Development of Cell Therapies for Renal Disease and Regenerative Medicine
Abstract
1. Introduction
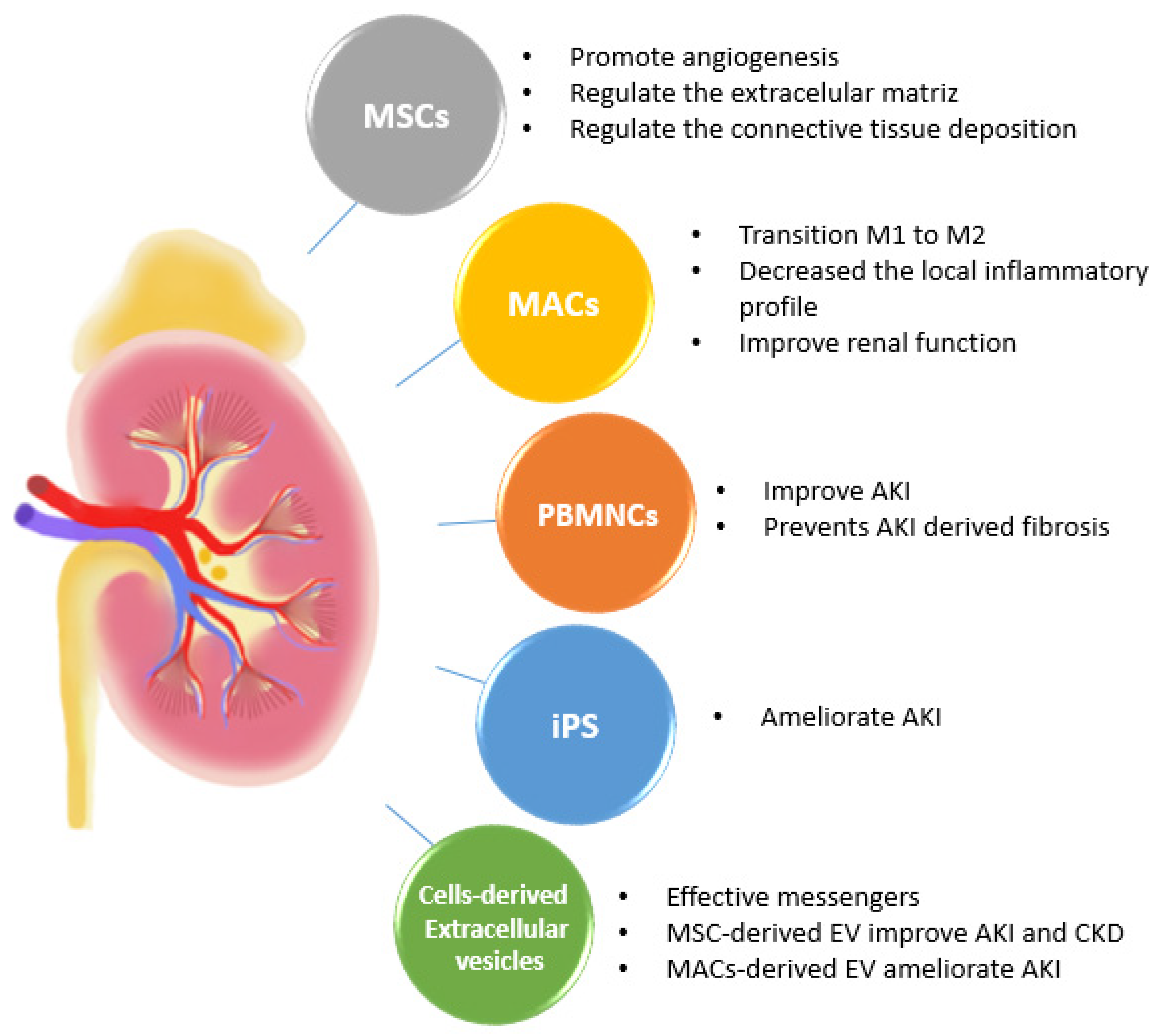
2. Cell Therapies in an Acute Kidney Injury (AKI)
2.1. Multipotent Mesenchymal Stromal Stem Cell Therapies
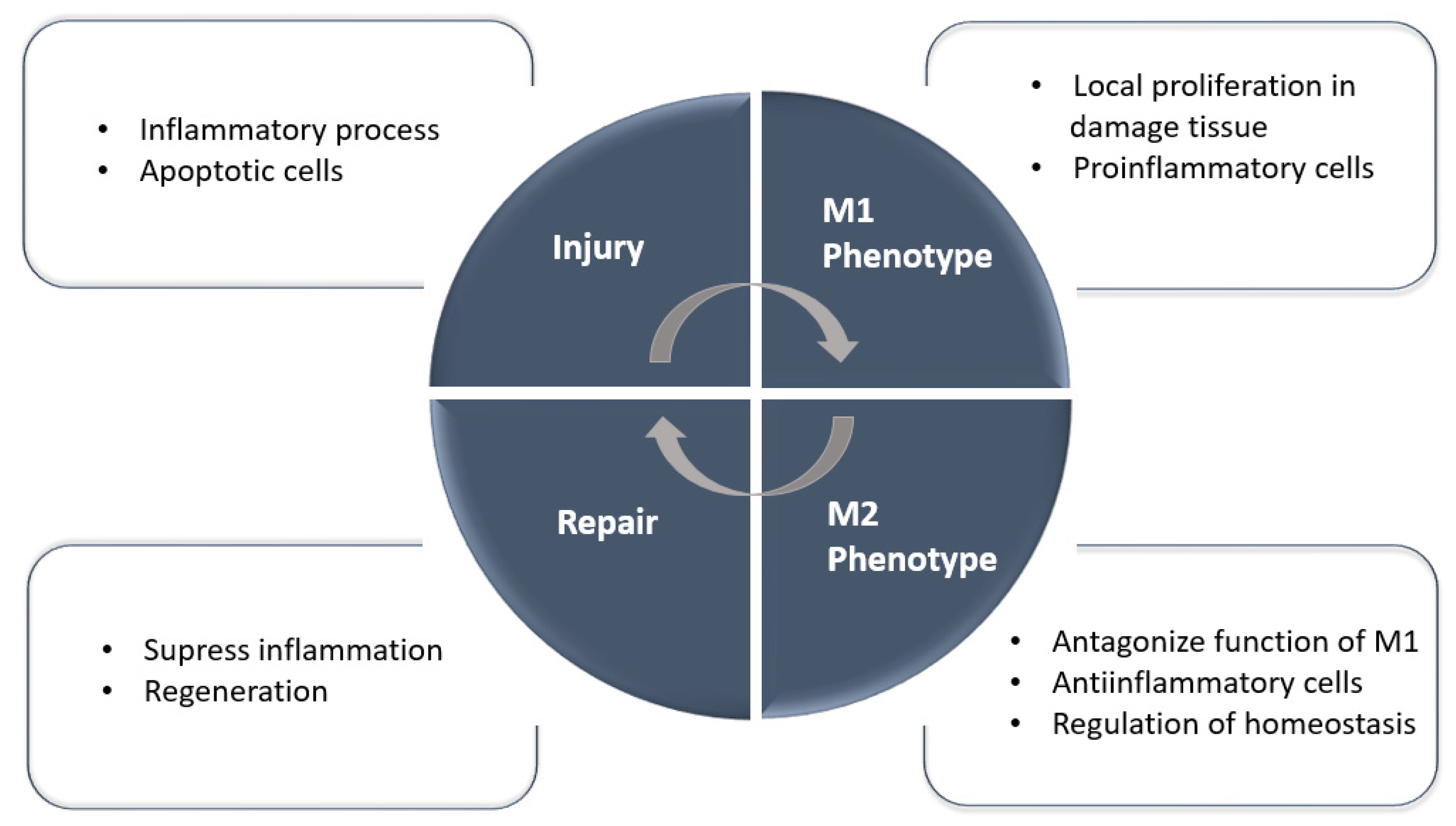
2.2. Mononuclear and Macrophage Cell Therapies
3. Cell Therapies in Chronic Kidney Disease (CKD)
4. Cell-Derived Extracellular Vesicles (EVs) as a Novel Therapeutic Strategy for Kidney Disease
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Remuzzi, G. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Investig. 2006, 116, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.D.S.; Roderick, P.J. Kidney disease in the Global Burden of Disease Study 2017. Nat. Rev. Nephrol. 2019, 15, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- McFetridge, M.L.; del Borgo, M.P.; Aguilar, M.-I.; Ricardo, S.D. The use of hydrogels for cell-based treatment of chronic kidney disease. Clin. Sci. 2018, 132, 1977–1994. [Google Scholar] [CrossRef]
- Gilbertson, D.T.; Liu, J.; Xue, J.L.; Louis, T.A.; Solid, C.A.; Ebben, J.P.; Collins, A.J. Projecting the Number of Patients with End-Stage Renal Disease in the United States to the Year 2015. JASN 2005, 16, 3736–3741. [Google Scholar] [CrossRef]
- Remuzzi, G.; Weening, J.J. Albuminuria as early test for vascular disease. Lancet 2005, 365, 556–557. [Google Scholar] [CrossRef]
- Peired, A.J.; Antonelli, G.; Angelotti, M.L.; Allinovi, M.; Guzzi, F.; Sisti, A.; Semeraro, R.; Conte, C.; Mazzinghi, B.; Nardi, S.; et al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci. Transl. Med. 2020, 12, eaaw6003. [Google Scholar] [CrossRef]
- Lazzeri, E.; Angelotti, M.L.; Conte, C.; Anders, H.-J.; Romagnani, P. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol. Med. 2019, 25, 366–381. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mapping kidney cellular complexity. Science 2018, 360, 709–710. [Google Scholar] [CrossRef]
- Gorostidi, M.; Sánchez-Martínez, M.; Ruilope, L.M.; Graciani, A.; de la Cruz, J.J.; Santamaría, R.; del Pino, M.D.; Guallar-Castillón, P.; de Álvaro, F.; Rodríguez-Artalejo, F.; et al. Prevalencia de enfermedad renal crónica en España: Impacto de la acumulación de factores de riesgo cardiovascular. Nefrología 2018, 38, 606–615. [Google Scholar] [CrossRef]
- Hewitson, T.D. Renal tubulointerstitial fibrosis: Common but never simple. Am. J. Physiol. Ren. Physiol. 2009, 296, F1239–F1244. [Google Scholar] [CrossRef] [PubMed]
- Moffat, D.B.; Fourman, J. The vascular pattern of the rat kidney. J. Am. Soc. Nephrol. 1963, 97, 543. [Google Scholar]
- McDonald-Hyman, C.; Turka, L.A.; Blazar, B.R. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci. Transl. Med. 2015, 7, 280rv2. [Google Scholar] [CrossRef] [PubMed]
- Zucchini, A. Willem Kolff: Médico e inventor. Medicina 2009, 69, 288–290. [Google Scholar] [PubMed]
- Cabral, B.P.; Bonventre, J.V.; Wieringa, F.; Mota, F.B. Probing expert opinions on the future of kidney replacement therapies. Artif. Organs 2021, 45, 79–87. [Google Scholar] [CrossRef]
- Trapnell, C. Defining cell types and states with single-cell genomics. Genome Res. 2015, 25, 1491–1498. [Google Scholar] [CrossRef]
- Han, X.; Wang, R.; Zhou, Y.; Fei, L.; Sun, H.; Lai, S.; Saadatpour, A.; Zhou, Z.; Chen, H.; Ye, F.; et al. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 2018, 172, 1091–1107.e17. [Google Scholar] [CrossRef]
- Tögel, F.E.; Westenfelder, C. Kidney Protection and Regeneration Following Acute Injury: Progress Through Stem Cell Therapy. Am. J. Kidney Dis. 2012, 60, 1012–1022. [Google Scholar] [CrossRef]
- Li, B.; Cohen, A.; Hudson, T.E.; Motlagh, D.; Amrani, D.L.; Duffield, J.S. Mobilized Human Hematopoietic Stem/Progenitor Cells Promote Kidney Repair After Ischemia/Reperfusion Injury. Circulation 2010, 121, 2211–2220. [Google Scholar] [CrossRef]
- de Almeida, D.C.; Donizetti-Oliveira, C.; Barbosa-Costa, P.; Origassa, C.S.; Câmara, N.O. In Search of Mechanisms Associated with Mesenchymal Stem Cell-Based Therapies for Acute Kidney Injury. Clin. Biochem. Rev. 2013, 34, 131. [Google Scholar]
- Toyohara, T.; Mae, S.-I.; Sueta, S.-I.; Inoue, T.; Yamagishi, Y.; Kawamoto, T.; Kasahara, T.; Hoshina, A.; Toyoda, T.; Tanaka, H.; et al. Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem Cells Transl. Med. 2015, 4, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, D.; Chen, X.; Han, C.; Xu, L.; Huang, T.; Dong, Z.; Zhang, M. Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCS) protect against renal ischemia/reperfusion injury via delivering specifity propteis (SP1) and trasxcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 2017, 8, 3200. [Google Scholar] [PubMed]
- Wei, X.; Zhang, J.; Gu, Q.; Huang, M.; Zhang, W.; Guo, J.; Zhou, X. Reciprocal Expression of IL-35 and IL-10 Defines Two Distinct Effector Treg Subsets that Are Required for Maintenance of Immune Tolerance. Cell Rep. 2017, 21, 1853–1869. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Fan, G. Stem cell-based treatment of kidney diseases. Exp. Biol. Med. 2020, 245, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.; Lee, S. Potential and Therapeutic Efficacy of Cell-based Therapy Using Mesenchymal Stem Cells for Acute/chronic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 1619. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J. Cell Physiol. 2018, 233, 1913–1928. [Google Scholar] [CrossRef]
- Safwani, W.K.Z.W.; Choi, J.R.; Yong, K.W.; Ting, I.; Adenan, N.A.M.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99. [Google Scholar] [CrossRef]
- Prockop, D.J. Concise Review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- Eggenhofer, E.; Luk, F.; Dahlke, M.H.; Hoogduijn, M.J. The Life and Fate of Mesenchymal Stem Cells. Front. Immunol. 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Kojima, Y.; Ikarashi, S.; Seino, S.; Watanabe, Y.; Kawata, Y.; Terai, S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm. Regen. 2017, 37, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, W.; Liao, L.; Xu, T.; Wu, W.; Yang, S.; Tan, J. Mesenchymal Stem Cells Ameliorate Ischemia-Reperfusion-Induced Renal Dysfunction by Improving the Antioxidant/Oxidant Balance in the Ischemic Kidney. Urol. Int. 2011, 86, 191–196. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press. Res. 2016, 41, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Ryu, M.-O.; Seo, M.-S.; Park, S.-B.; Ahn, J.-O.; Han, S.-M.; Kang, K.-S.; Bhang, D.-H.; Youn, H.-Y. Mesenchymal Stem Cells Contribute to Improvement of Renal Function in a Canine Kidney Injury Model. In Vivo 2017, 31, 1115–1124. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, C.E.; Capcha, J.M.C.; de Bragança, A.C.; Sanches, T.R.; Gouveia, P.Q.; de Oliveira, P.A.F.; Malheiros, D.M.A.C.; Volpini, R.A.; Santinho, M.A.R.; Santana, B.A.A.; et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res. Ther. 2017, 8, 19. [Google Scholar] [CrossRef]
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal Cells Protect against Acute Tubular Injury via an Endocrine Effect. JASN 2007, 18, 2486–2496. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Remuzzi, G. Mesenchymal Stromal Cells for AKI after Cardiac Surgery. JASN 2018, 29, 7–9. [Google Scholar] [CrossRef]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. JASN 2018, 29, 260–267. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, B. The multi-differentiation potential of peripheral blood mononuclear cells. Stem Cell Res. Ther. 2012, 3, 48. [Google Scholar] [CrossRef]
- Mevorach, D.; Zuckerman, T.; Reiner, I.; Shimoni, A.; Samuel, S.; Nagler, A.; Rowe, J.M.; Or, R. Single Infusion of Donor Mononuclear Early Apoptotic Cells as Prophylaxis for Graft-versus-Host Disease in Myeloablative HLA-Matched Allogeneic Bone Marrow Transplantation: A Phase I/IIa Clinical Trial. Biol. Blood Marrow Transplant. 2014, 20, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Yoon, J.-H.; Kim, W.; Lee, J.M.; Bin Lee, Y.; Cho, Y.; Lee, D.H.; Lee, M.; Yoo, J.-J.; Cho, E.J.; et al. Ultrasound-guided percutaneous portal transplantation of peripheral blood monocytes in patients with liver cirrhosis. Korean J. Int. Med. 2017, 32, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.S.A.; Ismail, N.A.; Jamaludin, W.F.W.; Muhamad, N.A.; Idris, M.A.M.; Lai, N.M. Efficacy and Safety of Autologous Cell-based Therapy in Patients with No-option Critical Limb Ischaemia: A Meta-Analysis. Curr. Stem Cell Res. Ther. 2018, 13, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Sermsathanasawadi, N.; Pruekprasert, K.; Chruewkamlow, N.; Kittisares, K.; Warinpong, T.; Chinsakchai, K.; Wongwanit, C.; Ruangsetakit, C.; Mutirangura, P. Peripheral blood mononuclear cell transplantation to treat no-option critical limb ischaemia: Effectiveness and safety. J. Wound Care 2021, 30, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Kobayashi, S.; Slavin, S.; Mochida, Y.; Ishioka, K.; Moriya, H.; Hidaka, S.; Matsuura, R.; Sumida, M.; Katagiri, D.; et al. Human Peripheral Blood Mononuclear Cells Incubated in Vasculogenic Conditioning Medium Dramatically Improve Ischemia/Reperfusion Acute Kidney Injury in Mice. Cell Transpl. 2018, 27, 520–530. [Google Scholar] [CrossRef]
- Játiva, S.; Torrico, S.; Calle, P.; Muñoz, Á.; García, M.; Larque, A.B.; Poch, E.; Hotter, G. NGAL release from peripheral blood mononuclear cells protects against acute kidney injury and prevents AKI induced fibrosis. Biomed. Pharmacother. 2022, 153, 113415. [Google Scholar] [CrossRef]
- Okabe, Y.; Medzhitov, R. Tissue biology perspective on macrophages. Nat. Immunol. 2016, 17, 9–17. [Google Scholar] [CrossRef]
- Gosselin, D.; Link, V.M.; Romanoski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment Drives Selection and Function of Enhancers Controlling Tissue-Specific Macrophage Identities. Cell 2014, 159, 1327–1340. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs.Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Tiruppathi, C.; Nepal, S.; Zhao, Y.-Y.; Grzych, D.; Soni, D.; Prockop, D.J.; Malik, A.B. TNFα-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc. Natl. Acad. Sci. USA 2016, 113, E8151–E8158. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cao, Q.; Wang, Y.; Harris, D.C.H. M2 macrophages in kidney disease: Biology, therapies, and perspectives. Kidney Int. 2019, 95, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Wang, C.; Zhang, F.; Zhao, M.; Liu, S.; Liao, G.; Li, L.; Chen, Y.; Cheng, J.; Liu, J.; et al. Peritoneal M2 macrophage transplantation as a potential cell therapy for enhancing renal repair in acute kidney injury. J. Cell Mol. Med. 2020, 24, 3314–3327. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Ruedl, C.; Karjalainen, K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity 2015, 43, 382–393. [Google Scholar] [CrossRef]
- Jang, H.-S.; Kim, J.I.; Jung, K.-J.; Kim, J.; Han, K.-H.; Park, K.M. Bone marrow-derived cells play a major role in kidney fibrosis via proliferation and differentiation in the infiltrated site. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 817–825. [Google Scholar] [CrossRef]
- Malone, A.F. Monocytes and Macrophages in Kidney Transplantation and Insights from Single Cell RNA-Seq Studies. Kidney360 2021, 2, 1654–1659. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Y.; Li, Z.; Ji, J.; You, A.; Jin, S.; Ma, Y.; Zhao, Y.; Wang, J.; Qu, L.; et al. Single Cell RNA Sequencing Identifies a Unique Inflammatory Macrophage Subset as a Druggable Target for Alleviating Acute Kidney Injury. Adv. Sci. 2022, 9, 2103675. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, Y.; Zheng, D.; Sun, Y.; Wang, Y.; Lee, V.; Zheng, G.; Tan, T.K.; Ince, J.; Alexander, S.I.; et al. IL-10/TGF-β–Modified Macrophages Induce Regulatory T Cells and Protect against Adriamycin Nephrosis. JASN 2010, 21, 933–942. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Ramdas, V.; Spencer, N.; Marson, L.; Anegon, I.; Hughes, J.; Kluth, D.C. Macrophages Expressing Heme Oxygenase-1 Improve Renal Function in Ischemia/Reperfusion Injury. Mol. Ther. 2010, 18, 1706–1713. [Google Scholar] [CrossRef]
- Jung, M.; Sola, A.; Hughes, J.; Kluth, D.C.; Vinuesa, E.; Viñas, J.L.; Pérez-Ladaga, A.; Hotter, G. Infusion of IL-10–expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 2012, 81, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.V.; Jayakumar, C.; Ramesh, G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am. J. Physiol. Ren. Physiol. 2013, 304, F948–F957. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhang, L.; Fu, B.; Zhang, J.; Hong, Q.; Hu, J.; Li, D.; Luo, C.; Cui, S.; Zhu, F.; et al. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res. Ther. 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Tsuboi, N.; Shi, Y.; Ito, S.; Sugiyama, Y.; Furuhashi, K.; Endo, N.; Kim, H.; Katsuno, T.; Akiyama, S.; et al. Transfusion of CD206+ M2 Macrophages Ameliorates Antibody-Mediated Glomerulonephritis in Mice. Am. J. Pathol. 2016, 186, 3176–3188. [Google Scholar] [CrossRef]
- Taguchi, K.; Okada, A.; Hamamoto, S.; Unno, R.; Moritoki, Y.; Ando, R.; Mizuno, K.; Tozawa, K.; Kohri, K.; Yasui, T. M1/M2-macrophage phenotypes regulate renal calcium oxalate crystal development. Sci. Rep. 2016, 6, 35167. [Google Scholar] [CrossRef]
- Jung, M.; Brüne, B.; Hotter, G.; Sola, A. Macrophage-derived Lipocalin-2 contributes to ischemic resistance mechanisms by protecting from renal injury. Sci. Rep. 2016, 6, 21950. [Google Scholar] [CrossRef]
- Singbartl, K.; Formeck, C.L.; Kellum, J.A. Kidney-Immune System Crosstalk in AKI. Semin. Nephrol. 2019, 39, 96–106. [Google Scholar] [CrossRef]
- Lech, M.; Gröbmayr, R.; Ryu, M.; Lorenz, G.; Hartter, I.; Mulay, S.R.; Susanti, E.; Kobayashi, K.S.; Flavell, R.A.; Anders, H.-J. Macrophage Phenotype Controls Long-Term AKI Outcomes—Kidney Regeneration versus Atrophy. JASN 2014, 25, 292–304. [Google Scholar] [CrossRef]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef]
- Gong, L.; Pan, Q.; Yang, N. Autophagy and Inflammation Regulation in Acute Kidney Injury. Front. Physiol. 2020, 11, 576463. [Google Scholar] [CrossRef]
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.A.; HogenEsch, J.B.; Cao, R.; Liu, A.C. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018, 14, e1007369. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A. Immunopathogenesis of Acute Kidney Injury. Toxicol. Pathol. 2018, 46, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yan, Y.; Liang, Z.; Tandra, N.; Zhang, B.; Wang, J.; Xu, W.; Qian, H. Autophagy: A new treatment strategy for MSC-based therapy in acute kidney injury (Review). Mol. Med. Rep. 2017, 17, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Anders, H.-J.; Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011, 80, 915–925. [Google Scholar] [CrossRef]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2017, 5, 2013. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 2016, 20, 345–359.e5. [Google Scholar] [CrossRef]
- Yoon, Y.M.; Han, Y.-S.; Yun, C.W.; Lee, J.H.; Kim, R.; Lee, S.H. Pioglitazone Protects Mesenchymal Stem Cells against P-Cresol-Induced Mitochondrial Dysfunction via Up-Regulation of PINK-1. Int. J. Mol. Sci. 2018, 19, 2898. [Google Scholar] [CrossRef]
- Lira, R.; Oliveira, M.; Martins, M.; Silva, C.; Carvalho, S.; Stumbo, A.C.; Cortez, E.; Verdoorn, K.; Einicker-Lamas, M.; Thole, A.; et al. Transplantation of bone marrow-derived MSCs improves renal function and Na++K+-ATPase activity in rats with renovascular hypertension. Cell Tissue Res. 2017, 369, 287–301. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Lin, S.-L.; Kobayashi, A.; Hudson, T.E.; Nowlin, B.T.; Bonventre, J.V.; Valerius, M.T.; McMahon, A.P.; Duffield, J.S. Fate Tracing Reveals the Pericyte and Not Epithelial Origin of Myofibroblasts in Kidney Fibrosis. Am. J. Pathol. 2010, 176, 85–97. [Google Scholar] [CrossRef]
- Lemos, D.R.; Duffield, J.S. Tissue-resident mesenchymal stromal cells: Implications for tissue-specific antifibrotic therapies. Sci. Transl. Med. 2018, 10, eaan5174. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.; Pires, M.J.; Oliveira, P.A. Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. In Vivo 2017, 31, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.; Barron, L. Macrophages: Master Regulators of Inflammation and Fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Castaño, A.P.; Lin, S.L.; Surowy, T.; Nowlin, B.T.; Turlapati, S.A.; Patel, T.; Singh, A.; Li, S.; Lupher, M.L., Jr.; Duffield, J.S. Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci. Transl. Med. 2009, 1, 5ra13. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Lin, S.L.; Castaño, A.P.; Nowlin, B.T.; Lupher, M.L.; Duffield, J.S. Bone Marrow Ly6C high Monocytes Are Selectively Recruited to Injured Kidney and Differentiate into Functionally Distinct Populations. J. Immunol. 2009, 183, 6733–6743. [Google Scholar] [CrossRef]
- Nishida, M.; Okumura, Y.; Fujimoto, S.; Shiraishi, I.; Itoi, T.; Hamaoka, K. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem. Biophys. Res. Commun. 2005, 332, 11–16. [Google Scholar] [CrossRef]
- Moreira, A.P.; Cavassani, K.A.; Hullinger, R.; Rosada, R.S.; Fong, D.J.; Murray, L.; Hesson, D.P.; Hogaboam, C.M. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore–induced allergic airway disease. J. Allergy Clin. Immunol. 2010, 126, 712–721.e7. [Google Scholar] [CrossRef]
- van Strien, M.E.; Mercier, D.; Drukarch, B.; Brevé, J.J.P.; Poole, S.; Binnekade, R.; Bol, J.G.J.M.; Blits, B.; Verhaagen, J.; van Dam, A.-M. Anti-inflammatory effect by lentiviral-mediated overexpression of IL-10 or IL-1 receptor antagonist in rat glial cells and macrophages. Gene Ther. 2010, 17, 662–671. [Google Scholar] [CrossRef]
- Calle, P.; Muñoz, A.; Sola, A.; Hotter, G. CPT1a gene expression reverses the inflammatory and anti-phagocytic effect of 7-ketocholesterol in RAW264.7 macrophages. Lipids Health Dis. 2019, 18, 215. [Google Scholar] [CrossRef]
- Calle, P.; Játiva, S.; Torrico, S.; Muñoz, A.; García, M.; Sola, A.; Serra, D.; Mera, P.; Herrero, L.; Hotter, G. Infusion of Phagocytic Macrophages Overexpressing CPT1a Ameliorates Kidney Fibrosis in the UUO Model. Cells 2021, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Calle, P.; Hotter, G. Macrophage Phenotype and Fibrosis in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 2806. [Google Scholar] [CrossRef] [PubMed]
- Karpman, D.; Ståhl, A.; Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, X.; Zhang, H.; Yao, Q.; Liu, Y.; Dong, Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am. J. Physiol. Ren. Physiol. 2016, 311, F844–F851. [Google Scholar] [CrossRef] [PubMed]
- Kumate, J.; Sepúlveda-Amor, J.; Valdespino, J.L.; De Mucha, J.; Díaz-Ortega, J.L.; A García-Sáinz, J.; Ruiz-Puente, J.; Jiménez-Paredes, J.; Ruiz-Arriaga, A.; Gutiérrez, G. Mexican contributions to vaccines. Gac. Med. Mex. 1988, 124, 73–97. [Google Scholar] [PubMed]
- Cantaluppi, V.; Gatti, S.; Medica, D.; Figliolini, F.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012, 82, 412–427. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Miao, S.; Chen, J.; Du, T.; Zhong, L.; Ju, G.; Liu, G.; Zhu, Y. The Anti-Oxidative Role of Micro-Vesicles Derived from Human Wharton-Jelly Mesenchymal Stromal Cells through NOX2/gp91(phox) Suppression in Alleviating Renal Ischemia-Reperfusion Injury in Rats. PLoS ONE 2014, 9, e92129. [Google Scholar] [CrossRef]
- Bruno, S.; Tapparo, M.; Collino, F.; Chiabotto, G.; Deregibus, M.C.; Lindoso, R.S.; Neri, F.; Kholia, S.; Giunti, S.; Wen, S.; et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng. Part A 2017, 1262, 23. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Lu, X.; Zhu, B.; Pei, X.; Wu, J.; Zhao, W. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology 2015, 20, 591–600. [Google Scholar] [CrossRef]
- Eirin, A.; Zhu, X.-Y.; Jonnada, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Mesenchymal Stem Cell-Derived Extracellular Vesicles Improve the Renal Microvasculature in Metabolic Renovascular Disease in Swine. Cell Transpl. 2018, 27, 1080–1095. [Google Scholar] [CrossRef] [PubMed]
- Ucero, A.C.; Benito-Martin, A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Sanz, A.B.; Ramos, A.M.; Berzal, S.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Unilateral ureteral obstruction: Beyond obstruction. Int. Urol. Nephrol. 2014, 46, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Qian, F.; Zheng, D.; He, W.; Gong, J.; He, Q. Mesenchymal Stem Cells Attenuate Renal Fibrosis via Exosomes-Mediated Delivery of microRNA Let-7i-5p Antagomir. IJN 2021, 16, 3565–3578. [Google Scholar] [CrossRef] [PubMed]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.-N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yan, H.-R.; Wang, B.; Liu, B.-C. Macrophage Heterogeneity in Kidney Injury and Fibrosis. Front. Immunol. 2021, 12, 681748. [Google Scholar] [CrossRef]
- Tang, D.; Cao, F.; Yan, C.; Fang, K.; Ma, J.; Gao, L.; Sun, B.; Wang, G. Extracellular Vesicle/Macrophage Axis: Potential Targets for Inflammatory Disease Intervention. Front. Immunol. 2022, 13, 705472. [Google Scholar] [CrossRef]
- Lv, L.-L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.-L.; Zhong, X.; Wu, W.-J.; Chen, J.; Ni, H.-F.; Tang, T.-T.; et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef]
- Tang, T.-T.; Wang, B.; Wu, M.; Li, Z.-L.; Feng, Y.; Cao, J.-Y.; Yin, D.; Liu, H.; Tang, R.-N.; Crowley, S.D.; et al. Extracellular vesicle–encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci. Adv. 2020, 6, eaaz0748. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
| Animal Model | Machophage | Genetic Modific (Y/N) | Treatment | Effects | Year | Ref |
|---|---|---|---|---|---|---|
| BALB/c mice | CD11b+cells isolated from spleen | N | IL-10 1/TGF-β 2 modification | Significantly attenuated renal inflammation, structural injury and functional | 2010 | [59] |
| FVB/nj mice (Harlan) | Bone marrow | Y | Overexpress HO-1 3 | Preserved renal function and reduced microvascular platelet deposition | 2010 | [60] |
| Sprague–Dawley rat | Bone marrow | Y | Overexpress IL-10 | Decreased the local inflammatory profile and improve renal function | 2012 | [61] |
| Netrin-1 transgenic mice/ C57BL/6J mice | Bone marrow | N | Netrin-1 treated Mac | Suppressed inflammation and kidney injury | 2013 | [62] |
| C57BL/6 mice | Raw 264.7 | N | MSCs 4 modification | Supports the transition from tubule injury to tubule repair | 2014 | [63] |
| C57BL/6J mice | Bone marrow | N | IL-4 5/IL-13 6 stimulated | Protected against renal injury and decreased proteinuria | 2016 | [64] |
| C57BL/6J wild- type mice | Bone marrow | N | IL-4/M-CSF 7 stimulated IL-4/IL-13 injection | Suppressed renal crystal formation | 2016 | [65] |
| Brown Norway rat/Sprague-Dawley rat | Bone marrow | Y | Overexpress LCN-2 8 | Lower susceptibility to ischemic injury | 2016 | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrico, S.; Hotter, G.; Játiva, S. Development of Cell Therapies for Renal Disease and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 15943. https://doi.org/10.3390/ijms232415943
Torrico S, Hotter G, Játiva S. Development of Cell Therapies for Renal Disease and Regenerative Medicine. International Journal of Molecular Sciences. 2022; 23(24):15943. https://doi.org/10.3390/ijms232415943
Chicago/Turabian StyleTorrico, Selene, Georgina Hotter, and Soraya Játiva. 2022. "Development of Cell Therapies for Renal Disease and Regenerative Medicine" International Journal of Molecular Sciences 23, no. 24: 15943. https://doi.org/10.3390/ijms232415943
APA StyleTorrico, S., Hotter, G., & Játiva, S. (2022). Development of Cell Therapies for Renal Disease and Regenerative Medicine. International Journal of Molecular Sciences, 23(24), 15943. https://doi.org/10.3390/ijms232415943





