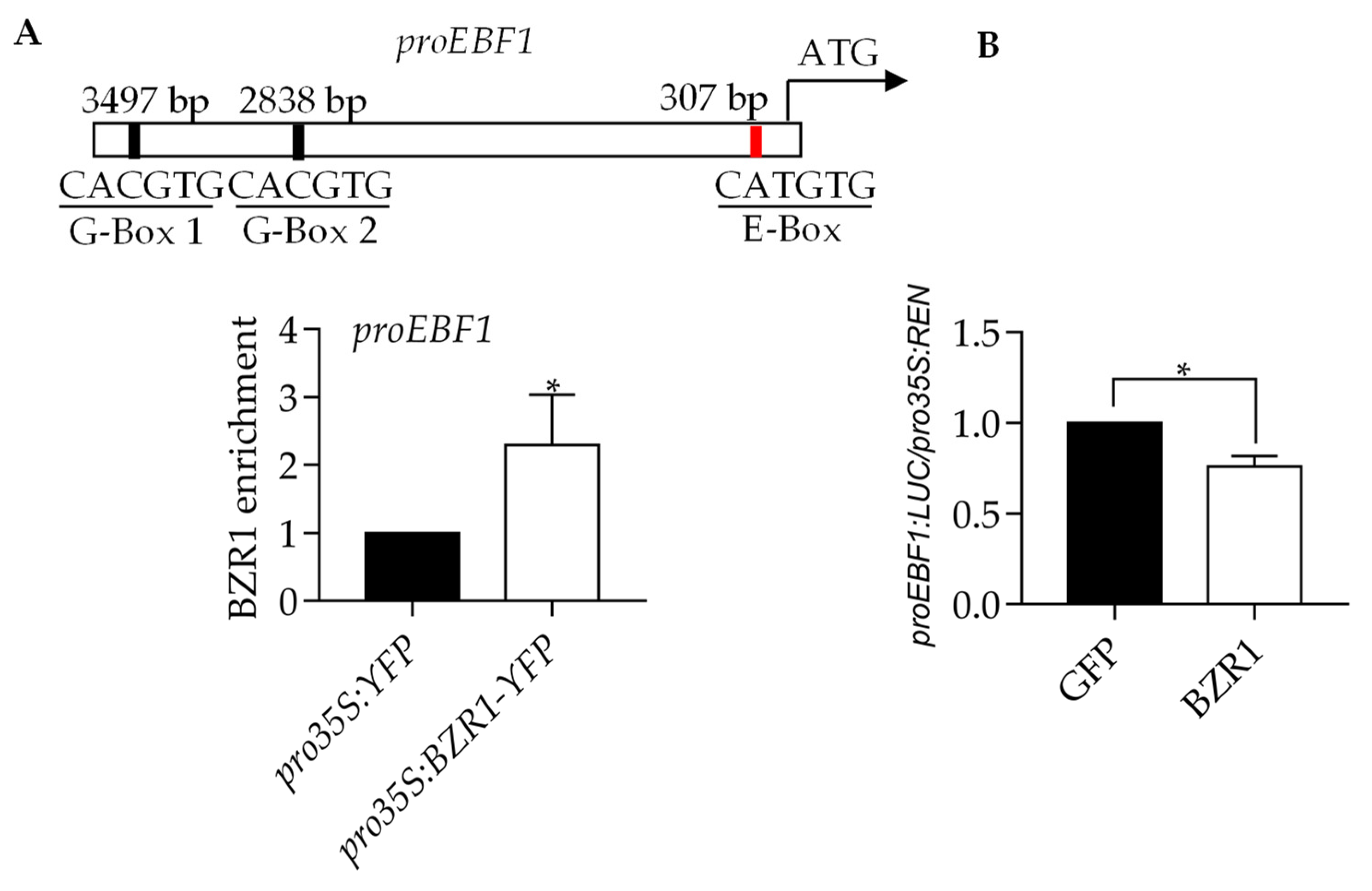
2.3. EBF1 Inhibits the Expression of BZR1 Target Genes
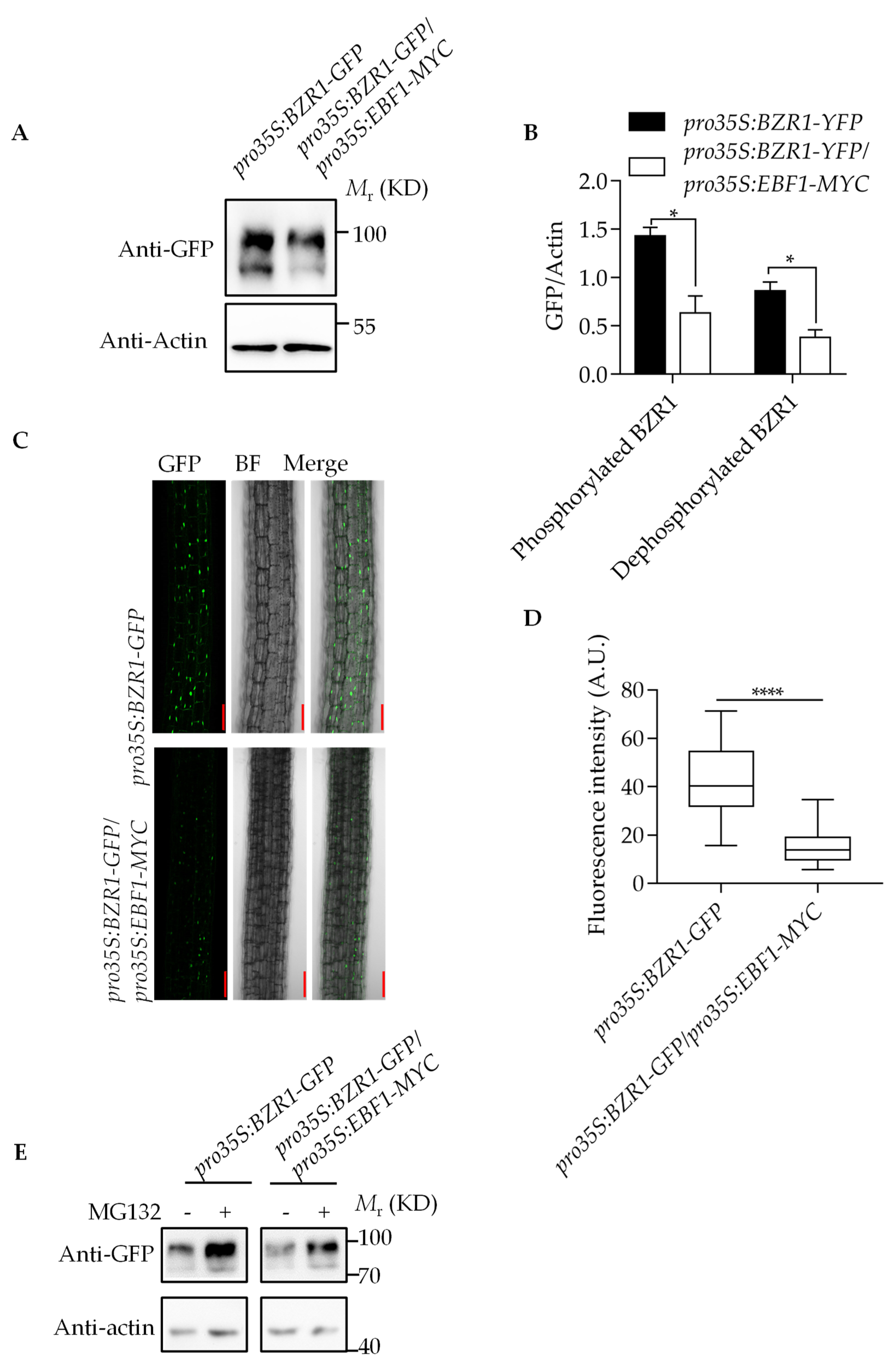
2.4. EBF1 Promotes the Degradation of BZR1
2.5. BZR1 Reduces EBF1 Expression
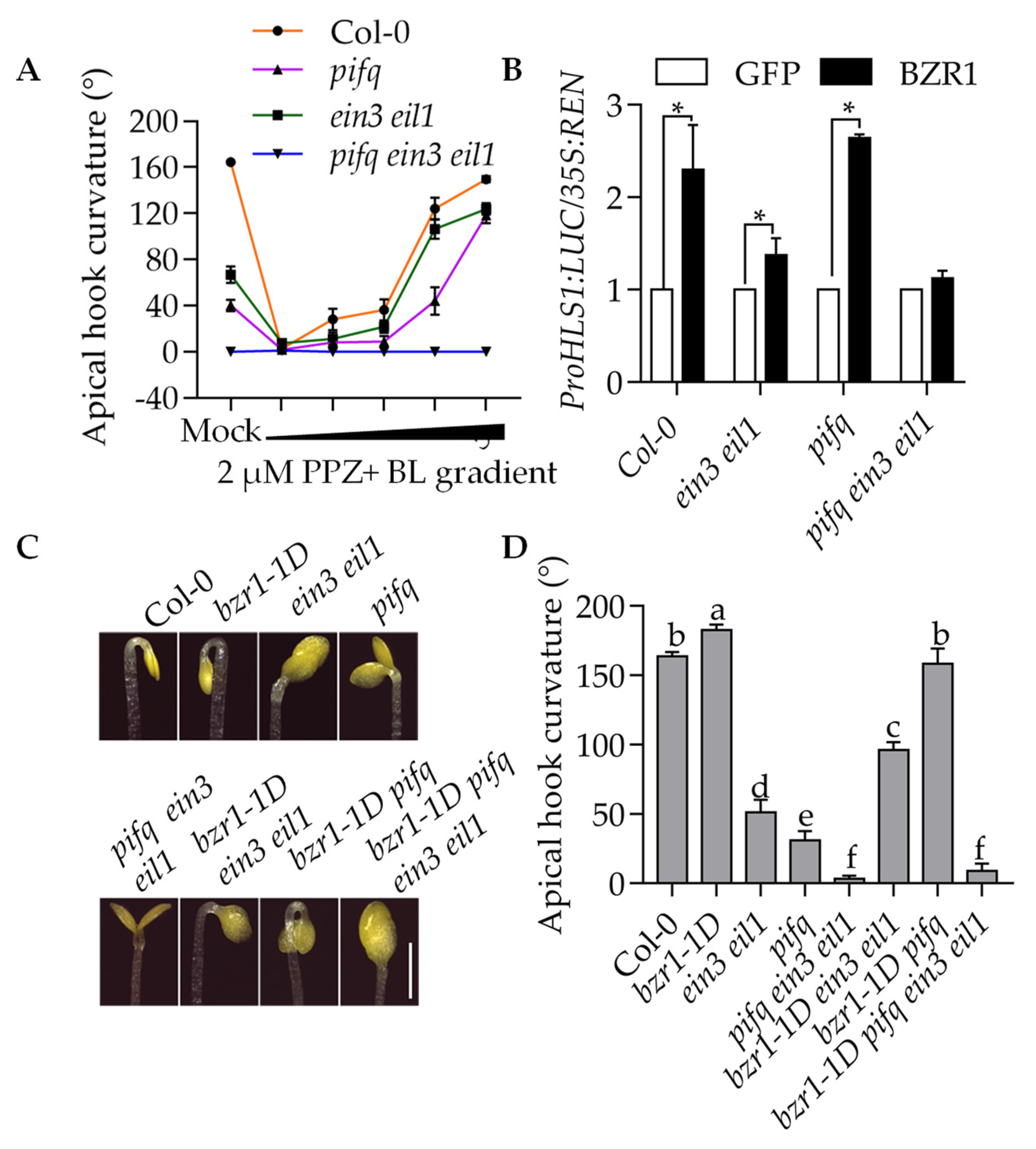
2.6. BZR1 Needs at Least One Class of Transcription Factor EIN3 or PIFs to Promote Apical Hook Development
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Solution Preparation
4.3. Hook Curvature Measurement
4.4. Hypocotyl Length Measurement
4.5. RNA Extraction, Reverse Transcription and Real-Time PCR
4.6. Yeast Two-Hybrid Assay
4.7. Pull-Down Assays
4.8. BiFC Assays
4.9. Co-Immunoprecipitation Assays
4.10. ChIP-PCR
4.11. Transient Gene Expression Assays
4.12. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BR | Brassinosteroid |
| PPZ | propiconazole |
| BZR1 | BRASSINAZOLE-RESISTANT 1 |
| EBF1 | EIN3 BINDING F-BOX PROTEIN1 |
| EIN3 | ETHYLENE-INSENSITIVE 3 |
| PIF4 | PHYTOCHROME INTERACTING FACTOR 4 |
| LUC | Luciferase |
| REN | Renilla luciferase |
| GST | Glutathione S-transferase |
| YFP | Yellow fluorescence protein |
| MBP | Maltose-binding protein |
| 3-AT | 3-amino-1,2,4-triazole |
| BIFC | Bimolecular fluorescence complementation |
| BD | Binding domain |
| AD | Acting domain |
| RT-PCR | Quantitative real-time PCR |
| COIP | Co-immunoprecipitation |
References
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Bai, M.Y.; Oh, E.; Zhu, J.Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Ann. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, R.; Liu, M.; Yuan, W.; Zhao, Z.; Liu, X.; Peng, Y.; Yang, X.; Sun, Y.; Tang, W. Nucleocytoplasmic trafficking and turnover mechanisms of BRASSINAZOLE RESISTANT1 in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2101838118. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef]
- Oh, E.; Zhu, J.Y.; Bai, M.Y.; Arenhart, R.A.; Sun, Y.; Wang, Z.Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 2014, 3, e03031. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, M.; Tian, Y.; Wang, Y.; Han, C.; Fan, M.; Guo, H.; Bai, M.Y. Interaction between BZR1 and EIN3 mediates signalling crosstalk between brassinosteroids and ethylene. New Phytol. 2021, 232, 2308–2323. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, S.H.; Park, C.H.; Kim, S.H.; Hsu, C.C.; Xu, S.; Wang, Z.Y.; Kim, S.K.; Kim, T.W. Plant U-Box40 mediates degradation of the brassinosteroid-responsive transcription factor BZR1 in Arabidopsis roots. Plant Cell 2019, 31, 791–808. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, Y.J.; Corvalan, C.; Fujioka, S.; Cho, S.; Park, T.; Choe, S. Darkness and gulliver2/phyB mutation decrease the abundance of phosphorylated BZR1 to activate brassinosteroid signaling in Arabidopsis. Plant J. 2014, 77, 737–747. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.; Cai, Z.; Hu, Y.; Nolan, T.; Yu, F.; Yin, Y.; Xie, Q.; Tang, G.; Wang, X. SINAT E3 ligases control the light-mediated stability of the brassinosteroid-activated transcription factor BES1 in Arabidopsis. Dev. Cell 2017, 41, 47–58.e44. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, J.Y.; Roh, J.; Marchive, C.; Kim, S.K.; Meyer, C.; Sun, Y.; Wang, W.; Wang, Z.Y. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr. Biol. 2016, 26, 1854–1860. [Google Scholar] [CrossRef]
- Xiao, W.; Jang, J. F-box proteins in Arabidopsis. Trends Plant. Sci. 2000, 5, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Potuschak, T.; Lechner, E.; Parmentier, Y.; Yanagisawa, S.; Grava, S.; Koncz, C.; Genschik, P. EIN3-dependent regulation of plant ethylene hormone signaling by two arabidopsis F box proteins: EBF1 and EBF2. Cell 2003, 115, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, Y.G.; Chang, H.C.; Pellequer, J.L.; La Valle, R.; Lanker, S.; Wittenberg, C. F-box protein Grr1 interacts with phosphorylated targets via the cationic surface of its leucine-rich repeat. Mol. Cell Biol. 2001, 21, 2506–2520. [Google Scholar] [CrossRef][Green Version]
- Guo, H.; Ecker, J.R. Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 2003, 115, 667–677. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E.; Oka, Y.; Liu, T.; Carle, C.; Castillon, A.; Huq, E.; Quail, P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008, 18, 1815–1823. [Google Scholar] [CrossRef]
- Dong, J.; Ni, W.; Yu, R.; Deng, X.W.; Chen, H.; Wei, N. Light-dependent degradation of PIF3 by SCF(EBF1/2) promotes a photomorphogenic response in Arabidopsis. Curr. Biol. 2017, 27, 2420–2430.e2426. [Google Scholar] [CrossRef]
- Bai, M.Y.; Shang, J.X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.P.; Wang, Z.Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Shi, Y.; Zhang, X.; Xin, X.; Qi, L.; Guo, H.; Li, J.; Yang, S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E6695–E6702. [Google Scholar] [CrossRef] [PubMed]
- Lehman, A.; Black, R.; Ecker, J.R. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 1996, 85, 183–194. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, K.M.; Thomas, S.G.; Soule, J.D.; Strader, L.C.; Zale, J.M.; Sun, T.P.; Steber, C.M. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 2003, 15, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Dill, A.; Thomas, S.G.; Hu, J.; Steber, C.M.; Sun, T.P. The Arabidopsis F-box protein SLEEPY1 targets gibberellin signaling repressors for gibberellin-induced degradation. Plant Cell 2004, 16, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, N.; Zheng, L.; Zhang, F.; Xiang, M.; Chen, H.; Deng, X.W.; Wei, N. Brassinosteroids promote etiolated apical structures in darkness by amplifying the ethylene response via the EBF-EIN3/PIF3 circuit. Plant Cell 2022, online. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, R.; Xue, C.; Shen, X.; Wei, N.; Deng, X.W.; Zhong, S. Seedlings transduce the depth and mechanical pressure of covering soil using COP1 and ethylene to regulate EBF1/EBF2 for soil emergence. Curr. Biol. 2016, 26, 139–149. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; An, F.; Hao, D.; Li, P.; Song, J.; Yi, C.; Guo, H. Jasmonate-activated MYC2 represses ETHYLENE INSENSITIVE3 activity to antagonize ethylene-promoted apical hook formation in Arabidopsis. Plant Cell 2014, 26, 1105–1117. [Google Scholar] [CrossRef]
- Zhu, Q.; Zadnikova, P.; Smet, D.; Van Der Straeten, D.; Benkova, E. Real-time analysis of the apical hook development. Methods Mol. Biol. 2017, 1497, 1–8. [Google Scholar]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich—A simpler Arabidopsis protoplast isolation method. Plant. Methods 2009, 5, 16. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Zhao, M.; Wang, L.; Han, C.; Bai, M.; Fan, M. EBF1 Negatively Regulates Brassinosteroid-Induced Apical Hook Development and Cell Elongation through Promoting BZR1 Degradation. Int. J. Mol. Sci. 2022, 23, 15889. https://doi.org/10.3390/ijms232415889
Zhao N, Zhao M, Wang L, Han C, Bai M, Fan M. EBF1 Negatively Regulates Brassinosteroid-Induced Apical Hook Development and Cell Elongation through Promoting BZR1 Degradation. International Journal of Molecular Sciences. 2022; 23(24):15889. https://doi.org/10.3390/ijms232415889
Chicago/Turabian StyleZhao, Na, Min Zhao, Lingyan Wang, Chao Han, Mingyi Bai, and Min Fan. 2022. "EBF1 Negatively Regulates Brassinosteroid-Induced Apical Hook Development and Cell Elongation through Promoting BZR1 Degradation" International Journal of Molecular Sciences 23, no. 24: 15889. https://doi.org/10.3390/ijms232415889
APA StyleZhao, N., Zhao, M., Wang, L., Han, C., Bai, M., & Fan, M. (2022). EBF1 Negatively Regulates Brassinosteroid-Induced Apical Hook Development and Cell Elongation through Promoting BZR1 Degradation. International Journal of Molecular Sciences, 23(24), 15889. https://doi.org/10.3390/ijms232415889





