Functional Characterization of Novel Bony Fish Lipoxygenase Isoforms and Their Possible Involvement in Inflammation
Abstract
:1. Introduction
2. Results
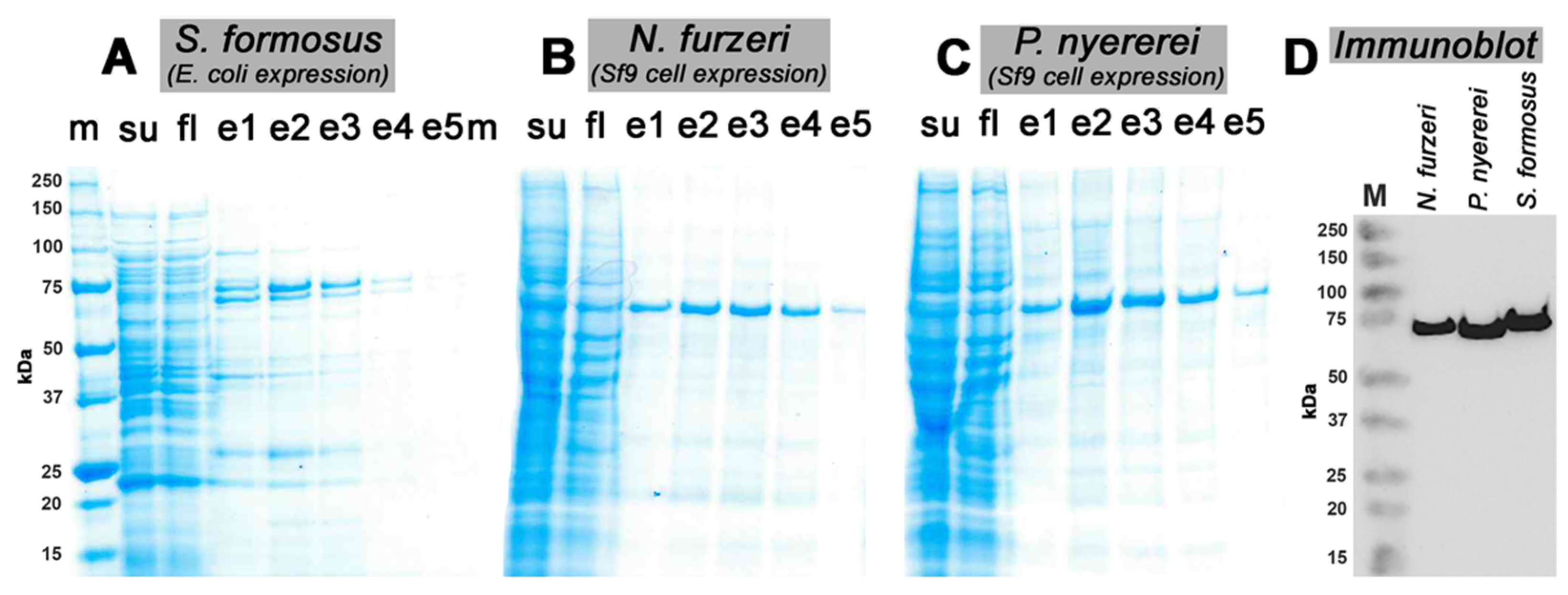
2.1. Recombinant Expression of Putative Bony Fish ALOX15-Isoforms
2.2. Product Specificity with Different PUFAs
2.3. Temperature Dependence and Determination of the Activation Energy
2.4. pH-Dependence of the Catalytic Activity of Bony Fish ALOX Isoforms
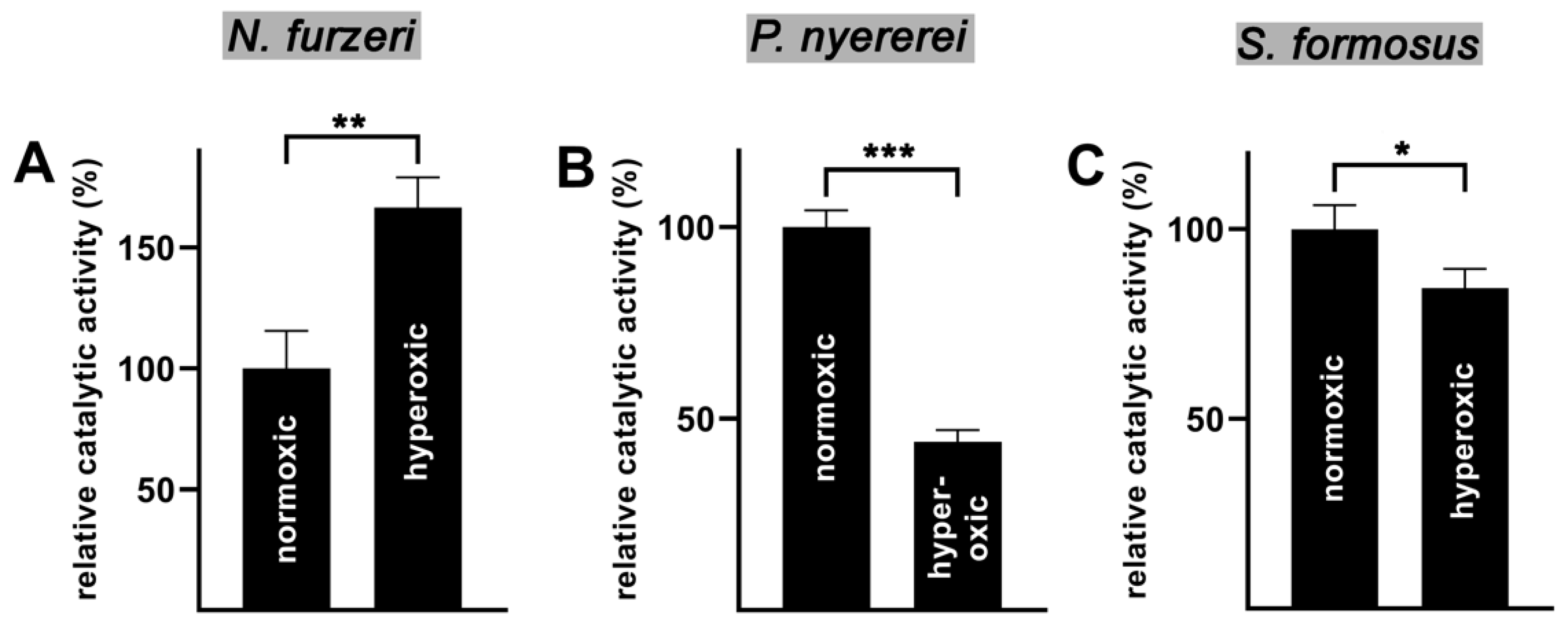
2.5. Oxygen Affinity
2.6. Oxygenation of Complex Substrates by the Putative Bony Fish ALOX-Isoforms
3. Discussion
3.1. Degree of Novelty, Advancement of Knowledge and Limitations
3.2. Catalytic Properties of Bony Fish ALOX-Isoforms
3.3. Evolutionary Aspects of Bony Fish ALOX-Isoforms
3.4. Possible Roles of ALOX-Isoforms in Bony Fish Inflammation
4. Materials and Methods
4.1. Chemicals
4.2. Database Searches and Amino Acid Alignments
4.3. Cloning of Bony Fish ALOX Isoforms
4.4. Expression of Bony Fish ALOX Isoforms
4.5. Ni-Agarose Based Affinity Purification of Recombinant ALOX-Isoforms
4.6. Immunoblotting
4.7. In Vitro Activity Assays and HPLC Analyses of the Reaction Products
4.8. LC-MS/MS Analysis of the Reaction Products
4.9. Kinetic Studies Using Recombinant Bony Fish ALOX-Isoforms
4.10. Miscellaneous Methods
4.11. Statistics Evaluation of the Experimental Raw Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Fujino, H. The Biased Activities of Prostanoids and Their Receptors: Review and Beyond. Biol. Pharm. Bull. 2022, 45, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Chang, W.A.; Chuang, C.H.; Wu, K.L.; Cheng, C.H.; Sheu, C.C.; Hsu, Y.L.; Hung, J.Y. Cysteinyl Leukotriene Pathway and Cancer. Int. J. Mol. Sci. 2021, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jin, M.; Lou, L.; Yang, S.; Li, C.; Li, X.; Zhou, M.; Cai, C. Role of arachidonic acid lipoxygenase pathway in Asthma. Prostaglandins Other Lipid Mediat. 2022, 158, 106609. [Google Scholar] [CrossRef]
- Guo, T.C.; Gamil, A.A.; Koenig, M.; Evensen, O. Sequence analysis and identification of new isoform of EP4 receptors in different atlantic salmon tissues (Salmo salar L.) and its role in PGE2 induced immunomodulation in vitro. PLoS ONE 2015, 10, e0120483. [Google Scholar] [CrossRef] [Green Version]
- Kwok, A.H.; Wang, Y.; Wang, C.Y.; Leung, F.C. Molecular cloning and characterization of chicken prostaglandin E receptor subtypes 2 and 4 (EP2 and EP4). Gen. Comp. Endocrinol. 2008, 157, 99–106. [Google Scholar] [CrossRef]
- Takahashi, T.; Fujimori, C.; Hagiwara, A.; Ogiwara, K. Recent advances in the understanding of teleost medaka ovulation: The roles of proteases and prostaglandins. Zool. Sci. 2013, 30, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Alam, M. Ionophore A23187 stimulates Entamoeba histolytica to release prostaglandin F2 alpha. Prostaglandins Leukot Med. 1986, 22, 259–264. [Google Scholar] [CrossRef]
- Hawkins, D.J.; Brash, A.R. Eggs of the sea urchin, Strongylocentrotus purpuratus, contain a prominent (11R) and (12R) lipoxygenase activity. J. Biol. Chem. 1987, 262, 7629–7634. [Google Scholar] [CrossRef]
- Koljak, R.; Jarving, I.; Kurg, R.; Boeglin, W.E.; Varvas, K.; Valmsen, K.; Ustav, M.; Brash, A.R.; Samel, N. The basis of prostaglandin synthesis in coral: Molecular cloning and expression of a cyclooxygenase from the Arctic soft coral Gersemia fruticosa. J. Biol. Chem. 2001, 276, 7033–7040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brash, A.R.; Boeglin, W.E.; Chang, M.S.; Shieh, B.H. Purification and molecular cloning of an 8R-lipoxygenase from the coral Plexaura homomalla reveal the related primary structures of R- and S-lipoxygenases. J. Biol. Chem. 1996, 271, 20949–20957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.W. Recent investigations into the lipoxygenase pathway of plants. Biochim. Biophys. Acta 1991, 1084, 221–239. [Google Scholar] [CrossRef]
- Haeggström, J.Z.; Funk, C.D. Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Horn, T.; Adel, S.; Schumann, R.; Sur, S.; Kakularam, K.R.; Polamarasetty, A.; Redanna, P.; Kuhn, H.; Heydeck, D. Evolutionary aspects of lipoxygenases and genetic diversity of human leukotriene signaling. Prog. Lipid Res. 2015, 57, 13–39. [Google Scholar] [CrossRef]
- Garreta, A.; Val-Moraes, S.P.; Garcia-Fernandez, Q.; Busquets, M.; Juan, C.; Oliver, A.; Ortiz, A.; Gaffney, B.J.; Fita, I.; Manresa, A.; et al. Structure and interaction with phospholipids of a prokaryotic lipoxygenase from Pseudomonas aeruginosa. FASEB J. 2013, 27, 4811–4821. [Google Scholar] [CrossRef] [Green Version]
- Banthiya, S.; Pekarova, M.; Kuhn, H.; Heydeck, D. Secreted lipoxygenase from Pseudomonas aeruginosa exhibits biomembrane oxygenase activity and induces hemolysis in human red blood cells. Arch. Biochem. Biophys. 2015, 584, 116–124. [Google Scholar] [CrossRef]
- Kalms, J.; Banthiya, S.; Galemou Yoga, E.; Hamberg, M.; Holzhutter, H.G.; Kuhn, H.; Scheerer, P. The crystal structure of Pseudomonas aeruginosa lipoxygenase Ala420Gly mutant explains the improved oxygen affinity and the altered reaction specificity. Biochim. Biophys. Acta 2017, 1862, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, J.D.; Ogunsola, A.F.; Jameson, J.B., 2nd; Yasgar, A.; Flitter, B.A.; Freedman, C.J.; Melvin, J.A.; Nguyen, J.V.; Maloney, D.J.; Jadhav, A.; et al. Biochemical and Cellular Characterization and Inhibitor Discovery of Pseudomonas aeruginosa 15-Lipoxygenase. Biochemistry 2016, 55, 3329–3340. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.C.; Tyurin, V.A.; Krieger, J.; St Croix, C.M.; Watkins, S.; Bayir, E.; et al. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Investig. 2018, 128, 4639–4653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irie, N.; Satoh, N.; Kuratani, S. The phylum Vertebrata: A case for zoological recognition. Zool. Lett. 2018, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Fang, Z.; Li, L.; Luo, L. Using zebrafish as the model organism to understand organ regeneration. Sci. China Life Sci. 2015, 58, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A marvel of high-throughput biology for 21 century toxicology. Curr. Environ. Health Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Adel, S.; Heydeck, D.; Kuhn, H.; Ufer, C. The lipoxygenase pathway in zebrafish. Expression and characterization of zebrafish ALOX5 and comparison with its human ortholog. Biochim. Biophys. Acta 2016, 1861, 1–11. [Google Scholar] [CrossRef]
- Haas, U.; Raschperger, E.; Hamberg, M.; Samuelsson, B.; Tryggvason, K.; Haeggstrom, J.Z. Targeted knock-down of a structurally atypical zebrafish 12S-lipoxygenase leads to severe impairment of embryonic development. Proc. Natl. Acad. Sci. USA 2011, 108, 20479–20484. [Google Scholar] [CrossRef] [Green Version]
- Jansen, C.; Hofheinz, K.; Vogel, R.; Roffeis, J.; Anton, M.; Reddanna, P.; Kuhn, H.; Walther, M. Stereocontrol of arachidonic acid oxygenation by vertebrate lipoxygenases: Newly cloned zebrafish lipoxygenase 1 does not follow the Ala-versus-Gly concept. J. Biol. Chem. 2011, 286, 37804–37812. [Google Scholar] [CrossRef] [Green Version]
- German, J.B.; Bruckner, G.G.; Kinsella, J.E. Lipoxygenase in trout gill tissue acting on arachidonic, eicosapentaenoic and docosahexaenoic acids. Biochim. Biophys. Acta 1986, 875, 12–20. [Google Scholar] [CrossRef]
- Holland, J.W.; Taylor, G.W.; Rowley, A.F. The eicosanoid generating capacity of isolated cell populations from the gills of the rainbow trout, Oncorhynchus mykiss. Comp. Biochem. Physiol. C Pharm. Toxicol. Endocrinol. 1999, 122, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, T.R.; Rowley, A.F.; Barrow, S.E.; Mallet, A.I.; Secombes, C.J. Synthesis of lipoxins and other lipoxygenase products by macrophages from the rainbow trout, Oncorhynchus mykiss. J. Biol. Chem. 1991, 266, 8720–8726. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Griffiths, D.H.; Rowley, A.F. Trout thrombocytes contain 12- but not 5-lipoxygenase activity. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 1999, 1437, 63–70. [Google Scholar] [CrossRef]
- Rowley, A.F.; Lloyd-Evans, P.; Barrow, S.E.; Serhan, C.N. Lipoxin biosynthesis by trout macrophages involves the formation of epoxide intermediates. Biochemistry 1994, 33, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.F. Lipoxin formation in fish leucocytes. Biochim. Biophys. Acta 1991, 1084, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zuo, R.; Mai, K.; Xu, W.; Ai, Q. Molecular cloning and functional characterization of arachidonate 5-lipoxygenase (Alox5), and its expression in response to the ratio of linolenic acid to linoleic acid in diets of large yellow croaker (Larmichthys crocea). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 201, 21–28. [Google Scholar] [CrossRef]
- Schewe, T.; Halangk, W.; Hiebsch, C.; Rapoport, S.M. A lipoxygenase in rabbit reticulocytes which attacks phospholipids and intact mitochondria. FEBS Lett. 1975, 60, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Kühn, H.; Barnett, J.; Grunberger, D.; Baecker, P.; Chow, J.; Nguyen, B.; Bursztyn-Pettegrew, H.; Chan, H.; Sigal, E. Overexpression, purification and characterization of human recombinant 15-lipoxygenase. Biochim. Biophys. Acta 1993, 1169, 80–89. [Google Scholar] [CrossRef]
- Unsworth, L.D.; van der Oost, J.; Koutsopoulos, S. Hyperthermophilic enzymes--stability, activity and implementation strategies for high temperature applications. FEBS J. 2007, 274, 4044–4056. [Google Scholar] [CrossRef]
- Leuschner, C.; Antranikian, G. Heat-Stable Enzymes from Extremely Thermophilic and Hyperthermophilic Microorganisms. World J. Microb. Biot. 1995, 11, 95–114. [Google Scholar] [CrossRef]
- Tappel, A.L.; Lundberg, W.O.; Boyer, P.D. Effect of Temperature and Antioxidants Upon the Lipoxidase-Catalyzed Oxidation of Sodium Linoleate. Arch. Biochem. Biophys. 1953, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Gotze, R.; Schewe, T.; Rapoport, S.M. Quasi-lipoxygenase activity of haemoglobin. A model for lipoxygenases. Eur. J. Biochem. 1981, 120, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Banthiya, S.; Kalms, J.; Galemou Yoga, E.; Ivanov, I.; Carpena, X.; Hamberg, M.; Kuhn, H.; Scheerer, P. Structural and functional basis of phospholipid oxygenase activity of bacterial lipoxygenase from Pseudomonas aeruginosa. Biochim. Biophys. Acta 2016, 1861, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, N.; Humeniuk, L.; Ufer, C.; Ivanov, I.; Golovanov, A.; Stehling, S.; Heydeck, D.; Kuhn, H. Functional characterization of novel ALOX15 orthologs representing key steps in mammalian evolution supports the Evolutionary Hypothesis of reaction specificity. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Madshus, I.H. Regulation of intracellular pH in eukaryotic cells. Biochem. J. 1988, 250, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asokan, A.; Cho, M.J. Exploitation of intracellular pH gradients in the cellular delivery of macromolecules. J. Pharm. Sci. 2002, 91, 903–913. [Google Scholar] [CrossRef]
- Ludwig, P.; Holzhutter, H.G.; Colosimo, A.; Silvestrini, M.C.; Schewe, T.; Rapoport, S.M. A kinetic model for lipoxygenases based on experimental data with the lipoxygenase of reticulocytes. Eur. J. Biochem. 1987, 168, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Gardner, H.W. Soybean lipoxygenase-1 enzymically forms both (9S)- and (13S)-hydroperoxides from linoleic acid by a pH-dependent mechanism. Biochim. Biophys. Acta 1989, 1001, 274–281. [Google Scholar] [CrossRef]
- Ivanov, I.; Heydeck, D.; Hofheinz, K.; Roffeis, J.; O’Donnell, V.B.; Kuhn, H.; Walther, M. Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 2010, 503, 161–174. [Google Scholar] [CrossRef]
- Minor, W.; Steczko, J.; Stec, B.; Otwinowski, Z.; Bolin, J.T.; Walter, R.; Axelrod, B. Crystal structure of soybean lipoxygenase L-1 at 1.4 A resolution. Biochemistry 1996, 35, 10687–10701. [Google Scholar] [CrossRef]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, N.C.; Bartlett, S.G.; Waight, M.T.; Neau, D.B.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. The structure of human 5-lipoxygenase. Science 2011, 331, 217–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobe, M.J.; Neau, D.B.; Mitchell, C.E.; Bartlett, S.G.; Newcomer, M.E. The structure of human 15-lipoxygenase-2 with a substrate mimic. J. Biol. Chem. 2014, 289, 8562–8569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Mueser, T.C.; Marnett, L.J.; Funk, M.O., Jr. Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure 2012, 20, 1490–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saam, J.; Ivanov, I.; Walther, M.; Holzhutter, H.G.; Kuhn, H. Molecular dioxygen enters the active site of 12/15-lipoxygenase via dynamic oxygen access channels. Proc. Natl. Acad. Sci. USA 2007, 104, 13319–13324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juranek, I.; Suzuki, H.; Yamamoto, S. Affinities of various mammalian arachidonate lipoxygenases and cyclooxygenases for molecular oxygen as substrate. Biochim. Biophys. Acta 1999, 1436, 509–518. [Google Scholar] [CrossRef]
- Van Os, C.P.; Rijke-Schilder, G.P.; Van Halbeek, H.; Verhagen, J.; Vliegenthart, J.F. Double dioxygenation of arachidonic acid by soybean lipoxygenase-1. Kinetics and regio-stereo specificities of the reaction steps. Biochim. Biophys. Acta 1981, 663, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, H.; Wiesner, R.; Stender, H.; Schewe, T.; Lankin, V.Z.; Nekrasov, A.; Rapoport, S.M. Requirement of monohydroperoxy fatty acids for the oxygenation of 15LS-HETE by reticulocyte lipoxygenase. FEBS Lett. 1986, 203, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Pettitt, T.R.; Rowley, A.F. Fatty acid composition and lipoxygenase metabolism in blood cells of the lesser spotted dogfish, Scyliorhinus canicula. Comp. Biochem. Physiol. B 1991, 99, 647–652. [Google Scholar] [CrossRef]
- Bryant, R.W.; Bailey, J.M.; Schewe, T.; Rapoport, S.M. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15-S-hydroperoxy-eicosatetraenoic acid. J. Biol. Chem. 1982, 257, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Heydeck, D.; Reisch, F.; Schäfer, M.; Kakularam, K.R.; Roigas, S.A.; Stehling, S.; Püschel, G.P.; Kuhn, H. The Reaction Specificity of Mammalian ALOX15 Orthologs is Changed During Late Primate Evolution and These Alterations Might Offer Evolutionary Advantages for Hominidae. Front. Cell Dev. Biol. 2022, 10, 871585. [Google Scholar] [CrossRef] [PubMed]
- Borngraber, S.; Browner, M.; Gillmor, S.; Gerth, C.; Anton, M.; Fletterick, R.; Kuhn, H. Shape and specificity in mammalian 15-lipoxygenase active site. The functional interplay of sequence determinants for the reaction specificity. J. Biol. Chem. 1999, 274, 37345–37350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.; Kuhn, H.; Heydeck, D. Structural and functional biology of arachidonic acid 15-lipoxygenase-1 (ALOX15). Gene 2015, 573, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Wiesner, R.; Schewe, T.; Rapoport, S.M. Reticulocyte lipoxygenase exhibits both n-6 and n-9 activities. FEBS Lett. 1983, 153, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falgueyret, J.P.; Denis, D.; Macdonald, D.; Hutchinson, J.H.; Riendeau, D. Characterization of the arachidonate and ATP binding sites of human 5-lipoxygenase using photoaffinity labeling and enzyme immobilization. Biochemistry 1995, 34, 13603–13611. [Google Scholar] [CrossRef]
- Nugteren, D.H. Arachidonate lipoxygenase in blood platelets. Biochim. Biophys. Acta 1975, 380, 299–307. [Google Scholar] [CrossRef]
- Pettitt, T.R.; Rowley, A.F.; Barrow, S.E. Synthesis of leukotriene B and other conjugated triene lipoxygenase products by blood cells of the rainbow trout, Salmo gairdneri. Biochim. Biophys. Acta 1989, 1003, 1–8. [Google Scholar] [CrossRef]
- Kuhn, H.; Humeniuk, L.; Kozlov, N.; Roigas, S.; Adel, S.; Heydeck, D. The evolutionary hypothesis of reaction specificity of mammalian ALOX15 orthologs. Prog. Lipid Res. 2018, 72, 55–74. [Google Scholar] [CrossRef]
- Funk, C.D.; Chen, X.-S.; Johnson, E.N.; Zhao, L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002, 68, 303–312. [Google Scholar] [CrossRef]
- Funk, C.D.; Keeney, D.S.; Oliw, E.H.; Boeglin, W.E.; Brash, A.R. Functional expression and cellular localization of a mouse epidermal lipoxygenase. J. Biol. Chem. 1996, 271, 23338–23344. [Google Scholar] [CrossRef]
- Sun, D.; Funk, C.D. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J. Biol. Chem. 1996, 271, 24055–24062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, E.N.; Brass, L.F.; Funk, C.D. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc. Natl. Acad. Sci. USA 1998, 95, 3100–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.S.; Sheller, J.R.; Johnson, E.N.; Funk, C.D. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature 1994, 372, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Epp, N.; Furstenberger, G.; Muller, K.; de Juanes, S.; Leitges, M.; Hausser, I.; Thieme, F.; Liebisch, G.; Schmitz, G.; Krieg, P. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 2007, 177, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Krieg, P.; Rosenberger, S.; de Juanes, S.; Latzko, S.; Hou, J.; Dick, A.; Kloz, U.; van der Hoeven, F.; Hausser, I.; Esposito, I.; et al. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J. Investig. Dermatol. 2013, 133, 172–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfardan, R.; Guo, C.; Toth, L.A.; Nie, D. Impaired Recovery from Influenza A/X-31(H3N2) Infection in Mice with 8-Lipoxygenase Deficiency. Med. Sci 2019, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Krieg, P.; Dick, A.; Latzko, S.; Rosenberger, S.; Meyer, J.; Crumrine, D.; Hielscher, T.; Elias, P.M.; Rauh, M.; Schneider, H. Conditional Alox12b Knockout: Degradation of the Corneocyte Lipid Envelope in a Mouse Model of Autosomal Recessive Congenital Ichthyoses. J. Investig. Dermatol. 2020, 140, 249–253.e246. [Google Scholar] [CrossRef] [Green Version]
- Morgan, E.L.; Maskrey, B.H.; Rowley, A.F. At what stage in metazoan evolution did leukotriene generation first appear?--key insights from cartilaginous fish. Dev. Comp. Immunol. 2005, 29, 53–59. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef] [Green Version]
- Adel, S.; Karst, F.; Gonzalez-Lafont, A.; Pekarova, M.; Saura, P.; Masgrau, L.; Lluch, J.M.; Stehling, S.; Horn, T.; Kuhn, H.; et al. Evolutionary alteration of ALOX15 specificity optimizes the biosynthesis of antiinflammatory and proresolving lipoxins. Proc. Natl. Acad. Sci. USA 2016, 113, E4266–E4275. [Google Scholar] [CrossRef]
- Bender, G.; Schexnaydre, E.E.; Murphy, R.C.; Uhlson, C.; Newcomer, M.E. Membrane-dependent Activities of Human 15-LOX-2 and Its Murine Counterpart: Implications for Murine Models of Atherosclerosis. J. Biol. Chem. 2016, 291, 19413–19424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisch, F.; Kakularam, K.R.; Stehling, S.; Heydeck, D.; Kuhn, H. Eicosanoid biosynthesis in marine mammals. FEBS J. 2021, 288, 1387–1406. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Kakularam, K.R.; Reisch, F.; Rothe, M.; Stehling, S.; Heydeck, D.; Puschel, G.P.; Kuhn, H. Male Knock-in Mice Expressing an Arachidonic Acid Lipoxygenase 15B (Alox15B) with Humanized Reaction Specificity Are Prematurely Growth Arrested When Aging. Biomedicines 2022, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Fan, Y.; Gu, T.; Heydeck, D.; Stehling, S.; Ivanov, I.; Yao, Y.G.; Kuhn, H. The lipoxygenase pathway of Tupaia belangeri representing Scandentia. Genomic multiplicity and functional characterization of the ALOX15 orthologs in the tree shrew. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158550. [Google Scholar] [CrossRef] [PubMed]







| Substrate | Relative Oxygenase Activity (%) | Main Product | ||
|---|---|---|---|---|
| N. furzeri | P. nyererei | S. formosus | ||
| AA | 100 | 100 | 100 | 12S-HETE |
| 5(R/S)-HETE | 209.9 | 242.6 | 393.3 | 5,12-DiHETE |
| 15(R/S)-HETE | 78.2 | 202.4 | 508.3 | 8,15-DiHETE |
| 18(S)-HEPE | 9.5 | 20.9 | 1.0 | n.i. |
| 18(R)-HEPE | 3.7 | 4.8 | 1.0 | n.i. |
| 5(S),15(S)-DiHETE | 1.0 | 1.0 | 1.0 | n.i. |
| 5(S),6(R)-DiHETE | 1.0 | 1.0 | 1.0 | n.i. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roigas, S.; Heydeck, D.; Kuhn, H. Functional Characterization of Novel Bony Fish Lipoxygenase Isoforms and Their Possible Involvement in Inflammation. Int. J. Mol. Sci. 2022, 23, 16026. https://doi.org/10.3390/ijms232416026
Roigas S, Heydeck D, Kuhn H. Functional Characterization of Novel Bony Fish Lipoxygenase Isoforms and Their Possible Involvement in Inflammation. International Journal of Molecular Sciences. 2022; 23(24):16026. https://doi.org/10.3390/ijms232416026
Chicago/Turabian StyleRoigas, Sophie, Dagmar Heydeck, and Hartmut Kuhn. 2022. "Functional Characterization of Novel Bony Fish Lipoxygenase Isoforms and Their Possible Involvement in Inflammation" International Journal of Molecular Sciences 23, no. 24: 16026. https://doi.org/10.3390/ijms232416026
APA StyleRoigas, S., Heydeck, D., & Kuhn, H. (2022). Functional Characterization of Novel Bony Fish Lipoxygenase Isoforms and Their Possible Involvement in Inflammation. International Journal of Molecular Sciences, 23(24), 16026. https://doi.org/10.3390/ijms232416026






