Circulating Extracellular Vesicles: Their Role in Patients with Abdominal Aortic Aneurysm (AAA) Undergoing EndoVascular Aortic Repair (EVAR)
Abstract
1. Introduction
2. Results
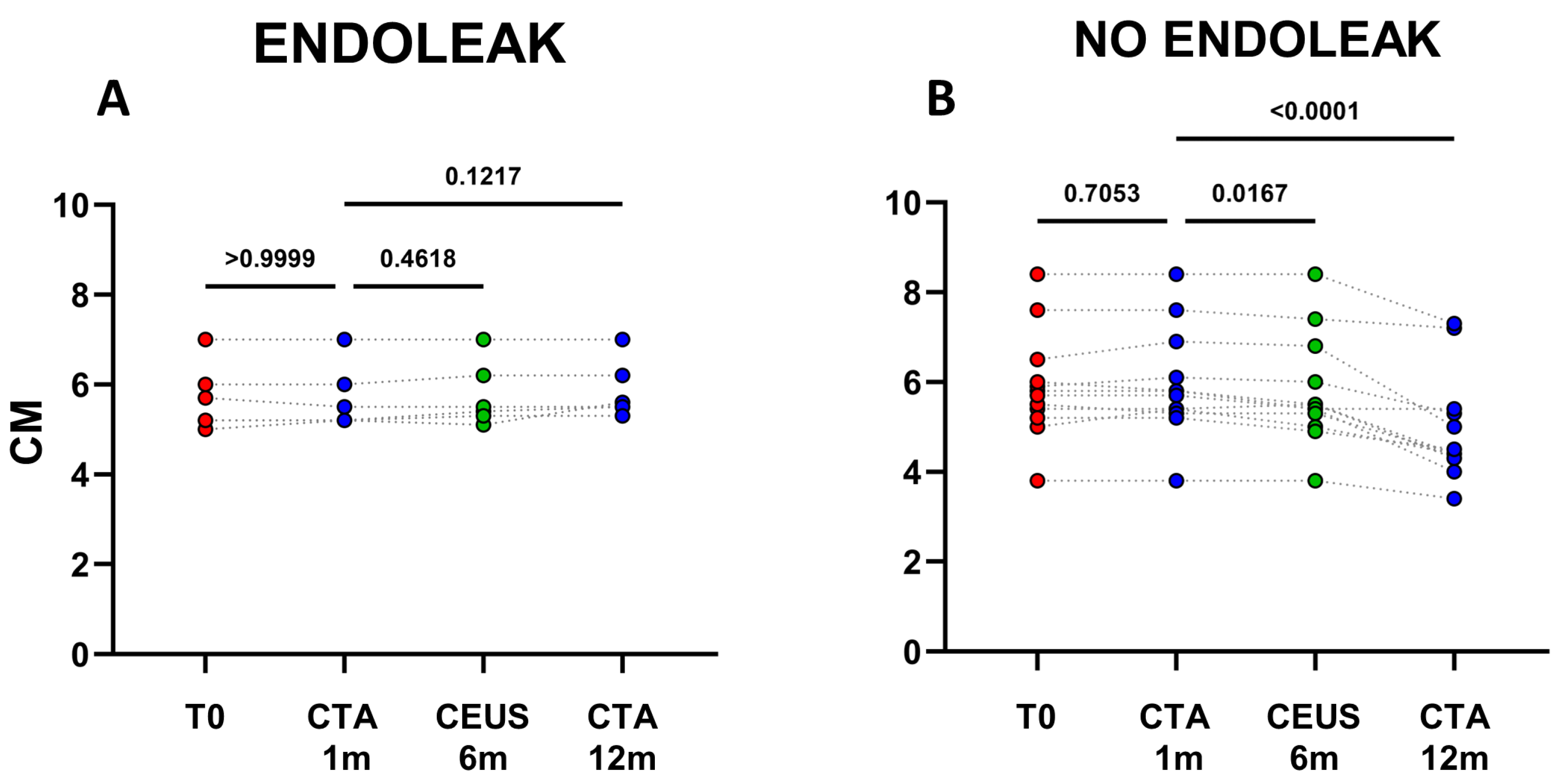
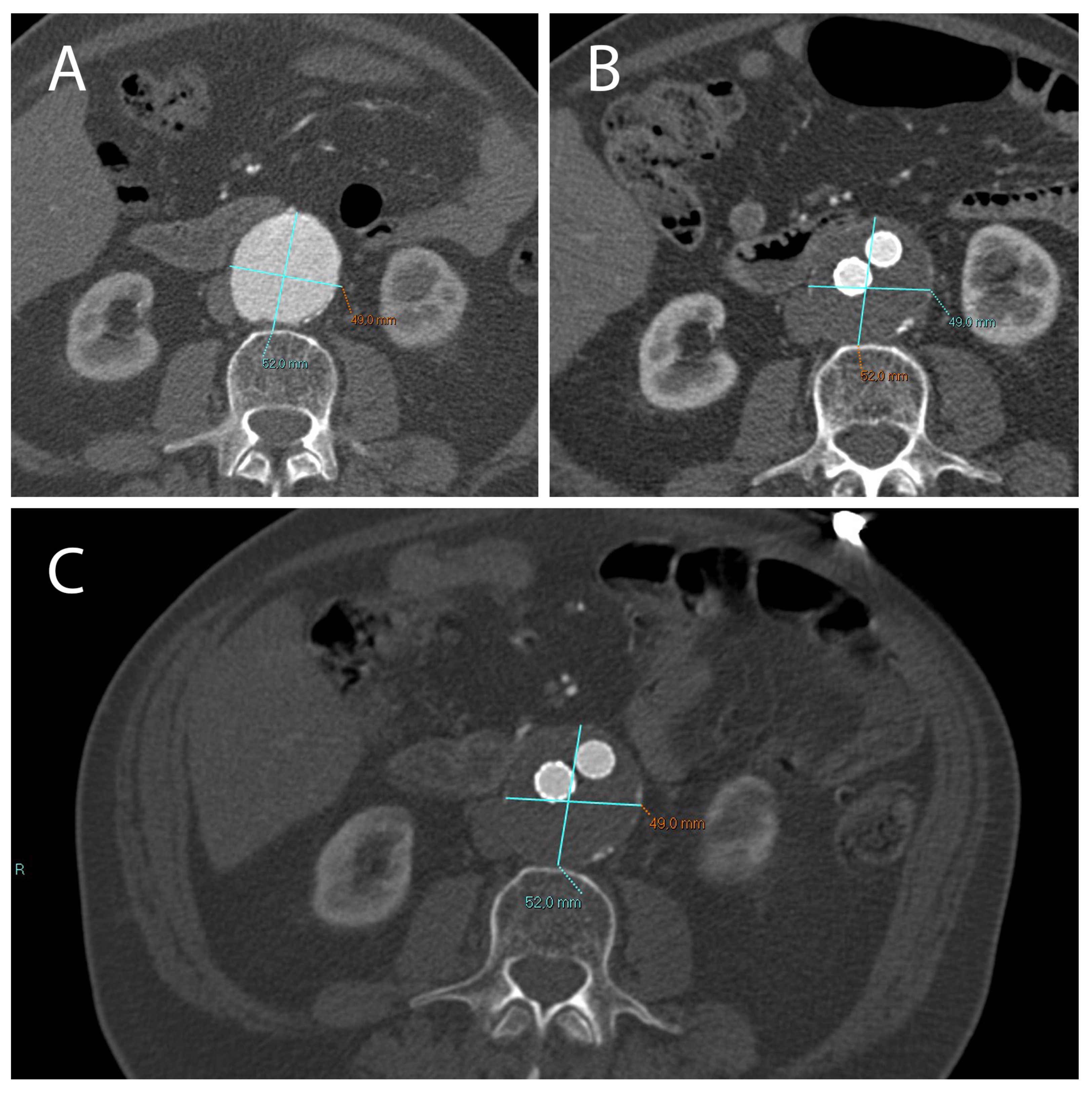
2.1. A Stable Aneurysm Diameter Is Associated with Persistence of Endoleaks
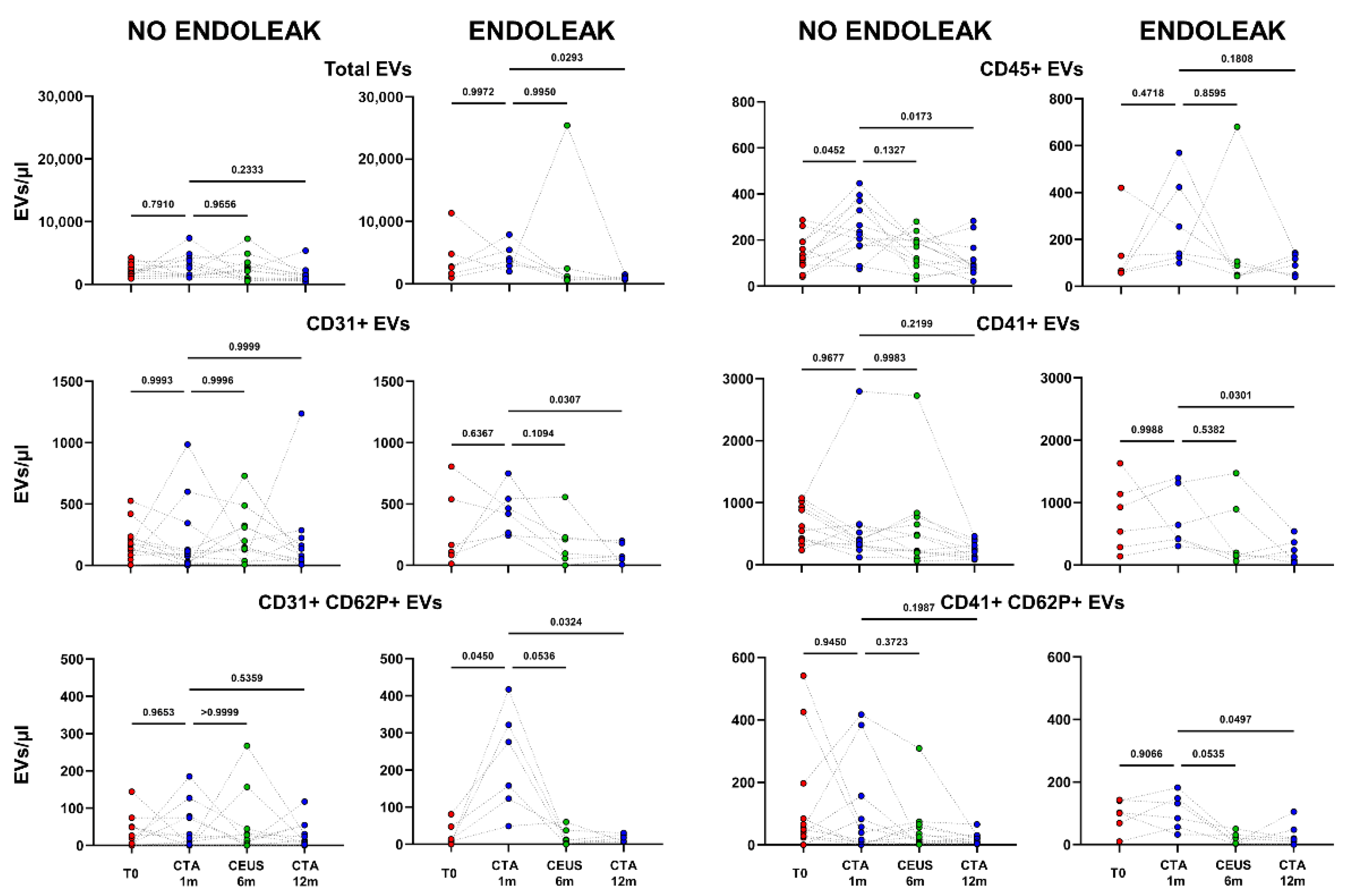
2.2. Extracellular Vesicle Release in Patients Undergoing EVAR Implantation
2.3. Endoleak Onset Induces the Release of EVs
3. Discussion
4. Materials and Methods
4.1. Study Population and Design
- At the AAA diagnosis, eligible for EVAR;
- After 1 month folllowing EVAR implantation (at the same time point patients underwent a follow-up CTA of the abdominal aorta);
- After 6 months following EVAR implantation (at the same time point patients underwent a follow-up CEUS of the abdominal aorta);
- After 12 months following EVAR implantation (at the same time point patients underwent a follow-up CTA of the abdominal aorta).
4.2. CT Scan Protocol
4.3. CEUS Protocol
4.4. Flow Cytometry Analysis of EVs
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, M.; Powell, J.; Greenhalgh, R.M. Limitations of ultrasonography in surveillance of small abdominal aortic aneurysms. Br. J. Surg. 1991, 78, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A. Selective Screening for Abdominal Aortic Aneurysms with Physical Examination and Ultrasound. Arch. Intern. Med. 1988, 148, 1753. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Vammen, S.; Juul, S.; Henneberg, E.W.; Fasting, H. The Validity of Ultrasonographic Scanning as Screening Method for. Eur. J. Vasc. Endovasc. Surg. 1999, 475, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, C.; Esposito, D.; Apostolou, D.; Attisani, L.; Bellosta, R.; Benedetto, F.; Blangetti, I.; Bonardelli, S.; Casini, A.; Fargion, A.T.; et al. Guidelines on the management of abdominal aortic aneurysms: Updates from the Italian Society of Vascular and Endovascular Surgery (SICVE). J. Cardiovasc. Surg. 2022, 63, 328–352. [Google Scholar] [CrossRef] [PubMed]
- Sidloff, D.; Gokani, V.; Stather, P.; Choke, E.; Bown, M.; Sayers, R. Editor’s Choice—Type II Endoleak: Conservative Management Is a Safe Strategy. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 391–399. [Google Scholar] [CrossRef]
- Jones, J.E.; Atkins, M.D.; Brewster, D.C.; Chung, T.; Kwolek, C.J.; LaMuraglia, G.M.; Hodgman, T.M.; Cambria, R.P. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J. Vasc. Surg. 2007, 46, 1–8. [Google Scholar] [CrossRef]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, 2514. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Eitan, E.; Suire, C.; Zhang, S.; Mattson, M.P. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 2016, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.-J.; Cavallini, L.; Ciardiello, C.; Sobreiro, M.R.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef] [PubMed]
- Vagner, T.; Spinelli, C.; Minciacchi, V.R.; Balaj, L.; Zandian, M.; Conley, A.; Zijlstra, A.; Freeman, M.R.; Demichelis, F.; De, S.; et al. Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma. J. Extracell. Vesicles 2018, 7, 1505403. [Google Scholar] [CrossRef]
- Connor, D.E.; Exner, T.; Ma, D.D.F.; Joseph, J.E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 2010, 103, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, 1. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. N. Engl. J. Med. 2018, 379, 958–966. [Google Scholar] [CrossRef]
- Kakarla, R.; Hur, J.; Kim, Y.J.; Kim, J.; Chwae, Y.-J. Apoptotic cell-derived exosomes: Messages from dying cells. Exp. Mol. Med. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- Totani, L.; Plebani, R.; Piccoli, A.; Di Silvestre, S.; Lanuti, P.; Recchiuti, A.; Cianci, E.; Dell’Elba, G.; Sacchetti, S.; Patruno, S.; et al. Mechanisms of endothelial cell dysfunction in cystic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3243–3253. [Google Scholar] [CrossRef]
- Helmke, A.; Von Vietinghoff, S. Extracellular vesicles as mediators of vascular inflammation in kidney disease. World J. Nephrol. 2016, 5, 125–138. [Google Scholar] [CrossRef]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramírez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; Cicalini, I.; Lanuti, P.; Ercolino, E.; Di Ioia, M.; Zucchelli, M.; Zappacosta, R.; Miscia, S.; Marchisio, M.; Sacchetta, P.; et al. Enhanced release of acid sphingomyelinase-enriched exosomes generates a lipidomics signature in CSF of Multiple Sclerosis patients. Sci. Rep. 2018, 8, 3071. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Lv, Y.; Tan, J.; Miao, Y.; Zhang, Q. The role of microvesicles and its active molecules in regulating cellular biology. J. Cell. Mol. Med. 2019, 23, 7894–7904. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.R.; Bentivoglio, A.R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction and Aging: Insights from the Analysis of Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, 805. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, N.; Neytchev, O.; Selman, C.; Shiels, P.G. Extracellular Vesicles, Ageing, and Therapeutic Interventions. Cells 2018, 7, 110. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Chen, C.; Lin, Z.; Liu, J.; Lin, F. Extracellular vesicle-derived circ_SLC19A1 promotes prostate cancer cell growth and invasion through the miR-497/septin 2 pathway. Cell Biol. Int. 2020, 44, 1037–1045. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Borgmann, K.; Edara, V.V.; Stacy, S.; Ghorpade, A.; Ikezu, T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J. Extracell. Vesicles 2020, 9, 1706801. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, A.; Bentivegna, M.; Calfa, G.; Filipello, F.; Pomilio, C.; Bonaventura, M.M.; Lux-Lantos, V.; Matzkin, M.E.; Gregosa, A.; Presa, J.; et al. Early Exposure to a High-Fat Diet Impacts on Hippocampal Plasticity: Implication of Microglia-Derived Exosome-like Extracellular Vesicles. Mol. Neurobiol. 2018, 56, 5075–5094. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J.; Šućurović, S.; Mulac-Jeričević, B. The Role of Extracellular Vesicles and PIBF in Embryo-Maternal Immune-Interactions. Front. Immunol. 2018, 9, 2890. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions1. Biol. Reprod. 2016, 94, 38. [Google Scholar] [CrossRef] [PubMed]
- Sagini, K.; Costanzi, E.; Emiliani, C.; Buratta, S.; Urbanelli, L. Extracellular Vesicles as Conveyors of Membrane-Derived Bioactive Lipids in Immune System. Int. J. Mol. Sci. 2018, 19, 1227. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Xiao, J. Exosomes in Cardiovascular Diseases and Treatment: Experimental and Clinical Aspects. J. Cardiovasc. Transl. Res. 2019, 12, 1–2. [Google Scholar] [CrossRef]
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular vesicles in metabolic disease. Diabetologia 2019, 62, 2179–2187. [Google Scholar] [CrossRef]
- Di Tomo, P.; Lanuti, P.; Di Pietro, N.; Baldassarre, M.P.A.; Marchisio, M.; Pandolfi, A.; Consoli, A.; Formoso, G. Liraglutide mitigates TNF-α induced pro-atherogenic changes and microvesicle release in HUVEC from diabetic women. Diabetes/Metabolism. Res. Rev. 2017, 33, e2925. [Google Scholar] [CrossRef]
- Codagnone, M.; Recchiuti, A.; Lanuti, P.; Pierdomenico, A.M.; Cianci, E.; Patruno, S.; Mari, V.C.; Simiele, F.; Di Tomo, P.; Pandolfi, A.; et al. Lipoxin A 4 stimulates endothelial miR-126–5p expression and its transfer via microvesicles. FASEB J. 2017, 31, 1856–1866. [Google Scholar] [CrossRef]
- Clemmens, H.; Lambert, D.W. Extracellular vesicles: Translational challenges and opportunities. Biochem. Soc. Trans. 2018, 46, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Puca, V.; Ercolino, E.; Celia, C.; Bologna, G.; Di Marzio, L.; Mincione, G.; Marchisio, M.; Miscia, S.; Muraro, R.; Lanuti, P.; et al. Detection and Quantification of eDNA-Associated Bacterial Membrane Vesicles by Flow Cytometry. Int. J. Mol. Sci. 2019, 20, 5307. [Google Scholar] [CrossRef] [PubMed]
- Karasu, E.; Eisenhardt, S.U.; Harant, J.; Huber-Lang, M. Extracellular Vesicles: Packages Sent with Complement. Front. Immunol. 2018, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Marchisio, M.; Lanuti, P.; Boccatonda, A.; Miscia, S.; Davì, G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb. Haemost. 2016, 116, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, C.; Leone, A.; Lanuti, P.; Roca, M.S.; Moccia, T.; Minciacchi, V.R.; Minopoli, M.; Gigantino, V.; De Cecio, R.; Rippa, M.; et al. Large oncosomes overexpressing integrin alpha-V promote prostate cancer adhesion and invasion via AKT activation. J. Exp. Clin. Cancer Res. 2019, 38, 317. [Google Scholar] [CrossRef]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Thompson, A.G.; Gray, E.; Heman-Ackah, S.M.; Mäger, I.; Talbot, K.; El Andaloussi, S.; Wood, M.J.; Turner, M.R. Extracellular vesicles in neurodegenerative disease—Pathogenesis to biomarkers. Nat. Rev. Neurol. 2016, 12, 346–357. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.-L.; Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef]
- Szabo, G.; Momen-Heravi, F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 455–466. [Google Scholar] [CrossRef]
- Tian, J.; Casella, G.; Zhang, Y.; Rostami, A.; Li, X. Potential roles of extracellular vesicles in the pathophysiology, diagnosis, and treatment of autoimmune diseases. Int. J. Biol. Sci. 2020, 16, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Piera-Velazquez, S.; Rosenbloom, J.; Jimenez, S.A. Existing and novel biomarkers for precision medicine in systemic sclerosis. Nat. Rev. Rheumatol. 2018, 14, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Linxweiler, J.; Junker, K. Extracellular vesicles in urological malignancies: An update. Nat. Rev. Urol. 2020, 17, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Lapitz, A.; Arbelaiz, A.; Olaizola, P.; Aranburu, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Extracellular Vesicles in Hepatobiliary Malignancies. Front. Immunol. 2018, 9, 2270. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Whiteside, T.L. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia 2017, 31, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, W.; Hua, S.; Liu, L. Roles of Extracellular Vesicles in Metastatic Breast Cancer. Breast Cancer Basic Clin. Res. 2018, 12, 1178223418767666. [Google Scholar] [CrossRef]
- Kadota, T.; Yoshioka, Y.; Fujita, Y.; Kuwano, K.; Ochiya, T. Extracellular vesicles in lung cancer—From bench to bedside. Semin. Cell Dev. Biol. 2017, 67, 39–47. [Google Scholar] [CrossRef]
- Chang, L.; Ni, J.; Zhu, Y.; Pang, B.; Graham, P.; Zhang, H.; Li, Y. Liquid biopsy in ovarian cancer: Recent advances in circulating extracellular vesicle detection for early diagnosis and monitoring progression. Theranostics 2019, 9, 4130–4140. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Brocco, D.; Lanuti, P.; Pieragostino, D.; Cufaro, M.; Simeone, P.; Bologna, G.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; Irtelli, L.; et al. Phenotypic and Proteomic Analysis Identifies Hallmarks of Blood Circulating Extracellular Vesicles in NSCLC Responders to Immune Checkpoint Inhibitors. Cancers 2021, 13, 585. [Google Scholar] [CrossRef]
- Brocco, D.; Lanuti, P.; Simeone, P.; Bologna, G.; Pieragostino, D.; Cufaro, M.C.; Graziano, V.; Peri, M.; Di Marino, P.; De Tursi, M.; et al. Circulating Cancer Stem Cell-Derived Extracellular Vesicles as a Novel Biomarker for Clinical Outcome Evaluation. J. Oncol. 2019, 2019, 5879616. [Google Scholar] [CrossRef] [PubMed]
- Brocco, D.; Simeone, P.; Buca, D.; Di Marino, P.; De Tursi, M.; Grassadonia, A.; De Lellis, L.; Martino, M.T.; Veschi, S.; Iezzi, M.; et al. Blood Circulating CD133+ Extracellular Vesicles Predict Clinical Outcomes in Patients with Metastatic Colorectal Cancer. Cancers 2022, 14, 1357. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Loy, L.M.; Chua, J.M.E.; Chong, T.T.; Chao, V.T.T.; Irani, F.G.; Damodharan, K.; Leong, S.; Chandramohan, S.; Venkatanarasimha, N.; Patel, A.; et al. Type 2 Endoleaks: Common and Hard to Eradicate yet Benign? Cardiovasc. Interv. Radiol. 2020, 43, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Lanuti, P.; Santilli, F.; Marchisio, M.; Pierdomenico, L.; Vitacolonna, E.; Santavenere, E.; Iacone, A.; Davì, G.; Romano, M.; Miscia, S. A novel flow cytometric approach to distinguish circulating endothelial cells from endothelial microparticles: Relevance for the evaluation of endothelial dysfunction. J. Immunol. Methods 2012, 380, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Dovizio, M.; Marcone, S.; Szklanna, P.B.; Bruno, A.; Ebhardt, H.A.; Cassidy, H.; Ní Áinle, F.; Caprodossi, A.; Lanuti, P.; et al. Platelet-Derived Microparticles from Obese Individuals: Characterization of Number, Size, Proteomics, and Crosstalk with Cancer and Endothelial Cells. Front. Pharmacol. 2019, 10, 7. [Google Scholar] [CrossRef]
- Lanuti, P.; Ciccocioppo, F.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Simeone, P.; Pieragostino, D.; Del Boccio, P.; Marchisio, M.; Miscia, S. Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen. Res. 2020, 15, 850–856. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanzini, M.; Cicalini, I.; Cufaro, M.C.; Damiani, V.; Mastropasqua, L.; De Laurenzi, V.; Nubile, M.; Lanuti, P.; Bologna, G.; et al. Tear proteomics reveals the molecular basis of the efficacy of human recombinant nerve growth factor treatment for Neurotrophic Keratopathy. Sci. Rep. 2022, 12, 1229. [Google Scholar] [CrossRef]
- Serafini, F.L.; Lanuti, P.; Pizzi, A.D.; Procaccini, L.; Villani, M.; Taraschi, A.L.; Pascucci, L.; Mincuzzi, E.; Izzi, J.; Chiacchiaretta, P.; et al. Diagnostic Impact of Radiological Findings and Extracellular Vesicles: Are We Close to Radiovesicolomics? Biology 2021, 10, 1265. [Google Scholar] [CrossRef]
- Oikonomou, E.; Stampouloglou, P.K.; Siasos, G.; Bletsa, E.; Vogiatzi, G.; Kalogeras, K.; Katsianos, E.; Vavuranakis, M.-A.; Souvaliotis, N.; Vavuranakis, M. The Role of Cell-derived Microparticles in Cardiovascular Diseases: Current Concepts. Curr. Pharm. Des. 2022, 28, 1745–1757. [Google Scholar] [CrossRef]
- Trisko, J.; Fleck, J.; Kau, S.; Oesterreicher, J.; Holnthoner, W. Lymphatic and Blood Endothelial Extracellular Vesicles: A Story Yet to Be Written. Life 2022, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Touat, Z.; Ollivier, V.; Dai, J.; Huisse, M.-G.; Bezeaud, A.; Sebbag, U.; Palombi, T.; Rossignol, P.; Meilhac, O.; Guillin, M.-C.; et al. Renewal of Mural Thrombus Releases Plasma Markers and Is Involved in Aortic Abdominal Aneurysm Evolution. Am. J. Pathol. 2006, 168, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef]
- Fernandez-García, C.-E.; Burillo, E.; Lindholt, J.S.; Martinez-Lopez, D.; Pilely, K.; Mazzeo, C.; Michel, J.-B.; Egido, J.; Garred, P.; Blanco-Colio, L.M.; et al. Association of ficolin-3 with abdominal aortic aneurysm presence and progression. J. Thromb. Haemost. 2017, 15, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Munthe-Fog, L.; Hummelshøj, T.; Honoré, C.; Madsen, H.O.; Permin, H.; Garred, P. Immunodeficiency Associated with FCN3 Mutation and Ficolin-3 Deficiency. N. Engl. J. Med. 2009, 360, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Nieuwland, R.; Siljander, P.R.M. 22—Platelet-Derived Extracellular Vesicles. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 401–416. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P. Coagulopathy in Critically Ill Patients. Chest 2009, 136, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Srivastava, A.; Pati, S.; Holcomb, J.B.; Wade, C.E. Platelet-Derived Microvesicles: A Potential Therapy for Trauma-Induced Coagulopathy. Shock 2018, 49, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-X.; Zhang, W.-M.; Li, T.-T.; Liu, Y.; Piao, C.-M.; Ma, Y.-C.; Lu, Y.; Wang, Y.; Liu, T.-T.; Qi, Y.-F.; et al. ER stress dependent microparticles derived from smooth muscle cells promote endothelial dysfunction during thoracic aortic aneurysm and dissection. Clin. Sci. 2017, 131, 1287–1299. [Google Scholar] [CrossRef]
- Jang, S.; Palzer, E.F.; Rudser, K.D.; Fox, C.K.; Hebbel, R.P.; Dengel, D.R.; Milbauer, L.; Kelly, A.S.; Ryder, J.R. Relationship of Endothelial Microparticles to Obesity and Cardiovascular Disease Risk in Children and Adolescents. J. Am. Hear. Assoc. 2022, 11, e026430. [Google Scholar] [CrossRef]
- Badrnya, S.; Assinger, A.; Baumgartner, R. Smoking alters circulating plasma microvesicle pattern and microRNA signatures. Thromb. Haemost. 2014, 112, 128–136. [Google Scholar] [CrossRef]
- Duftner, C.; Seiler, R.; Dejaco, C.; Chemelli-Steingruber, I.; Schennach, H.; Klotz, W.; Rieger, M.; Herold, M.; Falkensammer, J.; Fraedrich, G.; et al. Antiphospholipid Antibodies Predict Progression of Abdominal Aortic Aneurysms. PLoS ONE 2014, 9, e99302. [Google Scholar] [CrossRef]
- Cheng, C.; Bison, E.; Pontara, E.; Cattini, M.G.; Tonello, M.; Denas, G.; Pengo, V. Platelet- and endothelial-derived microparticles in the context of different antiphospholipid antibody profiles. Lupus 2022, 31, 1328–1334. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, H.; Park, J.K. Comparison of Clinical and Hematologic Factors Associated with Stenosis and Aneurysm Development in Patients with Atherosclerotic Arterial Disease. Ann. Vasc. Surg. 2019, 60, 165–170. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.F.; Shoenfeld, Y. Aneurysms in primary antiphospholipid syndrome: A case-based review. Clin. Rheumatol. 2021, 40, 3001–3006. [Google Scholar] [CrossRef]
- Marchisio, M.; Simeone, P.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Pieragostino, D.; Ventrella, A.; Antonini, F.; Del Zotto, G.; Vergara, D.; et al. Flow Cytometry Analysis of Circulating Extracellular Vesicle Subtypes from Fresh Peripheral Blood Samples. Int. J. Mol. Sci. 2020, 22, 48. [Google Scholar] [CrossRef]
- Simeone, P.; Celia, C.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Cilurzo, F.; Grande, R.; Diomede, F.; Vespa, S.; Canonico, B.; et al. Diameters and Fluorescence Calibration for Extracellular Vesicle Analyses by Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 7885. [Google Scholar] [CrossRef]
- Falasca, K.; Lanuti, P.; Ucciferri, C.; Pieragostino, D.; Cufaro, M.C.; Bologna, G.; Federici, L.; Miscia, S.; Pontolillo, M.; Auricchio, A.; et al. Circulating extracellular vesicles as new inflammation marker in HIV infection. AIDS 2021, 35, 595–604. [Google Scholar] [CrossRef]
- Pieragostino, D.; Lanuti, P.; Cicalini, I.; Cufaro, M.C.; Ciccocioppo, F.; Ronci, M.; Simeone, P.; Onofrj, M.; van der Pol, E.; Fontana, A.; et al. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J. Proteom. 2019, 204, 103403. [Google Scholar] [CrossRef]




| Complications | Definition | Estimated Frequency During 5-Year Follow-Up |
|---|---|---|
| Type I endoleak | Peri-graft flow occurring from attachment sites | 5% |
| A | Proximal end of stent graft | |
| B | Distal end of stent graft | |
| C | Iliac occluder | |
| Type II endoleak | Peri-graft flow occurring from collateral branches to the aneurysm; inferior mesenteric artery (IIA) and lumbar arteries (IIB) | 20–40%, 10% persistent at 2 years |
| Categorised as early or late/delayed (before or after 12 months) and as transient or persistent (resolved or not resolved ≤ 6 months) | ||
| Type III endoleak | Peri-graft flow occurring from stent graft defect or junction sites | 1–3% |
| A | Leak from junctions or modular disconnection | |
| B | Fabric holes | |
| Type IV endoleak | Peri-graft flow occurring from stent graft fabric porosity < 30 days after placement | 1% |
| Endotension | AAA sac enlargement without visualised endoleak | <1% |
| Migration | Movement of the stent graft in relation to proximal or distal landing zone | 1% |
| Limb kinking and occlusion | Graft thrombosis or stenosis | 4–8% |
| Infection | Stent graft infection | 0.5–1% |
| Rupture | Aortic rupture | 1–5% |
| Detection | Fluorochrome | Vendor | Ab Clone | Catalog | Amount per Test |
|---|---|---|---|---|---|
| Phalloidin | FITC | BD Biosciences | - | 626267 (custom kit) | 0.5 µL |
| CD41a | PE | BD Biosciences | HIP8 | 626266 (custom kit) | 5 µL |
| CD31 | PE-Cy7 | BD Biosciences | WM59 | 626266 (custom kit) | 5 µL |
| CD45 | BV510 | BD Biosciences | HI30 | 626266 (custom kit) | 5 µL |
| LCD | APC | BD Biosciences | 626267 (custom kit) | 0.5 µL | |
| CD62P | BV421 | BD Biosciences | AK4 | 564038 | 3 µL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafini, F.L.; Delli Pizzi, A.; Simeone, P.; Giammarino, A.; Mannetta, C.; Villani, M.; Izzi, J.; Buca, D.; Catitti, G.; Chiacchiaretta, P.; et al. Circulating Extracellular Vesicles: Their Role in Patients with Abdominal Aortic Aneurysm (AAA) Undergoing EndoVascular Aortic Repair (EVAR). Int. J. Mol. Sci. 2022, 23, 16015. https://doi.org/10.3390/ijms232416015
Serafini FL, Delli Pizzi A, Simeone P, Giammarino A, Mannetta C, Villani M, Izzi J, Buca D, Catitti G, Chiacchiaretta P, et al. Circulating Extracellular Vesicles: Their Role in Patients with Abdominal Aortic Aneurysm (AAA) Undergoing EndoVascular Aortic Repair (EVAR). International Journal of Molecular Sciences. 2022; 23(24):16015. https://doi.org/10.3390/ijms232416015
Chicago/Turabian StyleSerafini, Francesco Lorenzo, Andrea Delli Pizzi, Pasquale Simeone, Alberto Giammarino, Cristian Mannetta, Michela Villani, Jacopo Izzi, Davide Buca, Giulia Catitti, Piero Chiacchiaretta, and et al. 2022. "Circulating Extracellular Vesicles: Their Role in Patients with Abdominal Aortic Aneurysm (AAA) Undergoing EndoVascular Aortic Repair (EVAR)" International Journal of Molecular Sciences 23, no. 24: 16015. https://doi.org/10.3390/ijms232416015
APA StyleSerafini, F. L., Delli Pizzi, A., Simeone, P., Giammarino, A., Mannetta, C., Villani, M., Izzi, J., Buca, D., Catitti, G., Chiacchiaretta, P., Trebeschi, S., Miscia, S., Caulo, M., & Lanuti, P. (2022). Circulating Extracellular Vesicles: Their Role in Patients with Abdominal Aortic Aneurysm (AAA) Undergoing EndoVascular Aortic Repair (EVAR). International Journal of Molecular Sciences, 23(24), 16015. https://doi.org/10.3390/ijms232416015





