Mobilome of the Rhus Gall Aphid Schlechtendalia chinensis Provides Insight into TE Insertion-Related Inactivation of Functional Genes
Abstract
1. Introduction
2. Results
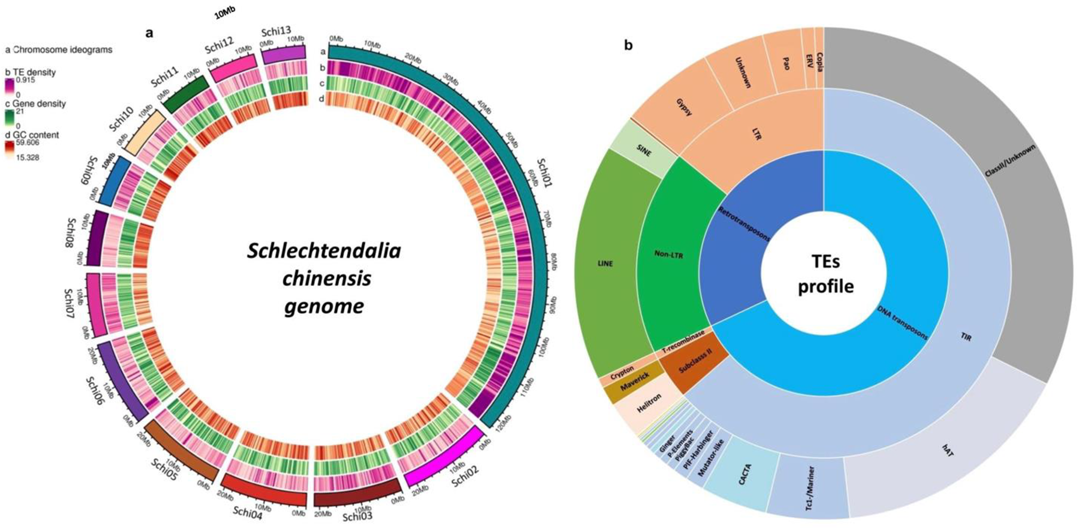
2.1. TE Identification and Annotation
2.2. Class I Retrotransposons
2.3. Class II DNA Transposons
2.4. Subclass 2 (Helitron and Maverick) DNA TEs
2.5. Transcriptomics Analysis of the Potentially Active Copies
2.6. Domesticated TE/Transposon-Derived Proteins in the Genome of S. chinensis
2.7. TE Insertions into Functional Genes
3. Discussion
4. Materials and Methods
4.1. Detection and Annotation of Transposable Elements
4.2. Phylogenetic Analysis
4.3. Sequence Analysis and Identification
4.4. TE Insertions Analysis in the Functional Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelley, J.L.; Peyton, J.T.; Fiston-Lavier, A.-S.; Teets, N.M.; Yee, M.-C.; Johnston, J.S.; Bustamante, C.D.; Lee, R.E.; Denlinger, D.L. Compact genome of the Antarctic midge is likely an adaptation to an extreme environment. Nat. Commun. 2014, 5, 4611. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.; Ruiz-Ruano, F.; Martín-Blázquez, R.; López-León, M.; Cabrero, J.; Lorite, P.; Cabral-de-Mello, D.; Bakkali, M. A step to the gigantic genome of the desert locust: Chromosome sizes and repeated DNAs. Chromosoma 2015, 124, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Wicker, T.; Sabot, F.; Hua-Van, A.; Bennetzen, J.L.; Capy, P.; Chalhoub, B.; Flavell, A.; Leroy, P.; Morgante, M.; Panaud, O. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 2007, 8, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Makałowski, W.; Gotea, V.; Pande, A.; Makałowska, I. Transposable elements: Classification, identification, and their use as a tool for comparative genomics. In Evolutionary Genomics; Springer: New York, NY, USA, 2019; pp. 177–207. [Google Scholar]
- Koonin, E.V.; Makarova, K.S.; Aravind, L. Horizontal gene transfer in prokaryotes: Quantification and classification. Annu. Rev. Microbiol. 2001, 55, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Kunze, R.; Saedler, H.; Lönnig, W.-E. Plant transposable elements. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 1997; Volume 27, pp. 331–470. [Google Scholar]
- Joly-Lopez, Z.; Bureau, T.E. Diversity and evolution of transposable elements in Arabidopsis. Chromosome Res. 2014, 22, 203–216. [Google Scholar] [CrossRef]
- Kojima, K.K. Hagfish genome reveals parallel evolution of 7SL RNA-derived SINEs. Mob. DNA 2020, 11, 18. [Google Scholar] [CrossRef]
- Almojil, D.; Bourgeois, Y.; Falis, M.; Hariyani, I.; Wilcox, J.; Boissinot, S. The structural, functional and evolutionary impact of transposable elements in eukaryotes. Genes 2021, 12, 918. [Google Scholar] [CrossRef]
- Bourque, G.; Burns, K.H.; Gehring, M.; Gorbunova, V.; Seluanov, A.; Hammell, M.; Imbeault, M.; Izsvák, Z.; Levin, H.L.; Macfarlan, T.S. Ten things you should know about transposable elements. Genome Biol. 2018, 19, 199. [Google Scholar] [CrossRef]
- Kojima, K.K. Structural and sequence diversity of eukaryotic transposable elements. Genes Genet. Syst. 2018, 94, 233–252. [Google Scholar] [CrossRef]
- Palazzo, A.; Caizzi, R.; Moschetti, R.; Marsano, R.M. What Have We Learned in 30 Years of Investigations on Bari Transposons? Cells 2022, 11, 583. [Google Scholar] [CrossRef]
- Horváth, V.; Merenciano, M.; González, J. Revisiting the relationship between transposable elements and the eukaryotic stress response. Trends Genet. 2017, 33, 832–841. [Google Scholar] [CrossRef]
- Sundaram, V.; Wysocka, J. Transposable elements as a potent source of diverse cis-regulatory sequences in mammalian genomes. Philos. Trans. R. Soc. B 2020, 375, 20190347. [Google Scholar] [CrossRef]
- Trizzino, M.; Park, Y.; Holsbach-Beltrame, M.; Aracena, K.; Mika, K.; Caliskan, M.; Perry, G.H.; Lynch, V.J.; Brown, C.D. Transposable elements are the primary source of novelty in primate gene regulation. Genome Res. 2017, 27, 1623–1633. [Google Scholar] [CrossRef]
- Martin, S.L. Nucleic acid chaperone properties of ORF1p from the non-LTR retrotransposon, LINE-1. RNA Biol. 2010, 7, 706–711. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, G.-J.; Yuan, J.-H.; Deng, C.-L.; Gao, W.-J. Repetitive sequences and epigenetic modification: Inseparable partners play important roles in the evolution of plant sex chromosomes. Planta 2016, 243, 1083–1095. [Google Scholar] [CrossRef]
- Bennetzen, J.L.; Wang, H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef]
- Harkess, A.; Mercati, F.; Abbate, L.; McKain, M.; Pires, J.C.; Sala, T.; Sunseri, F.; Falavigna, A.; Leebens-Mack, J. Retrotransposon proliferation coincident with the evolution of dioecy in Asparagus. G3 Genes Genomes Genet. 2016, 6, 2679–2685. [Google Scholar]
- Hollister, J.D.; Gaut, B.S. Epigenetic silencing of transposable elements: A trade-off between reduced transposition and deleterious effects on neighboring gene expression. Genome Res. 2009, 19, 1419–1428. [Google Scholar] [CrossRef]
- Zhang, C.X.; Tang, X.D.; Cheng, J.A. The utilization and industrialization of insect resources in China. Entomol. Res. 2008, 38, S38–S47. [Google Scholar] [CrossRef]
- Ren, Z.; Von Dohlen, C.D.; Harris, A.; Dikow, R.B.; Su, X.; Wen, J. Congruent phylogenetic relationships of Melaphidina aphids (Aphididae: Eriosomatinae: Fordini) according to nuclear and mitochondrial DNA data with taxonomic implications on generic limits. PLoS ONE 2019, 14, e0213181. [Google Scholar] [CrossRef]
- Moran, N.A. The evolution of aphid life cycles. Annu. Rev. Entomol. 1992, 37, 321–348. [Google Scholar] [CrossRef]
- Wool, D. Galling aphids: Specialization, biological complexity, and variation. Annu. Rev. Entomol. 2004, 49, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Scalzitti, N.; Kress, A.; Orhand, R.; Weber, T.; Moulinier, L.; Jeannin-Girardon, A.; Collet, P.; Poch, O. Spliceator: Multi-species splice site prediction using convolutional neural networks. BMC Bioinform. 2021, 22, 561. [Google Scholar]
- Maumus, F.; Fiston-Lavier, A.-S.; Quesneville, H. Impact of transposable elements on insect genomes and biology. Curr. Opin. Insect Sci. 2015, 7, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Legeai, F.; Shigenobu, S.; Gauthier, J.P.; Colbourne, J.; Rispe, C.; Collin, O.; Richards, S.; Wilson, A.C.; Murphy, T.; Tagu, D. AphidBase: A centralized bioinformatic resource for annotation of the pea aphid genome. Insect Mol. Biol. 2010, 19, 5–12. [Google Scholar] [CrossRef]
- Wenger, J.A.; Cassone, B.J.; Legeai, F.; Johnston, J.S.; Bansal, R.; Yates, A.D.; Coates, B.S.; Pavinato, V.A.; Michel, A. Whole genome sequence of the soybean aphid, Aphis glycines. Insect Biochem. Mol. Biol. 2020, 123, 102917. [Google Scholar] [CrossRef]
- Nicholson, S.J.; Nickerson, M.L.; Dean, M.; Song, Y.; Hoyt, P.R.; Rhee, H.; Kim, C.; Puterka, G.J. The genome of Diuraphis noxia, a global aphid pest of small grains. BMC Genom. 2015, 16, 429. [Google Scholar] [CrossRef]
- Biémont, C.; Lemeunier, F.; Guerreiro, M.G.; Brookfield, J.; Gautier, C.; Aulard, S.; Pasyukova, E. Population dynamics of the copia, mdg1, mdg3, gypsy, and P transposable elements in a natural population of Drosophila melanogaster. Genet. Res. 1994, 63, 197–212. [Google Scholar] [CrossRef]
- Fablet, M. Host control of insect endogenous retroviruses: Small RNA silencing and immune response. Viruses 2014, 6, 4447–4464. [Google Scholar] [CrossRef]
- Petersen, M.; Armisén, D.; Gibbs, R.A.; Hering, L.; Khila, A.; Mayer, G.; Richards, S.; Niehuis, O.; Misof, B. Diversity and evolution of the transposable element repertoire in arthropods with particular reference to insects. BMC Ecol. Evol. 2019, 19, 11. [Google Scholar] [CrossRef]
- Alberola, T.M.; de Frutos, R. Molecular structure of a gypsy element of Drosophila subobscura (gypsyDs) constituting a degenerate form of insect retroviruses. Nucleic Acids Res. 1996, 24, 914–923. [Google Scholar] [CrossRef]
- Tubio, J.M.C.; Tojo, M.; Bassaganyas, L.; Escaramis, G.; Sharakhov, I.V.; Sharakhova, M.V.; Tornador, C.; Unger, M.F.; Naveira, H.; Costas, J. Evolutionary dynamics of the Ty3/gypsy LTR retrotransposons in the genome of Anopheles gambiae. PLoS ONE 2011, 6, e16328. [Google Scholar] [CrossRef]
- Garner, K.J.; Hiremath, S.; Lehtoma, K.; Valaitis, A.P. Cloning and complete sequence characterization of two gypsy moth aminopeptidase-N cDNAs, including the receptor for Bacillus thuringiensis Cry1Ac toxin. Insect Biochem. Mol. Biol. 1999, 29, 527–535. [Google Scholar] [CrossRef]
- Ben Amara, W.; Quesneville, H.; Khemakhem, M.M. A Genomic Survey of Mayetiola destructor Mobilome Provides New Insights into the Evolutionary History of Transposable Elements in the Cecidomyiid Midges. PLoS ONE 2021, 16, e0257996. [Google Scholar] [CrossRef]
- Fernández-Medina, R.D.; Struchiner, C.J.; Ribeiro, J. Novel transposable elements from Anopheles gambiae. BMC Genom. 2011, 12, 260. [Google Scholar] [CrossRef]
- Gilbert, C.; Peccoud, J.; Cordaux, R. Transposable elements and the evolution of insects. Annu. Rev. Entomol. 2021, 66, 355–372. [Google Scholar] [CrossRef]
- Bouallègue, M.; Filée, J.; Kharrat, I.; Mezghani-Khemakhem, M.; Rouault, J.-D.; Makni, M.; Capy, P. Diversity and evolution of mariner-like elements in aphid genomes. BMC Genom. 2017, 18, 494. [Google Scholar] [CrossRef]
- Jurka, J. Mariner families from Acyrthosiphon pisum. Repbase Rep. 2008, 8, 340. [Google Scholar]
- Kharrat, I.; Mezghani, M.; Casse, N.; Denis, F.; Caruso, A.; Makni, H.; Capy, P.; Rouault, J.-D.; Chénais, B.; Makni, M. Characterization of mariner-like transposons of the mauritiana subfamily in seven tree aphid species. Genetica 2015, 143, 63–72. [Google Scholar] [CrossRef]
- Ahmad, A.; Wallau, G.L.; Ren, Z. Characterization of Mariner transposons in seven species of Rhus gall aphids. Sci. Rep. 2021, 11, 16349. [Google Scholar] [CrossRef]
- Ahmad, A.; Su, X.; Harris, A.; Ren, Z. Closing the Gap: Horizontal Transfer of Mariner Transposons between Rhus Gall Aphids and Other Insects. Biology 2022, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, A.; Marsano, R.M. Transposable elements: A jump toward the future of expression vectors. Crit. Rev. Biotechnol. 2021, 41, 792–808. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.J. DNA sequence insertion and evolutionary variation in gene regulation. Proc. Natl. Acad. Sci. USA 1996, 93, 9374–9377. [Google Scholar] [CrossRef]
- Nekrutenko, A.; Li, W.-H. Transposable elements are found in a large number of human protein-coding genes. Trends Genet. 2001, 17, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tao, X.; Yuan, S.; Zhang, Y.; Li, P.; Beilinson, H.A.; Zhang, Y.; Yu, W.; Pontarotti, P.; Escriva, H. Discovery of an active RAG transposon illuminates the origins of V (D) J recombination. Cell 2016, 166, 102–114. [Google Scholar] [CrossRef]
- Kapitonov, V.V.; Koonin, E.V. Evolution of the RAG1-RAG2 locus: Both proteins came from the same transposon. Biol. Direct 2015, 10, 20. [Google Scholar] [CrossRef]
- Frank, J.A.; Feschotte, C. Co-option of endogenous viral sequences for host cell function. Curr. Opin. Virol. 2017, 25, 81–89. [Google Scholar] [CrossRef]
- Marsano, R.M.; Caizzi, R.; Moschetti, R.; Junakovic, N. Evidence for a functional interaction between the Bari1 transposable element and the cytochrome P450 cyp12a4 gene in Drosophila melanogaster. Gene 2005, 357, 122–128. [Google Scholar] [CrossRef]
- Piriyapongsa, J.; Rutledge, M.T.; Patel, S.; Borodovsky, M.; Jordan, I.K. Evaluating the protein coding potential of exonized transposable element sequences. Biol. Direct 2007, 2, 31. [Google Scholar] [CrossRef]
- Yu, L.; Tang, W.; He, W.; Ma, X.; Vasseur, L.; Baxter, S.W.; Yang, G.; Huang, S.; Song, F.; You, M. Characterization and expression of cytochrome p450 gene family in diamondback moth, Plutella xylostella (L.). Sci. Rep. 2015, 5, 8952. [Google Scholar] [CrossRef]
- Flynn, J.M.; Hubley, R.; Goubert, C.; Rosen, J.; Clark, A.G.; Feschotte, C.; Smit, A.F. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl. Acad. Sci. USA 2020, 117, 9451–9457. [Google Scholar] [CrossRef]
- Bao, Z.; Eddy, S.R. Automated de novo identification of repeat sequence families in sequenced genomes. Genome Res. 2002, 12, 1269–1276. [Google Scholar] [CrossRef]
- Price, A.L.; Jones, N.C.; Pevzner, P.A. De novo identification of repeat families in large genomes. Bioinformatics 2005, 21, i351–i358. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Kurtz, S.; Willhoeft, U. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinform. 2008, 9, 18. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, H. LTR_FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007, 35, W265–W268. [Google Scholar] [CrossRef]
- Ou, S.; Jiang, N. LTR_retriever: A highly accurate and sensitive program for identification of long terminal repeat retrotransposons. Plant Physiol. 2018, 176, 1410–1422. [Google Scholar] [CrossRef]
- Jurka, J.; Kapitonov, V.V.; Pavlicek, A.; Klonowski, P.; Kohany, O.; Walichiewicz, J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 2005, 110, 462–467. [Google Scholar] [CrossRef]
- Neumann, P.; Novák, P.; Hoštáková, N.; Macas, J. Systematic survey of plant LTR-retrotransposons elucidates phylogenetic relationships of their polyprotein domains and provides a reference for element classification. Mob. DNA 2019, 10, 1. [Google Scholar] [CrossRef]
- Wheeler, T.J.; Clements, J.; Eddy, S.R.; Hubley, R.; Jones, T.A.; Jurka, J.; Smit, A.F.; Finn, R.D. Dfam: A database of repetitive DNA based on profile hidden Markov models. Nucleic Acids Res. 2012, 41, D70–D82. [Google Scholar] [CrossRef]
- Yamada, K.D.; Tomii, K.; Katoh, K. Application of the MAFFT sequence alignment program to large data—Reexamination of the usefulness of chained guide trees. Bioinformatics 2016, 32, 3246–3251. [Google Scholar] [CrossRef]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Kohany, O.; Gentles, A.J.; Hankus, L.; Jurka, J. Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinform. 2006, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]






| Class | Order | Superfamily | No. of Copies | Full-Length Copies | Total Length of All Copies | Percentage in Genome |
|---|---|---|---|---|---|---|
| Retrotransposons | LTR | Copia | 1796 | 100 | 463,436 | 0.13 |
| Gypsy | 18,239 | 2002 | 10,381,315 | 3.01 | ||
| ERV | 7463 | 0 | 513,028 | 0.15 | ||
| Pao | 2624 | 179 | 1,047,169 | 0.3 | ||
| Cassandra | 6 | 0 | 336 | 0 | ||
| Caulimovirus | 3 | 0 | 171 | 0 | ||
| Unknown | 11,892 | 220 | 3,023,472 | 0.88 | ||
| Non-LTR | DIRS | 481 | 0 | 26,185 | 0.01 | |
| Viper | 52 | 0 | 5262 | 0 | ||
| LINE | 46,644 | 276 | 16,342,622 | 4.74 | ||
| SINE | 6852 | - | 1,234,961 | 0.36 | ||
| Subtotal | 96,053 | 2777 | 33,038,958 | 9.59 | ||
| DNA transposons | TIR | Tc1-/Mariner | 16,200 | 571 | 3,923,428 | 1.14 |
| CACTA | 21,973 | 53 | 2,506,904 | 0.73 | ||
| hAT | 47,622 | 990 | 12,144,219 | 3.52 | ||
| Mutator-like | 3610 | 0 | 921,870 | 0.27 | ||
| PIF-Harbinger | 2929 | 11 | 458,516 | 0.13 | ||
| PiggyBac | 1774 | 37 | 415,325 | 0.12 | ||
| P elements | 1513 | 09 | 382,695 | 0.11 | ||
| Ginger | 1725 | 0 | 235,990 | 0.07 | ||
| Zisupton | 1081 | 0 | 62,632 | 0.02 | ||
| Novosib | 969 | 01 | 81,878 | 0.02 | ||
| Kolobok | 897 | 0 | 56,957 | 0.02 | ||
| Sola | 508 | 0 | 70,640 | 0.02 | ||
| IS3EU | 484 | 0 | 33,525 | 0.01 | ||
| Merlin | 343 | 07 | 69,223 | 0.02 | ||
| Dada | 332 | 0 | 28,627 | 0.01 | ||
| Zator | 68 | 0 | 4652 | 0 | ||
| Academ | 58 | 0 | 4019 | 0 | ||
| Tyrosine-recombinase | Crypton | 1929 | 0 | 244,861 | 0.07 | |
| Class II (Subclass 2) | ||||||
| Helitron | - | 7503 | 158 | 2,623,664 | 0.76 | |
| Maverick | - | 3899 | 218 | 4,729,237 | 1.37 | |
| Class II/Unknown | 97,190 | 2911 | 27,695,009 | 8.04 | ||
| Subtotal | 212,638 | 4966 | ||||
| Total | 308,196 | 7743 | 89,735,022 | 26.04 |
| Class | Superfamily | No. of Transcripts | Shortest Sequence Length | Longest Sequence Length | Average Length |
|---|---|---|---|---|---|
| Retrotransposons | |||||
| Pao | 10 | 103 | 1732 | 879.5 | |
| Copia | 2 | 25 | 222 | 148.5 | |
| DNA-transposons | |||||
| Mariner | 54 | 51 | 416 | 112.4 | |
| hAT | 8 | 54 | 600 | 316 | |
| PiggyBac | 22 | 56 | 508 | 128 | |
| Mutator-like | 4 | 156 | 648 | 454 | |
| Sub-class 2 | |||||
| Helitron | 9 | 55 | 134 | 106 |
| Cytochrome P450 | Carboxyesterase | Trypsin | |
|---|---|---|---|
| Number of genes | 36 | 21 | 44 |
| Total length of genes (bp) | 265,062 | 244,753 | 309,547 |
| Total number of exons | 284 | 176 | 320 |
| Average number of exons per gene | 7.88 | 8.8 | 7.27 |
| Average length of exons per gene (bp) | 1461.25 | 1771.40 | 1529 |
| Total length of exons (bp) | 52,605 | 35,428 | 67,281 |
| Total number of introns | 179 | 156 | 278 |
| Average length of introns per gene (bp) | 5901 | 10,466.25 | 5506 |
| Total length of introns (bp) | 212,457 | 209,325 | 242,266 |
| Total number of TE insertions (≥100 bp) | 186 | 195 | 151 |
| Total length of TE fragments (bp) | 31,774 | 33,497 | 25,710 |
| Number of TE insertions in introns | 180 | 192 | 133 |
| TE fragments in CDS | 6 | 3 | 18 |
| Number of pseudogenes | 1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Ren, Z. Mobilome of the Rhus Gall Aphid Schlechtendalia chinensis Provides Insight into TE Insertion-Related Inactivation of Functional Genes. Int. J. Mol. Sci. 2022, 23, 15967. https://doi.org/10.3390/ijms232415967
Ahmad A, Ren Z. Mobilome of the Rhus Gall Aphid Schlechtendalia chinensis Provides Insight into TE Insertion-Related Inactivation of Functional Genes. International Journal of Molecular Sciences. 2022; 23(24):15967. https://doi.org/10.3390/ijms232415967
Chicago/Turabian StyleAhmad, Aftab, and Zhumei Ren. 2022. "Mobilome of the Rhus Gall Aphid Schlechtendalia chinensis Provides Insight into TE Insertion-Related Inactivation of Functional Genes" International Journal of Molecular Sciences 23, no. 24: 15967. https://doi.org/10.3390/ijms232415967
APA StyleAhmad, A., & Ren, Z. (2022). Mobilome of the Rhus Gall Aphid Schlechtendalia chinensis Provides Insight into TE Insertion-Related Inactivation of Functional Genes. International Journal of Molecular Sciences, 23(24), 15967. https://doi.org/10.3390/ijms232415967





