BAC-FISH Based Physical Map of Endangered Catfish Clarias magur for Chromosome Cataloguing and Gene Isolation through Positional Cloning
Abstract
:1. Introduction
2. Results
2.1. BAC Clone Screening
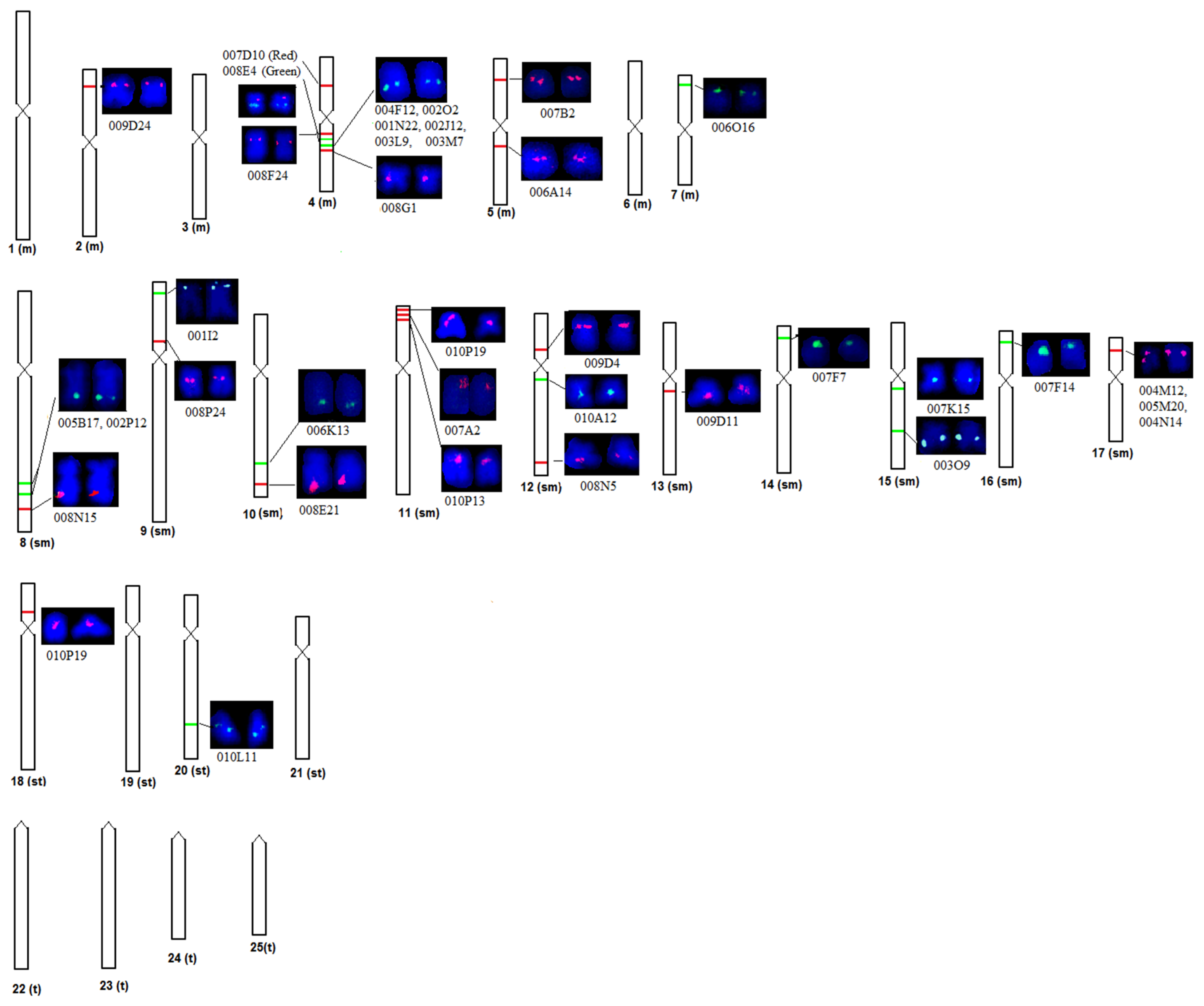
2.2. BAC Mapping on Chromosomes and Scaffolds
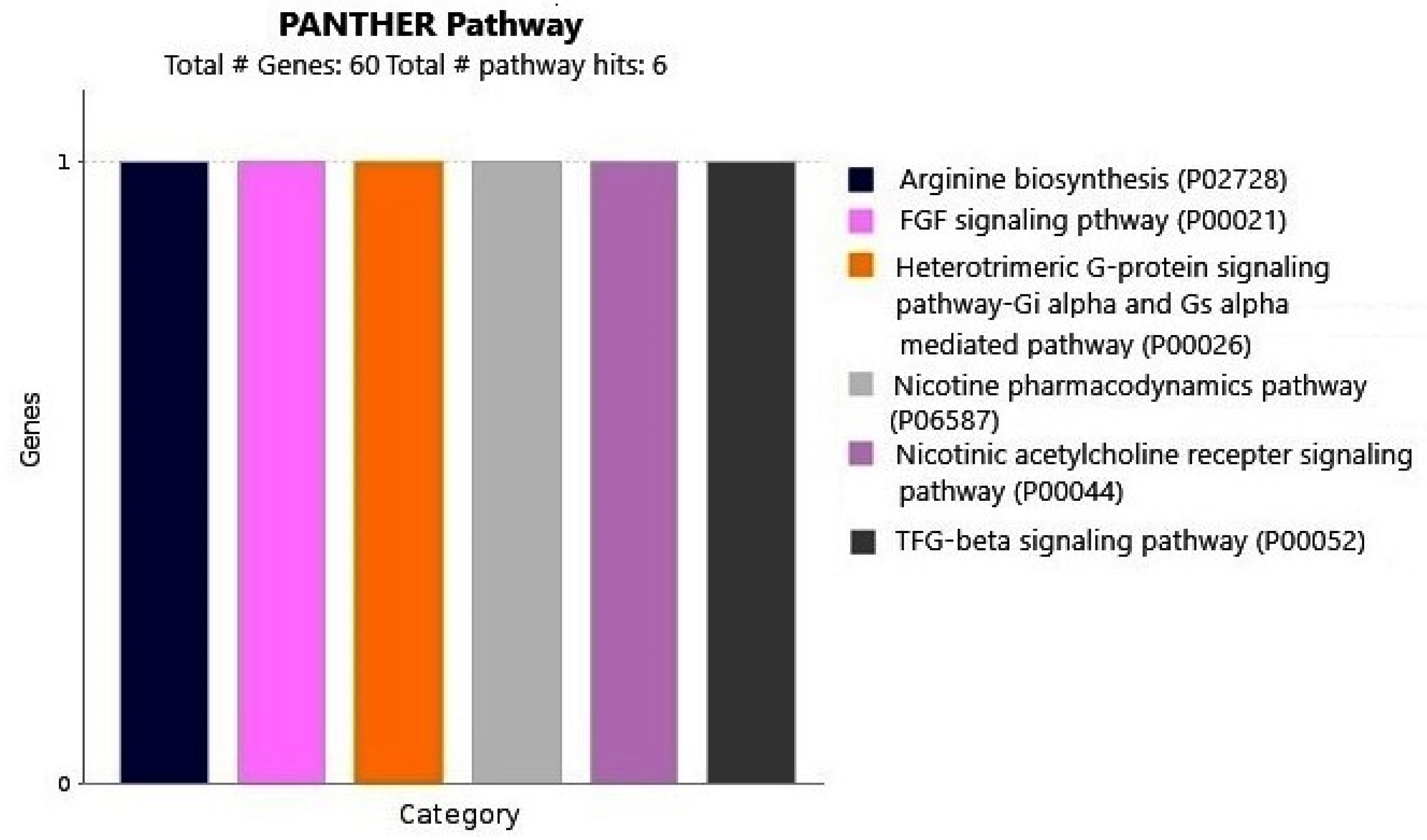
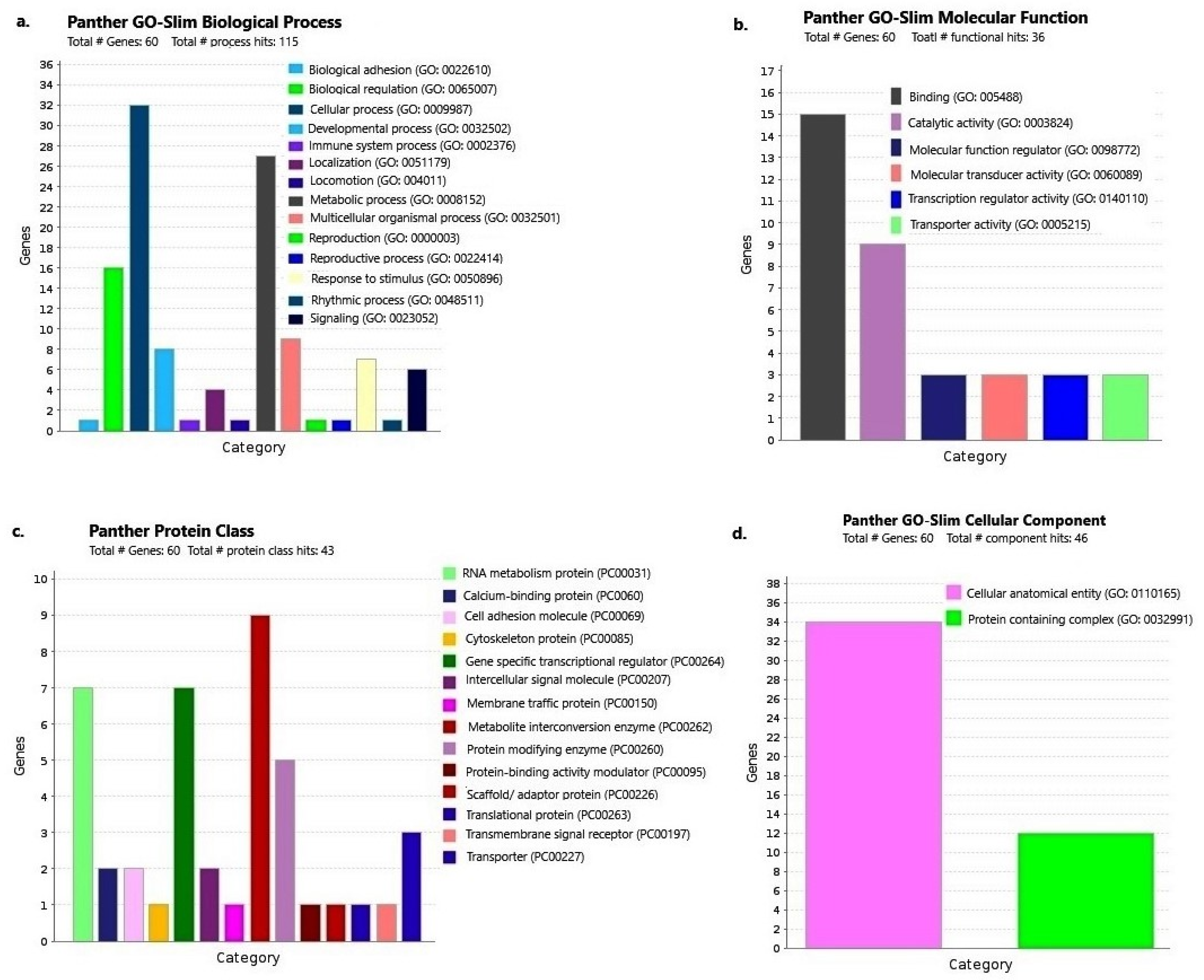
2.3. Gene Enrichment Analysis
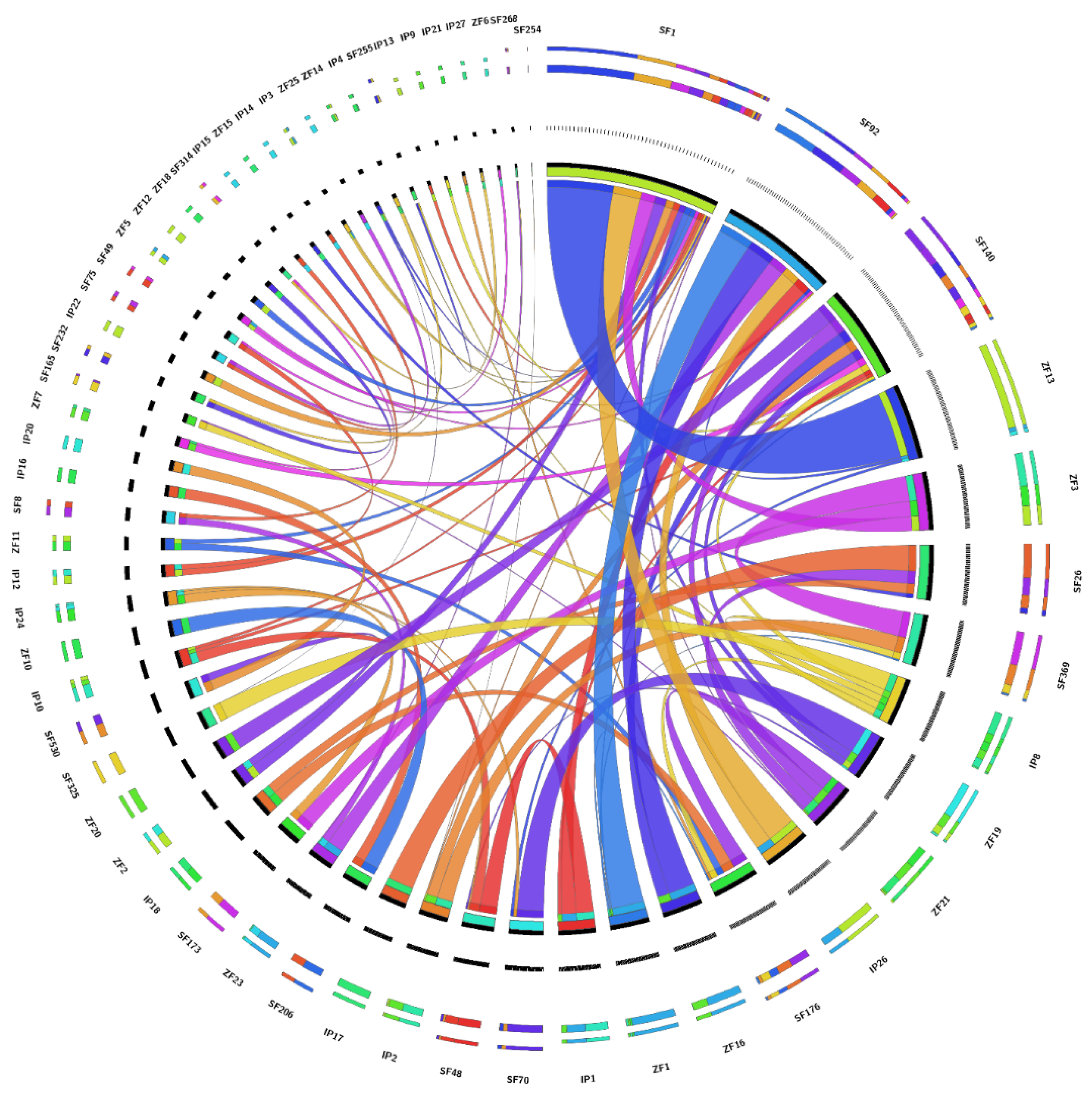
2.4. Comparative Genomics
3. Discussion
4. Material and Methods
4.1. Chromosome Preparation
4.2. BAC Library Construction
4.3. BAC Clone Culture, DNA Isolation
4.4. BAC End Sequencing
4.5. Probe Labelling and BAC-FISH
4.6. Functional Annotation of BAC Mapped Genes
4.7. Comparative Genomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sinha, M.; Mahapatra, B.K.; Saha, D.; Maitra, N.J. Mass scale seed production of Magur: Clarias batrachus at farm level through improvised modifications. Int. J. Fish. Aquat. Stud. 2014, 2, 210–214. [Google Scholar]
- Sahoo, S.K.; Ferosekhan, S.; Giri, S.S.; Swain, S.K. Recent trends in breeding and seed production of Magur in India. World Aquac. Mag. 2016, 47, 59–62. [Google Scholar]
- Baisvar, V.S.; Kushwaha, B.; Kumar, R.; Murali, S.K.; Pandey, M.; Singh, M. Chromosome analysis of walking catfish, Clarias magur (Teleostei, Siluriformes, Clariidae), using staining, FISH with 18S, 5S and BAC probes. J. Appl. Ichthyol. 2021, 37, 563–571. [Google Scholar] [CrossRef]
- Mohindra, V.; Singh, R.K.; Kumar, R.; Sah, R.S.; Lal, K.K. Complete mitochondrial genome sequences of two endangered Indian catfish species, Clarias batrachus and Pangasius pangasius. Mitochondrial DNA 2015, 26, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.; Kumar, R.; Agarwal, S.; Pandey, M.; Nagpure, N.S.; Singh, M.; Srivastava, S.; Joshi, C.G.; Das, P.; Sahoo, L.; et al. Assembly and variation analyses of Clarias batrachus mitogenome retrieved from WGS data and its phylogenetic relationship with other catfishes. Meta Gene 2015, 5, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, B.; Pandey, M.; Das, P.; Joshi, C.G.; Nagpure, N.S.; Kumar, R.; Kumar, D.; Agarwal, S.; Srivastava, S.; Singh, M.; et al. The genome ofwalking catfish Clarias magur (Hamilton, 1822) unveils the genetic basis that may have facilitated the development of environmental and terrestrial adaptation systems in air-breathing catfishes. DNA Res. 2021, 28. [Google Scholar] [CrossRef]
- Srivastava, S.; Kushwaha, B.; Prakash, J.; Kumar, R.; Nagpure, N.S.; Agarwal, S.; Pandey, M.; Das, P.; Joshi, C.; Jena, J.K. Development and characterization of genic SSR markers from low depth genome sequence of Clarias batrachus (Magur). J. Genet. 2016, 95, 603–609. [Google Scholar] [CrossRef]
- Mohindra, V.; Tripathi, R.K.; Singh, A.; Patangia, R.; Singh, R.K.; Lal, K.K.; Jena, J.K. Hypoxic stress-responsive genes in air breathing catfish, Clarias magur (Hamilton 1822) and their possible physiological adaptive function. Fish Shellfish Immunol. 2016, 59, 46–56. [Google Scholar] [CrossRef]
- Kumar, M.S.; Baisvar, V.S.; Kushwaha, B.; Kumar, R.; Singh, M.; Mishra, A.K. Thermal stress induces expression of Nuclear protein and Parkin genes in endangered catfish, Clarias magur. Gene 2022, 825, 146388. [Google Scholar] [CrossRef]
- Banerjee, B.; Koner, D.; Hasan, R.; Bhattacharya, S.; Saha, N. Transcriptome analysis reveals novel insights in air-breathing magur catfish (Clarias magur) in response to high environmental ammonia. Gene 2019, 703, 35–49. [Google Scholar] [CrossRef]
- Kumar, R.; Baisvar, V.S.; Kushwaha, B.; Pandey, M.; Singh, M. Individual chromosome identification in Clarias magur using BAC-FISH and comparative genes analyses. Nucleus 2021, 64, 203–209. [Google Scholar] [CrossRef]
- Dong, G.; Shen, J.; Zhang, Q.; Wang, J.; Yu, Q.; Ming, R.; Wang, K.; Zhang, J. Development and Applications of Chromosome-Specific Cytogenetic BAC-FISH Probes in S. spontaneum. Front. Plant Sci. 2018, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Bustamante, F.; do Nascimento, T.H.; Montenegro, C.; Dias, S.; do Vale Martins, L.; Braz, G.T.; Benko-Iseppon, A.M.; Jiang, J.; Pedrosa-Harand, A.; Brasileiro-Vidal, A.C. Oligo-FISH barcode in beans: A new chromosome identification system. Theor. Appl. Genet. 2021, 134, 3675–3686. [Google Scholar] [CrossRef]
- de Bello, C.M.; Molina, W.F.; Artoni, R.F.; Bertollo, L.A.C. Chromosomes as tools for discovering biodiversity—The case of Erythrinidae fish family. In Recent Trends in Cytogenetic Studies-Methodologies and Applications; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, D.; Gireesh, B.P.; Pavan, K.A.; Koringa, P.; Joshi, C.G.; Chaudhari, A. Transcriptome analysis of Clarias magur brain and gonads suggests neuro-endocrine inhibition of milt release from captive GnRH-induced males. Genomics 2020, 112, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Liu, F.; Peng, R.; Wang, C.; Li, S.; Zhang, X.; Wang, Y.; Wang, K. Individual chromosome identification, chromosomal collinearity and genetic-physical integrated map in Gossypium darwinii and four D genome cotton species revealed by BAC-FISH. Genes Genet Syst. 2012, 87, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, P.; Zhao, Z.; Wang, J.; Zhang, Y.; Sun, X.W. Construction and characterization of the BAC library for common carp Cyprinus carpio L. and establishment of microsynteny with zebrafish Danio rerio. Mar. Biotechnol. 2011, 13, 706–712. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Ji, P.; Zhang, X.; Zhao, Z.; Hou, G.; Huo, L.; Liu, G.; Li, C.; Xu, P.; et al. A Dense Genetic Linkage Map for Common Carp and Its Integration with a BAC-Based Physical Map. PLoS ONE 2013, 8, e63928. [Google Scholar] [CrossRef]
- Palti, Y.; Genet, C.; Luo, M.C.; Charlet, A.; Gao, G.; Hu, Y.; Castaño-Sánchez, C.T.; Tabet-Canale, K.; Krieg, F.; Yao, J.; et al. A first generation integrated map of the rainbow trout genome. BMC Genom. 2011, 12, 180. [Google Scholar] [CrossRef] [Green Version]
- Taboada, X.; Pansonato-Alves, J.C.; Foresti, F.; Martínez, P.; Viñas, A.; Pardo, B.G.; Bouza, C. Consolidation of the genetic and cytogenetic maps of turbot (Scophthalmus maximus) using FISH with BAC clones. Chromosoma 2014, 123, 281–291. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Zhang, X.; Zhou, W.; Yang, L. Construction of a pufferfish bacterial artificial chromosome library. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. Acta Acad. Med. Sin. 1998, 20, 161–166. [Google Scholar]
- Thorsen, J.; Zhu, B.; Frengen, E.; Osoegawa, K.; de Jong, P.J.; Koop, B.F.; Davidson, W.S.; Høyheim, B. A highly redundant BAC library of Atlantic salmon (Salmo salar): An important tool for salmon projects. BMC Genom. 2005, 6, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiniou, S.M.; Katagiri, T.; Miller, N.W.; Wilson, M.; Wolters, W.R.; Waldbieser, G.C. Construction and characterization of a BAC library from a gynogenetic channel catfish Ictalurus punctatus. Genet. Sel. Evol. 2003, 35, 673. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Asakawa, S.; Minagawa, S.; Shimizu, N.; Hirono, I.; Aoki, T. Construction and characterization of BAC libraries for three fish species; rainbow trout, carp and tilapia. Anim. Genet. 2001, 32, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khorasani, M.Z.; Hennig, S.; Imre, G.; Asakawa, S.; Palczewski, S.; Berger, A.; Hori, H.; Naruse, K.; Mitani, H.; Shima, A.; et al. A first generation physical map of the medaka genome in BACs essential for positional cloning and clone-by-clone based genomic sequencing. Mech. Dev. 2004, 121, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Liu, H.; Su, J.; Dong, F.; Xiong, F.; Liao, L.; Wang, Y.; Zhu, Z. Construction and characterization of two bacterial artificial chromosome libraries of Grass carp. Mar. Biotechnol. 2010, 12, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yim, Y.S.; Moak, P.; Sanchez-Villeda, H.; Musket, T.A.; Close, P.; Klein, P.E.; Mullet, J.E.; McMullen, M.D.; Fang, Z.; Schaeffer, M.L.; et al. A BAC pooling strategy combined with PCR-based screenings in a large, highly repetitive genome enables integration of the maize genetic and physical maps. BMC Genom. 2007, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- You, F.M.; Luo, M.C.; Xu, K.; Deal, K.R.; Anderson, O.D.; Dvorak, J. A new implementation of high-throughput five-dimensional clone pooling strategy for BAC library screening. BMC Genom. 2010, 11, 692. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, T.S.; Johnsen, H.E. BAC End Sequencing. In Methods in Molecular Biology. Vol. 255: Bacterial Artificial Chromosomes, Volume 1: Library Construction, Physical Mapping and Sequencing; Zhao, S., Stodolsky, M., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 157–161. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Liu, L.; Peatman, E.; Somridhivej, B.; Thimmapuram, J.; Gong, G.; Liu, Z. Channel catfish BAC-end sequences for marker development and assessment of syntenic conservation with other fish species. Anim. Genet. 2006, 37, 321–326. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Wang, S.; Ninwichian, P.; Somridhivej, B.; Xu, P.; Abernathy, J.; Kucuktas, H.; Liu, Z. Comparative analysis of catfish BAC end sequences with the zebrafish genome. BMC Genom. 2009, 10, 592. [Google Scholar] [CrossRef] [Green Version]
- Genet, C.; Dehais, P.; Palti, Y.; Gao, G.; Gavory, F.; Wincker, P.; Quillet, E.; Boussaha, M. Analysis of BAC-end sequences in rainbow trout: Content characterization and assessment of synteny between trout and other fish genomes. BMC Genom. 2011, 12, 314. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, X.; Liu, C.; Huan, P.; Li, F.; Xiang, J.; Huang, C. BAC end sequencing of Pacific white shrimp Litopenaeus vannamei: A glimpse into the genome of Penaeid shrimp. Chin. J. Oceanol. Limnol. 2012, 30, 456–470. [Google Scholar] [CrossRef]
- Li, N.; Bao, L.; Zhou, T.; Yuan, Z.; Liu, S.; Dunham, R.; Li, Y.; Wang, K.; Xu, X.; Jin, Y.; et al. Genome sequence of walking catfish (Clarias batrachus) provides insights into terrestrial adaptation. BMC Genom. 2018, 19, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohra, A.; Dubey, A.; Saxena, R.K.; Penmetsa, R.V.; Poornima, K.N.; Kumar, N.; Farmer, A.D.; Srivani, G.; Upadhyaya, H.D.; Gothalwal, R.; et al. Analysis of BAC-end sequences (BESs) and development of BES-SSR markers for genetic mapping and hybrid purity assessment in pigeonpea (Cajanus spp.). BMC Plant Biol. 2011, 11, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Kudrna, D.; Wing, R.A. Construction, characterization, and preliminary BAC-end sequence analysis of a Bacterial Artificial Chromosome library of the tea plant (Camellia sinensis). J. Biomed. Biotech. 2011, 2011, 476723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Zhao, Z.; Xu, P.; Li, Y.; Wang, S.; Sun, X. Construction of a BAC-FISH experimental system for localizing specific sequences on mirror carp chromosomes. Acta Hydrobiol. Sin. 2013, 37, 467–472. [Google Scholar]
- Szinay, D.; Chang, S.-B.; Khrustaleva, L.; Peters, S.; Schijlen, E.; Bai, Y.; Stiekema, W.J.; van Ham, R.C.H.J.; de Jong, H.; Lankhorst, R.M.K. High-resolution chromosome mapping of BACs using multi-colour FISH and pooled-BAC FISH as a backbone for sequencing tomato chromosome 6. Plant J. 2008, 56, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Clancy, S.; Shaw, K. DNA deletion and duplication and the associated genetic disorders. Nat. Educ. 2008, 1, 23. [Google Scholar]
- Talwar, P.K.; Jhingran, A.G. Inland Fishes of India and Adjacent Countries; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1991; Volume 2. [Google Scholar]
- Kumar, R.; Baisvar, V.S.; Kushwaha, B.; Waikhom, G.; Nagpure, N.S. Cytogenetic investigation of Cyprinus carpio (Linnaeus, 1758) using giemsa, silver nitrate, CMA3 staining and fluorescence in situ hybridization. Nucleus 2017, 60, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Baisvar, V.S.; Kushwaha, B.; Murali, S.; Singh, V.K. Improved protocols for BAC insert DNA isolation, BAC end sequencing and FISH for construction of BAC based physical map of genes on the chromosomes. Mol. Biol. Rep. 2020, 47, 2405–2413. [Google Scholar] [CrossRef]
- Pandey, M.; Kumar, R.; Srivastava, P.; Agarwal, S.; Srivastava, S.; Nagpure, N.S.; Jena, J.K.; Kushwaha, B. WGSSAT: A high-throughput computational pipeline for mining and annotation of SSR markers from whole genomes. J. Hered. 2018, 109, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baisvar, V.S.; Kushwaha, B.; Kumar, R.; Kumar, M.S.; Singh, M.; Rai, A.; Sarkar, U.K. BAC-FISH Based Physical Map of Endangered Catfish Clarias magur for Chromosome Cataloguing and Gene Isolation through Positional Cloning. Int. J. Mol. Sci. 2022, 23, 15958. https://doi.org/10.3390/ijms232415958
Baisvar VS, Kushwaha B, Kumar R, Kumar MS, Singh M, Rai A, Sarkar UK. BAC-FISH Based Physical Map of Endangered Catfish Clarias magur for Chromosome Cataloguing and Gene Isolation through Positional Cloning. International Journal of Molecular Sciences. 2022; 23(24):15958. https://doi.org/10.3390/ijms232415958
Chicago/Turabian StyleBaisvar, Vishwamitra Singh, Basdeo Kushwaha, Ravindra Kumar, Murali Sanjeev Kumar, Mahender Singh, Anil Rai, and Uttam Kumar Sarkar. 2022. "BAC-FISH Based Physical Map of Endangered Catfish Clarias magur for Chromosome Cataloguing and Gene Isolation through Positional Cloning" International Journal of Molecular Sciences 23, no. 24: 15958. https://doi.org/10.3390/ijms232415958




