From Low-Grade Inflammation in Osteoarthritis to Neuropsychiatric Sequelae: A Narrative Review
Abstract
1. Introduction
1.1. Current Understanding of the Causes and Development of Osteoarthritis
1.2. Osteoarthritis and Mood Disorders
2. Body Responses to Inflammation: Tissue Mediators and Signaling to the Brain
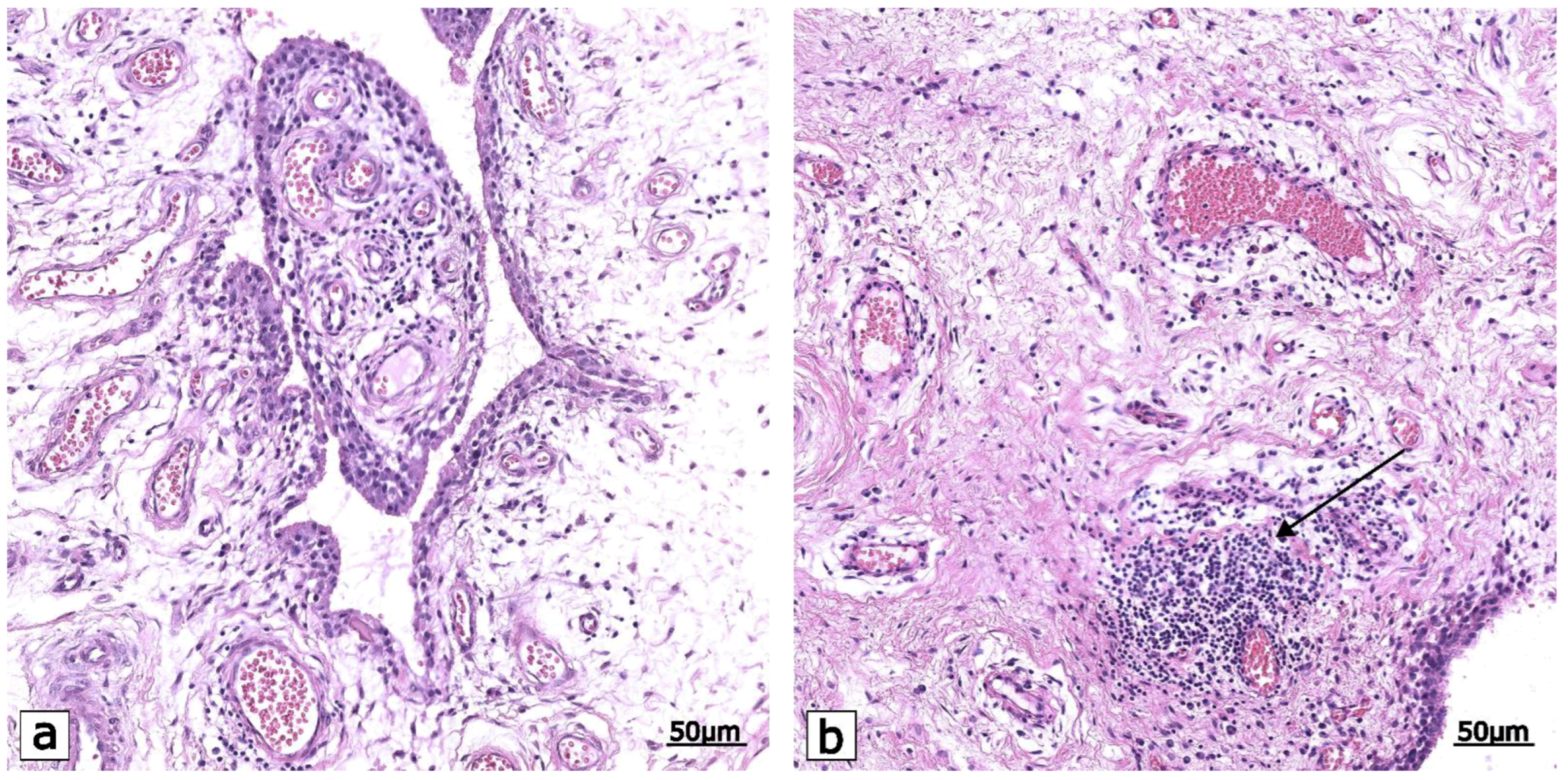
2.1. Pro-Inflammatory Mediators: Signaling in Osteoarthritis Patients
2.2. Mechanisms of Pain in Osteoarthritis
2.2.1. Peripheral Sensitization in Osteoarthritis
2.2.2. Central sensitization, the Spinal Dorsal Horn Pain Processing System
2.2.3. Central Sensitization, The Brainstem, Subcortex, and Cerebral Cortex
3. Neuroinflammation
3.1. Microglia
3.2. Astrocytes
4. Emerging Imaging Technologies Used for the Detection of CNS Alterations in Osteoarthritis
5. Neuropsychiatric Phenomena in the Context of Chronic Peripheral Inflammation
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Träger, U.; Tabrizi, S.J. Peripheral Inflammation in Neurodegeneration. J. Mol. Med. 2013, 91, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The Movers and Shapers in Immune Privilege of the CNS. Nat. Immunol. 2017, 18, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, D.; Tanaka, Y.; Hasebe, R.; Murakami, M. Bidirectional Communication between Neural and Immune Systems. Int. Immunol. 2020, 32, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Coppens, V.; Morrens, M.; Destoop, M.; Dom, G. The Interplay of Inflammatory Processes and Cognition in Alcohol Use Disorders—A Systematic Review. Front. Psychiatry 2019, 10, 632. [Google Scholar] [CrossRef]
- Lee, C.-H.; Giuliani, F. The Role of Inflammation in Depression and Fatigue. Front. Immunol. 2019, 10, 1696. [Google Scholar] [CrossRef]
- Süß, P.; Rothe, T.; Hoffmann, A.; Schlachetzki, J.C.M.; Winkler, J. The Joint-Brain Axis: Insights From Rheumatoid Arthritis on the Crosstalk Between Chronic Peripheral Inflammation and the Brain. Front. Immunol. 2020, 11, 612104. [Google Scholar] [CrossRef]
- Shin, S.Y.; Katz, P.; Wallhagen, M.; Julian, L. Cognitive Impairment in Persons with Rheumatoid Arthritis. Arthritis Care Res. 2012, 64, 1144–1150. [Google Scholar] [CrossRef]
- Meade, T.; Manolios, N.; Cumming, S.R.; Conaghan, P.G.; Katz, P. Cognitive Impairment in Rheumatoid Arthritis: A Systematic Review. Arthritis Care Res. 2018, 70, 39–52. [Google Scholar] [CrossRef]
- Vitturi, B.K.; Nascimento, B.A.C.; Alves, B.R.; de Campos, F.S.C.; Torigoe, D.Y. Cognitive Impairment in Patients with Rheumatoid Arthritis. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 69, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jones Amaowei, E.E.; Anwar, S.; Kavanoor Sridhar, K.; Shabbir, K.; Mohammed, E.H.; Bahar, A.R.; Talpur, A.S.; Bhat, S.; Zafar, S.; Qadar, L.T. Correlation of Depression and Anxiety With Rheumatoid Arthritis. Cureus 2022, 14, e23137. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Barberà, I.; Salman-Monte, T.C.; Vílchez-Oya, F.; Monfort, J. Neuropsychiatric Involvement in Systemic Lupus Erythematosus: A Review. Autoimmun. Rev. 2021, 20, 102780. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xu, Z.; Noordam, R.; van Heemst, D.; Li-Gao, R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-Sample Mendelian Randomization Study. J. Crohns Colitis 2022, 16, 633–642. [Google Scholar] [CrossRef]
- Lin, S.-S.; Verkhratsky, A. Systemic Inflammation and Neuronal Hyperexcitability: Deciphering Cellular Neuropathology of Sickness Behaviour. Brain. Behav. Immun. 2021, 97, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Peshkova, M.; Lychagin, A.; Lipina, M.; Di Matteo, B.; Anzillotti, G.; Ronzoni, F.; Kosheleva, N.; Shpichka, A.; Royuk, V.; Fomin, V.; et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022, 23, 2767. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis. Med. Clin. North Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Neogi, T.; Zhang, Y. Epidemiology of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Jackson, J.; Iyer, R.; Mellor, J.; Wei, W. The Burden of Pain Associated with Osteoarthritis in the Hip or Knee from the Patient’s Perspective: A Multinational Cross-Sectional Study. Adv. Ther. 2020, 37, 3985–3999. [Google Scholar] [CrossRef]
- Vitaloni, M.; Botto-van Bemden, A.; Sciortino Contreras, R.M.; Scotton, D.; Bibas, M.; Quintero, M.; Monfort, J.; Carné, X.; de Abajo, F.; Oswald, E.; et al. Global Management of Patients with Knee Osteoarthritis Begins with Quality of Life Assessment: A Systematic Review. BMC Musculoskelet. Disord. 2019, 20, 493. [Google Scholar] [CrossRef]
- Burgos-Vargas, R.; Aggarwal, J.; Johnson, K.D.; Ramey, D.; Lozano, F.; Macahilig, C.; Doshi, I.; Tunceli, K. Results from a Cross-Sectional, Observational Study to Assess Inadequate Pain Relief in Patients with Knee and/or Hip Osteoarthritis in Mexico. Reumatol. Clínica Engl. Ed. 2021, 17, 397–403. [Google Scholar] [CrossRef]
- Hiligsmann, M.; Cooper, C.; Guillemin, F.; Hochberg, M.C.; Tugwell, P.; Arden, N.; Berenbaum, F.; Boers, M.; Boonen, A.; Branco, J.C.; et al. A Reference Case for Economic Evaluations in Osteoarthritis: An Expert Consensus Article from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.B.; Cisternas, M.G.; Pasta, D.J.; Helmick, C.G.; Yelin, E.H. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res. 2018, 70, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C.R. Inflammation in Joint Injury and Post-Traumatic Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory Mediators in Osteoarthritis: A Critical Review of the State-of-the-Art, Current Prospects, and Future Challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; de Girolamo, L.; Filardo, G.; Oliveira, J.M.; Orth, P.; Pape, D.; Reboul, P. Basic Science of Osteoarthritis. J. Exp. Orthop. 2016, 3, 22. [Google Scholar] [CrossRef]
- Kruisbergen, N.N.L.; Di Ceglie, I.; van Gemert, Y.; Walgreen, B.; Helsen, M.M.A.; Slöetjes, A.W.; Koenders, M.I.; van de Loo, F.A.J.; Roth, J.; Vogl, T.; et al. Nox2 Deficiency Reduces Cartilage Damage and Ectopic Bone Formation in an Experimental Model for Osteoarthritis. Antioxidants 2021, 10, 1660. [Google Scholar] [CrossRef]
- Grandi, F.C.; Baskar, R.; Smeriglio, P.; Murkherjee, S.; Indelli, P.F.; Amanatullah, D.F.; Goodman, S.; Chu, C.; Bendall, S.; Bhutani, N. Single-Cell Mass Cytometry Reveals Cross-Talk between Inflammation-Dampening and Inflammation-Amplifying Cells in Osteoarthritic Cartilage. Sci. Adv. 2020, 6, eaay5352. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an Inflammatory Disease (Osteoarthritis Is Not Osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Siebuhr, A.; Bay-Jensen, A.; Jordan, J.; Kjelgaard-Petersen, C.; Christiansen, C.; Abramson, S.; Attur, M.; Berenbaum, F.; Kraus, V.; Karsdal, M. Inflammation (or Synovitis)-Driven Osteoarthritis: An Opportunity for Personalizing Prognosis and Treatment? Scand. J. Rheumatol. 2016, 45, 87–98. [Google Scholar] [CrossRef]
- Griffin, T.M.; Scanzello, C.R. Innate Inflammation and Synovial Macrophages in Osteoarthritis Pathophysiology. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 57–63. [Google Scholar] [PubMed]
- Pearson, M.J.; Herndler-Brandstetter, D.; Tariq, M.A.; Nicholson, T.A.; Philp, A.M.; Smith, H.L.; Davis, E.T.; Jones, S.W.; Lord, J.M. IL-6 Secretion in Osteoarthritis Patients Is Mediated by Chondrocyte-Synovial Fibroblast Cross-Talk and Is Enhanced by Obesity. Sci. Rep. 2017, 7, 3451. [Google Scholar] [CrossRef] [PubMed]
- Majoska, H.M.B.; Nicoline, M.K.; Gerrit, J.; van Spil, W.E. Synovial Macrophages: Potential Key Modulators of Cartilage Damage, Osteophyte Formation and Pain in Knee Osteoarthritis. J. Rheum. Dis. Treat. 2018, 4, 059. [Google Scholar] [CrossRef][Green Version]
- Moqbel, S.A.A.; He, Y.; Xu, L.; Ma, C.; Ran, J.; Xu, K.; Wu, L. Rat Chondrocyte Inflammation and Osteoarthritis Are Ameliorated by Madecassoside. Oxid. Med. Cell. Longev. 2020, 2020, 7540197. [Google Scholar] [CrossRef]
- Groma, V.; Tarasovs, M.; Skuja, S.; Semenistaja, S.; Nora-Krukle, Z.; Svirskis, S.; Murovska, M. Inflammatory Cytokine-Producing Cells and Inflammation Markers in the Synovium of Osteoarthritis Patients Evidenced in Human Herpesvirus 7 Infection. Int. J. Mol. Sci. 2020, 21, 6004. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural Elucidation and Physiologic Functions of Specialized Pro-Resolving Mediators and Their Receptors. Mol. Aspects Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Li, Y.-P. Osteoarthritis Genetic Factors Animal Models Mechanisms and Therapies. Front. Biosci. 2012, E4, 74–100. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and Therapeutic Implications of Cellular Senescence in Osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Zhu, W.-T.; Chen, B.-W.; Chen, Y.-H.; Ni, G.-X. Bidirectional Association between Metabolic Syndrome and Osteoarthritis: A Meta-Analysis of Observational Studies. Diabetol. Metab. Syndr. 2020, 12, 38. [Google Scholar] [CrossRef]
- Marchev, A.S.; Dimitrova, P.A.; Burns, A.J.; Kostov, R.V.; Dinkova-Kostova, A.T.; Georgiev, M.I. Oxidative Stress and Chronic Inflammation in Osteoarthritis: Can NRF2 Counteract These Partners in Crime? Ann. N. Y. Acad. Sci. 2017, 1401, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, S.; Zhao, L.; Chang, X.; Han, L.; Huang, J.; Chen, D. Acute Synovitis after Trauma Precedes and Is Associated with Osteoarthritis Onset and Progression. Int. J. Biol. Sci. 2020, 16, 970–980. [Google Scholar] [CrossRef]
- Yasuda, T. Cartilage Destruction by Matrix Degradation Products. Mod. Rheumatol. 2006, 16, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The Role of Metabolism in Chondrocyte Dysfunction and the Progression of Osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Choi, M.-C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-ΚB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Inflammation in Osteoarthritis: Is It Time to Dampen the Alarm(in) in This Debilitating Disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef]
- Chou, C.-H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.-M.; Gregory, S.; Kraus, V.B. Synovial Cell Cross-Talk with Cartilage Plays a Major Role in the Pathogenesis of Osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte Dedifferentiation and Osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Li, B.; Guan, G.; Mei, L.; Jiao, K.; Li, H. Pathological Mechanism of Chondrocytes and the Surrounding Environment during Osteoarthritis of Temporomandibular Joint. J. Cell. Mol. Med. 2021, 25, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Liu, S.-C.; Su, C.-M.; Wang, Y.-H.; Tsai, C.-H.; Tang, C.-H. Implications of Angiogenesis Involvement in Arthritis. Int. J. Mol. Sci. 2018, 19, 2012. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. The Growing Array of Innate Inflammatory Ignition Switches in Osteoarthritis. Arthritis Rheum. 2012, 64, 2055–2058. [Google Scholar] [CrossRef] [PubMed]
- van Lent, P.L.E.M.; Blom, A.B.; Schelbergen, R.F.P.; Slöetjes, A.; Lafeber, F.P.J.G.; Lems, W.F.; Cats, H.; Vogl, T.; Roth, J.; van den Berg, W.B. Active Involvement of Alarmins S100A8 and S100A9 in the Regulation of Synovial Activation and Joint Destruction during Mouse and Human Osteoarthritis. Arthritis Rheum. 2012, 64, 1466–1476. [Google Scholar] [CrossRef]
- Agarwal, P.; Sambamoorthi, U. Healthcare Expenditures Associated with Depression Among Individuals with Osteoarthritis: Post-Regression Linear Decomposition Approach. J. Gen. Intern. Med. 2015, 30, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, L.; Cicuttini, F.; Zhu, Z.; Han, W.; Antony, B.; Wluka, A.E.; Winzenberg, T.; Aitken, D.; Blizzard, L.; et al. Depression in Patients with Knee Osteoarthritis: Risk Factors and Associations with Joint Symptoms. BMC Musculoskelet. Disord. 2021, 22, 40. [Google Scholar] [CrossRef]
- Kye, S.-Y.; Park, K. Suicidal Ideation and Suicidal Attempts among Adults with Chronic Diseases: A Cross-Sectional Study. Compr. Psychiatry 2017, 73, 160–167. [Google Scholar] [CrossRef]
- Stubbs, B.; Aluko, Y.; Myint, P.K.; Smith, T.O. Prevalence of Depressive Symptoms and Anxiety in Osteoarthritis: A Systematic Review and Meta-Analysis. Age Ageing 2016, 45, 228–235. [Google Scholar] [CrossRef]
- Weber, A.; Mak, S.H.; Berenbaum, F.; Sellam, J.; Zheng, Y.-P.; Han, Y.; Wen, C. Association between Osteoarthritis and Increased Risk of Dementia: A Systemic Review and Meta-Analysis. Medicine (Baltimore) 2019, 98, e14355. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.-M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local Clearance of Senescent Cells Attenuates the Development of Post-Traumatic Osteoarthritis and Creates a pro-Regenerative Environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of Senescent Glial Cells Prevents Tau-Dependent Pathology and Cognitive Decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef]
- Sun, K.; Jing, X.; Guo, J.; Yao, X.; Guo, F. Mitophagy in Degenerative Joint Diseases. Autophagy 2021, 17, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Humo, M.; Lu, H.; Yalcin, I. The Molecular Neurobiology of Chronic Pain–Induced Depression. Cell Tissue Res 2019, 377, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.-H.; Jang, F.-L.; Ho, C.-H.; Chen, Y.-C.; Hsu, C.-C.; Lin, H.-J.; Wang, J.-J.; Huang, C.-C. Chronic Pain Increases the Risk of Dementia: A Nationwide Population-Based Cohort Study. Pain Physician 2021, 24, E849–E856. [Google Scholar] [PubMed]
- Ciaramella, A. Mood Spectrum Disorders and Perception of Pain. Psychiatr. Q. 2017, 88, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Tabarean, I. Central Thermoreceptors. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 121–127. ISBN 978-0-444-63912-7. [Google Scholar]
- Dinarello, C.A. The History of Fever, Leukocytic Pyrogen and Interleukin-1. Temperature 2015, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.E.; Cranston, W.I.; Honour, A.J. Observations on the Site and Mode of Action of Pyrogens in the Rabbit Brain. J. Physiol. 1967, 191, 325–337. [Google Scholar] [CrossRef]
- Flower, R.J.; Vane, J.R. Inhibition of Prostaglandin Synthetase in Brain Explains the Anti-Pyretic Activity of Paracetamol (4-Acetamidophenol). Nature 1972, 240, 410–411. [Google Scholar] [CrossRef]
- Flower, R.; Gryglewski, R.; Herbaczńska-Cedro, K.; Vane, J.R. Effects of Anti-Inflammatory Drugs on Prostaglandin Biosynthesis. Nature. New Biol. 1972, 238, 104–106. [Google Scholar] [CrossRef]
- Matsumura, K. Signaling the Brain in Inflammation: The Role of Endothelial Cells. Front. Biosci. 2004, 9, 2819. [Google Scholar] [CrossRef]
- Eskilsson, A.; Shionoya, K.; Engblom, D.; Blomqvist, A. Fever During Localized Inflammation in Mice Is Elicited by a Humoral Pathway and Depends on Brain Endothelial Interleukin-1 and Interleukin-6 Signaling and Central EP3 Receptors. J. Neurosci. 2021, 41, 5206–5218. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Simons, C.T.; Kulchitsky, V.A. “Biphasic” Fevers Often Consist of More than Two Phases. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1998, 275, R323–R331. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Blatteis, C.M. Biphasic Fever: What Triggers the Second Temperature Rise? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 269, R280–R286. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Central Circuitries for Body Temperature Regulation and Fever. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef] [PubMed]
- Murayama, S.; Kurganov, E.; Miyata, S. Activation of Microglia and Macrophages in the Circumventricular Organs of the Mouse Brain during TLR2-Induced Fever and Sickness Responses. J. Neuroimmunol. 2019, 334, 576973. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.A.; De Felice, F.G. The Crosstalk between Brain and Periphery: Implications for Brain Health and Disease. Neuropharmacology 2021, 197, 108728. [Google Scholar] [CrossRef]
- Steiner, A.A.; Ivanov, A.I.; Serrats, J.; Hosokawa, H.; Phayre, A.N.; Robbins, J.R.; Roberts, J.L.; Kobayashi, S.; Matsumura, K.; Sawchenko, P.E.; et al. Cellular and Molecular Bases of the Initiation of Fever. PLoS Biol. 2006, 4, e284. [Google Scholar] [CrossRef]
- Kis, B.; Isse, T.; Snipes, J.A.; Chen, L.; Yamashita, H.; Ueta, Y.; Busija, D.W. Effects of LPS Stimulation on the Expression of Prostaglandin Carriers in the Cells of the Blood-Brain and Blood-Cerebrospinal Fluid Barriers. J. Appl. Physiol. 2006, 100, 1392–1399. [Google Scholar] [CrossRef]
- Luheshi, G.N.; Bluthé, R.-M.; Rushforth, D.; Mulcahy, N.; Konsman, J.-P.; Goldbach, M.; Dantzer, R. Vagotomy Attenuates the Behavioural but Not the Pyrogenic Effects of Interleukin-1 in Rats. Auton. Neurosci. 2000, 85, 127–132. [Google Scholar] [CrossRef]
- Bluthé, R.M.; Walter, V.; Parnet, P.; Layé, S.; Lestage, J.; Verrier, D.; Poole, S.; Stenning, B.E.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide Induces Sickness Behaviour in Rats by a Vagal Mediated Mechanism. C. R. Acad. Sci. III 1994, 317, 499–503. [Google Scholar]
- Hansen, M.K.; Krueger, J.M. Subdiaphragmatic Vagotomy Blocks the Sleepand Fever-Promoting Effects of Interleukin-1β. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R1246–R1253. [Google Scholar] [CrossRef]
- Gaykema, R.P.A.; Goehler, L.E.; Hansen, M.K.; Maier, S.F.; Watkins, L.R. Subdiaphragmatic Vagotomy Blocks Interleukin-1β-Induced Fever but Does Not Reduce IL-1β Levels in the Circulation. Auton. Neurosci. 2000, 85, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Dunn, A.J. Effect of Subdiaphragmatic Vagotomy on the Noradrenergic and HPA Axis Activation Induced by Intraperitoneal Interleukin-1 Administration in Rats. Brain Res. 2006, 1101, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.K.; Daniels, S.; Goehler, L.E.; Gaykema, R.P.A.; Maier, S.F.; Watkins, L.R. Subdiaphragmatic Vagotomy Does Not Block Intraperitoneal Lipopolysaccharide-Induced Fever. Auton. Neurosci. 2000, 85, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Zanos, T.P.; Silverman, H.A.; Levy, T.; Tsaava, T.; Battinelli, E.; Lorraine, P.W.; Ashe, J.M.; Chavan, S.S.; Tracey, K.J.; Bouton, C.E. Identification of Cytokine-Specific Sensory Neural Signals by Decoding Murine Vagus Nerve Activity. Proc. Natl. Acad. Sci. USA 2018, 115, E4843–E4852. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.A.; Hansen, M.K.; Anderson, K.; Maier, S.F.; Watkins, L.R. Vagal Immune-to-Brain Communication: A Visceral Chemosensory Pathway. Auton. Neurosci. 2000, 85, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kollarik, M.; Ru, F.; Sun, H.; McNeil, B.; Dong, X.; Stephens, G.; Korolevich, S.; Brohawn, P.; Kolbeck, R.; et al. Distinct and Common Expression of Receptors for Inflammatory Mediators in Vagal Nodose versus Jugular Capsaicin-Sensitive/TRPV1-Positive Neurons Detected by Low Input RNA Sequencing. PLoS ONE 2017, 12, e0185985. [Google Scholar] [CrossRef]
- Roth, J.; Blatteis, C.M. Mechanisms of Fever Production and Lysis: Lessons from Experimental LPS Fever. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: New York, NY, USA, 2014; pp. 1563–1604. ISBN 978-0-470-65071-4. [Google Scholar]
- Aguiar, G.C.; Do Nascimento, M.R.; De Miranda, A.S.; Rocha, N.P.; Teixeira, A.L.; Scalzo, P.L. Effects of an Exercise Therapy Protocol on Inflammatory Markers, Perception of Pain, and Physical Performance in Individuals with Knee Osteoarthritis. Rheumatol. Int. 2015, 35, 525–531. [Google Scholar] [CrossRef]
- Loukov, D.; Karampatos, S.; Maly, M.R.; Bowdish, D.M.E. Monocyte Activation Is Elevated in Women with Knee-Osteoarthritis and Associated with Inflammation, BMI and Pain. Osteoarthr. Cartil. 2018, 26, 255–263. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Z.; Wang, M.; Guo, S.; Cong, H.; Liu, L. Analysis on the Expression and Value of CCL2 and CCL3 in Patients with Osteoarthritis. Exp. Mol. Pathol. 2021, 118, 104576. [Google Scholar] [CrossRef]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Aguirre, D.; Trawick, R.H.; Rasmussen, G.L.; Momberger, N.G. Serum Cytokines Are Increased and Circulating Micronutrients Are Not Altered in Subjects with Early Compared to Advanced Knee Osteoarthritis. Cytokine 2014, 68, 133–136. [Google Scholar] [CrossRef]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Dixon, B.M.; Momberger, N.G.; Rasmussen, G.L.; Trawick, R.H. Muscular-Based and Patient-Reported Outcomes Differentially Associate with Circulating Superoxide Dismutases and Cytokines in Knee Osteoarthritis. Cytokine 2019, 115, 45–49. [Google Scholar] [CrossRef]
- Attur, M.; Krasnokutsky, S.; Statnikov, A.; Samuels, J.; Li, Z.; Friese, O.; Hellio Le Graverand-Gastineau, M.-P.; Rybak, L.; Kraus, V.B.; Jordan, J.M.; et al. Low-Grade Inflammation in Symptomatic Knee Osteoarthritis: Prognostic Value of Inflammatory Plasma Lipids and Peripheral Blood Leukocyte Biomarkers. Arthritis Rheumatol. 2015, 67, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β Signaling in Osteoarthritis—Chondrocytes in Focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-Inflammatory Cytokines: The Link between Obesity and Osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, B.; Christin, M.; Tessandier, N.; Sotoudeh, C.; Bretheau, F.; Turmel, R.; Pellerin, È.; Wang, F.; Bories, C.; Joly-Beauparlant, C.; et al. Neuronal Interleukin-1 Receptors Mediate Pain in Chronic Inflammatory Diseases. J. Exp. Med. 2020, 217, e20191430. [Google Scholar] [CrossRef]
- Bevilaqua-Grossi, D.; Zanin, M.; Benedetti, C.; Florencio, L.; Oliveira, A. Thermal and Mechanical Pain Sensitization in Patients with Osteoarthritis of the Knee. Physiother. Theory Pract. 2019, 35, 139–147. [Google Scholar] [CrossRef]
- Attur, M.; Belitskaya-Lévy, I.; Oh, C.; Krasnokutsky, S.; Greenberg, J.; Samuels, J.; Smiles, S.; Lee, S.; Patel, J.; Al-Mussawir, H.; et al. Increased Interleukin-1β Gene Expression in Peripheral Blood Leukocytes Is Associated with Increased Pain and Predicts Risk for Progression of Symptomatic Knee Osteoarthritis. Arthritis Rheum. 2011, 63, 1908–1917. [Google Scholar] [CrossRef]
- Rosenberg, J.H.; Rai, V.; Dilisio, M.F.; Agrawal, D.K. Damage-Associated Molecular Patterns in the Pathogenesis of Osteoarthritis: Potentially Novel Therapeutic Targets. Mol. Cell. Biochem. 2017, 434, 171–179. [Google Scholar] [CrossRef]
- Ponchel, F.; Burska, A.N.; Hensor, E.M.A.; Raja, R.; Campbell, M.; Emery, P.; Conaghan, P.G. Changes in Peripheral Blood Immune Cell Composition in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1870–1878. [Google Scholar] [CrossRef]
- Al-Khazraji, B.K.; Appleton, C.T.; Beier, F.; Birmingham, T.B.; Shoemaker, J.K. Osteoarthritis, Cerebrovascular Dysfunction and the Common Denominator of Inflammation: A Narrative Review. Osteoarthr. Cartil. 2018, 26, 462–470. [Google Scholar] [CrossRef]
- Maly, M.R.; Cott, C.A. Being Careful: A Grounded Theory of Emergent Chronic Knee Problems. Arthritis Rheum. 2009, 61, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Stewart, L.; French, M.R.; Cibere, J.; Jordan, J.M.; March, L.; Suarez-Almazor, M.; Gooberman-Hill, R. Understanding the Pain Experience in Hip and Knee Osteoarthritis–An OARSI/OMERACT Initiative. Osteoarthr. Cartil. 2008, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L. Peripheral Pain Mechanisms in Osteoarthritis. Pain 2020, 161, S138–S146. [Google Scholar] [CrossRef] [PubMed]
- Mapp, P.I. Innervation of the Synovium. Ann. Rheum. Dis. 1995, 54, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Macefield, V.G. Physiological Characteristics of Low-Threshold Mechanoreceptors in Joints, Muscle and Skin in Human Subjects. Clin. Exp. Pharmacol. Physiol. 2005, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Tran, P.B.; Obeidat, A.M.; Raghu, P.; Ishihara, S.; Miller, R.J.; Malfait, A.-M. The Role of Peripheral Nociceptive Neurons in the Pathophysiology of Osteoarthritis Pain. Curr. Osteoporos. Rep. 2015, 13, 318–326. [Google Scholar] [CrossRef]
- Miyamoto, S.; Iida, S.; Miyashita, T.; Katou, K.; Kawarai, Y.; Nakamura, J.; Orita, S.; Ohtori, S. Mechanism of Chronic Pain of Symptomatic Hip Osteoarthritis by Association of Its Distribution, Nociceptive, Neuropathic, Nociplastic, or Mixed-Pain Screening, and the Prevalence of Lumbar Spinal Stenosis: A Cross-Sectional Study. Clin. J. Pain 2022, 38, 77–87. [Google Scholar] [CrossRef]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef]
- Woolf, C.J.; Ma, Q. Nociceptors—Noxious Stimulus Detectors. Neuron 2007, 55, 353–364. [Google Scholar] [CrossRef]
- Syx, D.; Tran, P.B.; Miller, R.E.; Malfait, A.-M. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr. Rheumatol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Liu, T.; Chen, G.; Wang, B.; Yu, X.-L.; Yin, C.; Ji, R.-R. TLR Signaling Adaptor Protein MyD88 in Primary Sensory Neurons Contributes to Persistent Inflammatory and Neuropathic Pain and Neuroinflammation. Sci. Rep. 2016, 6, 28188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Boyette-Davis, J.A.; Kosturakis, A.K.; Li, Y.; Yoon, S.-Y.; Walters, E.T.; Dougherty, P.M. Induction of Monocyte Chemoattractant Protein-1 (MCP-1) and Its Receptor CCR2 in Primary Sensory Neurons Contributes to Paclitaxel-Induced Peripheral Neuropathy. J. Pain 2013, 14, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Argoff, C.E.; Bennett, G.J.; Cummins, T.R.; Durieux, M.E.; Gerner, P.; Gold, M.S.; Porreca, F.; Strichartz, G.R. The Role of Sodium Channels in Chronic Inflammatory and Neuropathic Pain. J. Pain 2006, 7, S1–S29. [Google Scholar] [CrossRef] [PubMed]
- Nees, T.A.; Rosshirt, N.; Reiner, T.; Schiltenwolf, M.; Moradi, B. Die Rolle der Inflammation bei Arthroseschmerzen. Schmerz 2019, 33, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain Mechanisms: A New Theory: A Gate Control System Modulates Sensory Input from the Skin before It Evokes Pain Perception and Response. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of Spinal Circuits Transmitting and Gating Mechanical Pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zhang, Y.-Q.; Goulding, M.; Wang, Y.-Q.; Ma, Q. Timing Mechanisms Underlying Gate Control by Feedforward Inhibition. Neuron 2018, 99, 941–955.e4. [Google Scholar] [CrossRef]
- Alba-Delgado, C.; Mountadem, S.; Mermet-Joret, N.; Monconduit, L.; Dallel, R.; Artola, A.; Antri, M. 5-HT2A Receptor-Induced Morphological Reorganization of PKCγ-Expressing Interneurons Gates Inflammatory Mechanical Allodynia in Rat. J. Neurosci. 2018, 38, 10489–10504. [Google Scholar] [CrossRef]
- Koch, S.C.; Acton, D.; Goulding, M. Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol. 2018, 80, 189–217. [Google Scholar] [CrossRef]
- Laird, J.M.; Bennett, G.J. An Electrophysiological Study of Dorsal Horn Neurons in the Spinal Cord of Rats with an Experimental Peripheral Neuropathy. J. Neurophysiol. 1993, 69, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Ratté, S.; Prescott, S.A. Excitatory Neurons Are More Disinhibited than Inhibitory Neurons by Chloride Dysregulation in the Spinal Dorsal Horn. eLife 2019, 8, e49753. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Peng, J.; Xu, Y.-N.; Zeng, W.-J.; Zhang, J.; Wei, X.; Mai, C.-L.; Lin, Z.-J.; Liu, Y.; Murugan, M.; et al. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. 2019, 27, 3844–3859. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.-J.; Wang, J.; Li, D.; Ren, W.-J.; Peng, J.; Wei, X.; Xu, T.; Xin, W.-J.; Pang, R.-P.; et al. TNF-α Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury. J. Neurosci. 2017, 37, 871–881. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, X.; Shi, H.; Tian, J.; Sun, L.; Hu, X.; Cui, W.; Du, D. P2Y12 Regulates Microglia Activation and Excitatory Synaptic Transmission in Spinal Lamina II Neurons during Neuropathic Pain in Rodents. Cell Death Dis. 2019, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.A.; Rancic, V.; Whitlock, K.H.; Simmons, D.; Longo, F.M.; Ballanyi, K.; Smith, P.A. Receptor Dependence of BDNF Actions in Superficial Dorsal Horn: Relation to Central Sensitization and Actions of Macrophage Colony Stimulating Factor 1. J. Neurophysiol. 2019, 121, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Monif, M.; Reid, C.A.; Powell, K.L.; Drummond, K.J.; O’Brien, T.J.; Williams, D.A. Interleukin-1β Has Trophic Effects in Microglia and Its Release Is Mediated by P2X7R Pore. J. Neuroinflamm. 2016, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, X.; Huang, J.; Wang, Z.; Li, Z.; Liu, Z. The Actions and Mechanisms of P2X7R and P38 MAPK Activation in Mediating Bortezomib-Induced Neuropathic Pain. BioMed Res. Int. 2020, 2020, 8143754. [Google Scholar] [CrossRef]
- Yamashita, T.; Kamikaseda, S.; Tanaka, A.; Tozaki-Saitoh, H.; Caaveiro, J.M.M.; Inoue, K.; Tsuda, M. New Inhibitory Effects of Cilnidipine on Microglial P2X7 Receptors and IL-1β Release: An Involvement in Its Alleviating Effect on Neuropathic Pain. Cells 2021, 10, 434. [Google Scholar] [CrossRef]
- Wu, Q.; Yue, J.; Lin, L.; Yu, X.; Zhou, Y.; Ying, X.; Chen, X.; Tu, W.; Lou, X.; Yang, G.; et al. Electroacupuncture May Alleviate Neuropathic Pain via Suppressing P2X7R Expression. Mol. Pain 2021, 17, 174480692199765. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, S.; Li, Y.; Randall, A.; Kremer, E.J.; Pickering, A.E. Functional Dichotomy in Spinal- vs Prefrontal-Projecting Locus Coeruleus Modules Splits Descending Noradrenergic Analgesia from Ascending Aversion and Anxiety in Rats. eLife 2017, 6, e29808. [Google Scholar] [CrossRef] [PubMed]
- Obata, H. Analgesic Mechanisms of Antidepressants for Neuropathic Pain. Int. J. Mol. Sci. 2017, 18, 2483. [Google Scholar] [CrossRef] [PubMed]
- George, D.T.; Ameli, R.; Koob, G.F. Periaqueductal Gray Sheds Light on Dark Areas of Psychopathology. Trends Neurosci. 2019, 42, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Singh, P. Neuroanatomy, Periaqueductal Gray. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Crawford, L.S.; Mills, E.P.; Hanson, T.; Macey, P.M.; Glarin, R.; Macefield, V.G.; Keay, K.A.; Henderson, L.A. Brainstem Mechanisms of Pain Modulation: A within-Subjects 7T FMRI Study of Placebo Analgesic and Nocebo Hyperalgesic Responses. J. Neurosci. 2021, 41, 9794–9806. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.F.; Pei, J.; Zickella, M.; Rey, C.; Zickella, J.; Ramirez, A.; Taylor, N.E. D2 Receptors in the Periaqueductal Gray/Dorsal Raphe Modulate Peripheral Inflammatory Hyperalgesia via the Rostral Ventral Medulla. Neuroscience 2021, 463, 159–173. [Google Scholar] [CrossRef]
- Follansbee, T.; Akiyama, T.; Fujii, M.; Davoodi, A.; Nagamine, M.; Iodi Carstens, M.; Carstens, E. Effects of Pruritogens and Algogens on Rostral Ventromedial Medullary ON and OFF Cells. J. Neurophysiol. 2018, 120, 2156–2163. [Google Scholar] [CrossRef]
- Chen, Q.; Heinricher, M.M. Plasticity in the Link between Pain-Transmitting and Pain-Modulating Systems in Acute and Persistent Inflammation. J. Neurosci. 2019, 39, 2065–2079. [Google Scholar] [CrossRef]
- Suárez-Pereira, I.; Llorca-Torralba, M.; Bravo, L.; Camarena-Delgado, C.; Soriano-Mas, C.; Berrocoso, E. The Role of the Locus Coeruleus in Pain and Associated Stress-Related Disorders. Biol. Psychiatry 2022, 91, 786–797. [Google Scholar] [CrossRef]
- Hayashida, K.; Obata, H. Strategies to Treat Chronic Pain and Strengthen Impaired Descending Noradrenergic Inhibitory System. Int. J. Mol. Sci. 2019, 20, 822. [Google Scholar] [CrossRef]
- Benarroch, E.E. Locus Coeruleus. Cell Tissue Res. 2018, 373, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.S.; de Souza, G.R.; Kalil-Cutti, B.; Giusti-Paiva, A.; Vilela, F.C. Post-Traumatic Stress Disorder Increases Pain Sensitivity by Reducing Descending Noradrenergic and Serotoninergic Modulation. Behav. Brain Res. 2021, 411, 113367. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.S.; McCall, J.G.; Charney, D.S.; Murrough, J.W. The Role of the Locus Coeruleus in the Generation of Pathological Anxiety. Brain Neurosci. Adv. 2020, 4, 239821282093032. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. Brain Mechanisms of Insomnia: New Perspectives on Causes and Consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Bastuji, H. Pain and Consciousness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 193–199. [Google Scholar] [CrossRef]
- Lee, I.-S.; Necka, E.A.; Atlas, L.Y. Distinguishing Pain from Nociception, Salience, and Arousal: How Autonomic Nervous System Activity Can Improve Neuroimaging Tests of Specificity. NeuroImage 2020, 204, 116254. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
- Isenburg, K.; Mawla, I.; Loggia, M.L.; Ellingsen, D.-M.; Protsenko, E.; Kowalski, M.H.; Swensen, D.; O’Dwyer-Swensen, D.; Edwards, R.R.; Napadow, V.; et al. Increased Salience Network Connectivity Following Manual Therapy Is Associated with Reduced Pain in Chronic Low Back Pain Patients. J. Pain 2021, 22, 545–555. [Google Scholar] [CrossRef]
- Bishop, J.H.; Shpaner, M.; Kubicki, A.; Clements, S.; Watts, R.; Naylor, M.R. Structural Network Differences in Chronic Muskuloskeletal Pain: Beyond Fractional Anisotropy. NeuroImage 2018, 182, 441–455. [Google Scholar] [CrossRef]
- Ho, T.C.; Walker, J.C.; Teresi, G.I.; Kulla, A.; Kirshenbaum, J.S.; Gifuni, A.J.; Singh, M.K.; Gotlib, I.H. Default Mode and Salience Network Alterations in Suicidal and Non-Suicidal Self-Injurious Thoughts and Behaviors in Adolescents with Depression. Transl. Psychiatry 2021, 11, 38. [Google Scholar] [CrossRef]
- Pimontel, M.A.; Solomonov, N.; Oberlin, L.; Kanellopoulos, T.; Bress, J.N.; Hoptman, M.J.; Alexopoulos, G.S.; Gunning, F.M. Cortical Thickness of the Salience Network and Change in Apathy Following Antidepressant Treatment for Late-Life Depression. Am. J. Geriatr. Psychiatry 2021, 29, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Guo, R.-J.; Shi, H.-W. Altered Default Mode Network and Salience Network Functional Connectivity in Patients with Generalized Anxiety Disorders: An ICA-Based Resting-State FMRI Study. Evid. Based Complement. Alternat. Med. 2020, 2020, 4048916. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, W.; Chen, S.; Hu, G.; Ge, H.; Xue, C.; Qi, W.; Lin, X.; Chen, J. Functional MRI-Specific Alterations in Salience Network in Mild Cognitive Impairment: An ALE Meta-Analysis. Front. Aging Neurosci. 2021, 13, 695210. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Nana, A.L.; Toller, G.; Brown, J.A.; Deng, J.; Staffaroni, A.; Kim, E.-J.; Hwang, J.-H.L.; Li, L.; Park, Y.; et al. Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia. Cereb. Cortex 2020, 30, 5387–5399. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.-H.; Wang, J.-Y.; Luo, F. Current Understanding of the Involvement of the Insular Cortex in Neuropathic Pain: A Narrative Review. Int. J. Mol. Sci. 2021, 22, 2648. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, X.; Qiu, J.; Fu, X.; Niu, C. Gray and White Matter Abnormalities in Primary Trigeminal Neuralgia with and without Neurovascular Compression. J. Headache Pain 2020, 21, 136. [Google Scholar] [CrossRef]
- Liao, X.; Mao, C.; Wang, Y.; Zhang, Q.; Cao, D.; Seminowicz, D.A.; Zhang, M.; Yang, X. Brain Gray Matter Alterations in Chinese Patients with Chronic Knee Osteoarthritis Pain Based on Voxel-Based Morphometry. Medicine 2018, 97, e0145. [Google Scholar] [CrossRef]
- Kang, D.; McAuley, J.H.; Kassem, M.S.; Gatt, J.M.; Gustin, S.M. What Does the Grey Matter Decrease in the Medial Prefrontal Cortex Reflect in People with Chronic Pain? Eur. J. Pain 2019, 23, 203–219. [Google Scholar] [CrossRef]
- Fiore, N.T.; Austin, P.J. Peripheral Nerve Injury Triggers Neuroinflammation in the Medial Prefrontal Cortex and Ventral Hippocampus in a Subgroup of Rats with Coincident Affective Behavioural Changes. Neuroscience 2019, 416, 147–167. [Google Scholar] [CrossRef]
- IsHak, W.W.; Wen, R.Y.; Naghdechi, L.; Vanle, B.; Dang, J.; Knosp, M.; Dascal, J.; Marcia, L.; Gohar, Y.; Eskander, L.; et al. Pain and Depression: A Systematic Review. Harv. Rev. Psychiatry 2018, 26, 352–363. [Google Scholar] [CrossRef]
- Medeiros, P.; dos Santos, I.R.; Júnior, I.M.; Palazzo, E.; da Silva, J.A.; Machado, H.R.; Ferreira, S.H.; Maione, S.; Coimbra, N.C.; de Freitas, R.L. An Adapted Chronic Constriction Injury of the Sciatic Nerve Produces Sensory, Affective, and Cognitive Impairments: A Peripheral Mononeuropathy Model for the Study of Comorbid Neuropsychiatric Disorders Associated with Neuropathic Pain in Rats. Pain Med. 2021, 22, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Serafini, R.A.; Pryce, K.D.; Zachariou, V. The Mesolimbic Dopamine System in Chronic Pain and Associated Affective Comorbidities. Biol. Psychiatry 2020, 87, 64–73. [Google Scholar] [CrossRef]
- Taylor, A.M.W.; Castonguay, A.; Taylor, A.J.; Murphy, N.P.; Ghogha, A.; Cook, C.; Xue, L.; Olmstead, M.C.; De Koninck, Y.; Evans, C.J.; et al. Microglia Disrupt Mesolimbic Reward Circuitry in Chronic Pain. J. Neurosci. 2015, 35, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.-D.; Yao, M.; Huang, B.; Xu, L.-S.; Zheng, Y.; Chu, Y.-X.; Wang, H.-Q.; Liu, M.-J.; Xu, S.-J.; Li, H.-B. Glial Activation in the Periaqueductal Gray Promotes Descending Facilitation of Neuropathic Pain through the P38 MAPK Signaling Pathway: Glial Activation, Neuropathic Pain, and the P38 MAPK Signaling Pathway. J. Neurosci. Res. 2016, 94, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Kume, K.; Ohsawa, M. Role of Microglia in Mechanical Allodynia in the Anterior Cingulate Cortex. J. Pharmacol. Sci. 2017, 134, 158–165. [Google Scholar] [CrossRef]
- Taylor, A.M.W.; Mehrabani, S.; Liu, S.; Taylor, A.J.; Cahill, C.M. Topography of Microglial Activation in Sensory- and Affect-related Brain Regions in Chronic Pain. J. Neurosci. Res. 2017, 95, 1330–1335. [Google Scholar] [CrossRef]
- López-Ruiz, M.; Losilla, J.M.; Monfort, J.; Portell, M.; Gutiérrez, T.; Poca, V.; Garcia-Fructuoso, F.; Llorente, J.; Garcia-Fontanals, A.; Deus, J. Central Sensitization in Knee Osteoarthritis and Fibromyalgia: Beyond Depression and Anxiety. PLoS ONE 2019, 14, e0225836. [Google Scholar] [CrossRef]
- Willett, M.J.; Siebertz, M.; Petzke, F.; Erlenwein, J.; Rushton, A.; Soldini, E.; Barbero, M.; Falla, D. The Extent of Pain Is Associated With Signs of Central Sensitization in Patients With Hip Osteoarthritis. Pain Pract. 2020, 20, 277–288. [Google Scholar] [CrossRef]
- Soni, A.; Wanigasekera, V.; Mezue, M.; Cooper, C.; Javaid, M.K.; Price, A.J.; Tracey, I. Central Sensitization in Knee Osteoarthritis: Relating Presurgical Brainstem Neuroimaging and Pain DETECT -Based Patient Stratification to Arthroplasty Outcome. Arthritis Rheumatol. 2019, 71, 550–560. [Google Scholar] [CrossRef]
- Ohashi, Y.; Fukushima, K.; Inoue, G.; Uchida, K.; Koyama, T.; Tsuchiya, M.; Uchiyama, K.; Takahira, N.; Takaso, M. Central Sensitization Inventory Scores Correlate with Pain at Rest in Patients with Hip Osteoarthritis: A Retrospective Study. BMC Musculoskelet. Disord. 2020, 21, 595. [Google Scholar] [CrossRef]
- Railton, P.; Delaney, A.J.; Goodyear, B.G.; Matyas, J.; Lama, S.; Sutherland, G.R.; Powell, J.N. Altered Activity of Pain Processing Brain Regions in Association with Hip Osteoarthritis. Sci. Rep. 2022, 12, 2791. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, W.; Wang, L.; Ye, Y.; Li, T. Transcranial Direct Current Stimulation Alleviates the Chronic Pain of Osteoarthritis by Modulating NMDA Receptors in Midbrain Periaqueductal Gray in Rats. J. Pain Res. 2022, 15, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, Y.; Wang, L.; Zhou, W.; Chu, X.; Li, T. Botulinum Toxin Type a Combined with Transcranial Direct Current Stimulation Reverses the Chronic Pain Induced by Osteoarthritis in Rats. Toxicon 2022, 212, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-C.; Zhu, B.; Jing, X.-H.; Xiong, L.-Z.; Wu, C.-H.; Gao, F.; Li, H.-P.; Xiang, H.-C.; Zhu, H.; Zhou, B.; et al. Electroacupuncture Potentiates Cannabinoid Receptor-Mediated Descending Inhibitory Control in a Mouse Model of Knee Osteoarthritis. Front. Mol. Neurosci. 2018, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, H.; Cai, Y.; Zhou, Z. Pharmacological Use of Gamma-Aminobutyric Acid Derivatives in Osteoarthritis Pain Management: A Systematic Review. BMC Rheumatol. 2022, 6, 28. [Google Scholar] [CrossRef]
- Russell, M.D.; Barrick, T.R.; Howe, F.A.; Sofat, N. Reduced Anterior Cingulate Grey Matter Volume in Painful Hand Osteoarthritis. Rheumatol. Int. 2018, 38, 1429–1435. [Google Scholar] [CrossRef]
- Ushio, K.; Nakanishi, K.; Mikami, Y.; Yoshino, A.; Takamura, M.; Hirata, K.; Akiyama, Y.; Kimura, H.; Okamoto, Y.; Adachi, N. Altered Resting-State Connectivity with Pain-Related Expectation Regions in Female Patients with Severe Knee Osteoarthritis. J. Pain Res. 2020, 13, 3227–3234. [Google Scholar] [CrossRef]
- Cottam, W.J.; Iwabuchi, S.J.; Drabek, M.M.; Reckziegel, D.; Auer, D.P. Altered Connectivity of the Right Anterior Insula Drives the Pain Connectome Changes in Chronic Knee Osteoarthritis. Pain 2018, 159, 929–938. [Google Scholar] [CrossRef]
- Pan, T.; Pan, F.; Gao, W.; Hu, S.; Wang, D. Involvement of Macrophages and Spinal Microglia in Osteoarthritis Pain. Curr. Rheumatol. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, L.; Jiang, H.; Zhou, J.; Tang, Y. Inhibition of Interleukin-6 Function Attenuates the Central Sensitization and Pain Behavior Induced by Osteoarthritis. Eur. J. Pharmacol. 2017, 811, 260–267. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Wei, J.; Lao, L.; Shen, X. The Effects of Laser Moxibustion on Knee Osteoarthritis Pain in Rats. Photobiomodulation Photomed. Laser Surg. 2020, 38, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Mondelli, V.; Pariante, C.M. Genetic Contributions of Inflammation to Depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.G. The Emerging Immunogenetic Architecture of Schizophrenia. Schizophr. Bull. 2018, 44, 993–1004. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Jeon, S.W. Neuroinflammation and the Immune-Kynurenine Pathway in Anxiety Disorders. Curr. Neuropharmacol. 2018, 16, 574–582. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of Microglia and Astrocytes: A Roadway to Neuroinflammation and Alzheimer’s Disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Kam, T.-I.; Hinkle, J.T.; Dawson, T.M.; Dawson, V.L. Microglia and Astrocyte Dysfunction in Parkinson’s Disease. Neurobiol. Dis. 2020, 144, 105028. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Marsh, S.E.; Stevens, B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020, 41, 820–835. [Google Scholar] [CrossRef]
- Hendriks, J.J.A.; Teunissen, C.E.; de Vries, H.E.; Dijkstra, C.D. Macrophages and Neurodegeneration. Brain Res. Brain Res. Rev. 2005, 48, 185–195. [Google Scholar] [CrossRef]
- Grigoriadis, N.; Grigoriadis, S.; Polyzoidou, E.; Milonas, I.; Karussis, D. Neuroinflammation in Multiple Sclerosis: Evidence for Autoimmune Dysregulation, Not Simple Autoimmune Reaction. Clin. Neurol. Neurosurg. 2006, 108, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Serpe, C.J.; Coers, S.; Sanders, V.M.; Jones, K.J. CD4+ T, but Not CD8+ or B, Lymphocytes Mediate Facial Motoneuron Survival after Facial Nerve Transection. Brain. Behav. Immun. 2003, 17, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.; Means, T.K.; El Khoury, J. The Microglial Sensome Revealed by Direct RNA Sequencing. Nat. Neurosci. 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient Neuron-Microglia Signaling Results in Impaired Functional Brain Connectivity and Social Behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS Neurodegenerative Diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-G.; Chen, S.-D. The Changing Phenotype of Microglia from Homeostasis to Disease. Transl. Neurodegener. 2012, 1, 9. [Google Scholar] [CrossRef]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Sagar; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A New Fate Mapping System Reveals Context-Dependent Random or Clonal Expansion of Microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef]
- Caplan, H.W.; Cardenas, F.; Gudenkauf, F.; Zelnick, P.; Xue, H.; Cox, C.S.; Bedi, S.S. Spatiotemporal Distribution of Microglia After Traumatic Brain Injury in Male Mice. ASN Neuro 2020, 12, 1759091420911770. [Google Scholar] [CrossRef]
- Askew, K.; Li, K.; Olmos-Alonso, A.; Garcia-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.A.; Riecken, K.; Beccari, S.; et al. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017, 18, 391–405. [Google Scholar] [CrossRef]
- Lloyd, A.F.; Davies, C.L.; Holloway, R.K.; Labrak, Y.; Ireland, G.; Carradori, D.; Dillenburg, A.; Borger, E.; Soong, D.; Richardson, J.C.; et al. Central Nervous System Regeneration Is Driven by Microglia Necroptosis and Repopulation. Nat. Neurosci. 2019, 22, 1046–1052. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Toll-like Receptors in the Pathogenesis of Neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Batista, C.R.A.; Saliba, S.W.; Yousif, N.M.; de Oliveira, A.C.P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Chen, M.; Chen, J.; Gao, T.; Yao, S. Dexmedetomidine Inhibits Inflammation in Microglia Cells under Stimulation of LPS and ATP by C-Fos/NLRP3/Caspase-1 Cascades. EXCLI J. 2018, 17, 302–311. [Google Scholar] [CrossRef]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef]
- Campolo, M.; Paterniti, I.; Siracusa, R.; Filippone, A.; Esposito, E.; Cuzzocrea, S. TLR4 Absence Reduces Neuroinflammation and Inflammasome Activation in Parkinson’s Diseases in Vivo Model. Brain. Behav. Immun. 2019, 76, 236–247. [Google Scholar] [CrossRef]
- Yang, J.; Wise, L.; Fukuchi, K.-I. TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/ TLR4/ NF-ΚB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, Lifestyle Stress, and Neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D Self-Organized Microvascular Model of the Human Blood-Brain Barrier with Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.S.; Jackson, J.; Sheu, S.-H.; Chang, C.-L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Schuebel, K.E.; Jair, K.; Cimbro, R.; De Biase, L.M.; Goldman, D.; Bonci, A. Ventral Midbrain Astrocytes Display Unique Physiological Features and Sensitivity to Dopamine D2 Receptor Signaling. Neuropsychopharmacology 2019, 44, 344–355. [Google Scholar] [CrossRef]
- Tan, C.X.; Burrus Lane, C.J.; Eroglu, C. Role of Astrocytes in Synapse Formation and Maturation. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 142, pp. 371–407. ISBN 978-0-12-815281-2. [Google Scholar]
- Wilton, D.K.; Dissing-Olesen, L.; Stevens, B. Neuron-Glia Signaling in Synapse Elimination. Annu. Rev. Neurosci. 2019, 42, 107–127. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte Function from Information Processing to Cognition and Cognitive Impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef]
- John Lin, C.-C.; Yu, K.; Hatcher, A.; Huang, T.-W.; Lee, H.K.; Carlson, J.; Weston, M.C.; Chen, F.; Zhang, Y.; Zhu, W.; et al. Identification of Diverse Astrocyte Populations and Their Malignant Analogs. Nat. Neurosci. 2017, 20, 396–405. [Google Scholar] [CrossRef]
- Bayraktar, O.A.; Bartels, T.; Holmqvist, S.; Kleshchevnikov, V.; Martirosyan, A.; Polioudakis, D.; Ben Haim, L.; Young, A.M.H.; Batiuk, M.Y.; Prakash, K.; et al. Astrocyte Layers in the Mammalian Cerebral Cortex Revealed by a Single-Cell in Situ Transcriptomic Map. Nat. Neurosci. 2020, 23, 500–509. [Google Scholar] [CrossRef]
- Kumar, A.; Fontana, I.C.; Nordberg, A. Reactive Astrogliosis: A Friend or Foe in the Pathogenesis of Alzheimer’s Disease. J. Neurochem. 2022, jnc.15565. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, N.; Magami, S.; Inaba, T.; Ueno, Y.; Hira, K.; Kijima, C.; Nakajima, S.; Yamashiro, K.; Urabe, T.; Hattori, N. The Effects of A1/A2 Astrocytes on Oligodendrocyte Linage Cells against White Matter Injury under Prolonged Cerebral Hypoperfusion. Glia 2020, 68, 1910–1924. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Schrepf, A.; Kaplan, C.M.; Ichesco, E.; Larkin, T.; Harte, S.E.; Harris, R.E.; Murray, A.D.; Waiter, G.D.; Clauw, D.J.; Basu, N. A Multi-Modal MRI Study of the Central Response to Inflammation in Rheumatoid Arthritis. Nat. Commun. 2018, 9, 2243. [Google Scholar] [CrossRef] [PubMed]
- Opel, N.; Cearns, M.; Clark, S.; Toben, C.; Grotegerd, D.; Heindel, W.; Kugel, H.; Teuber, A.; Minnerup, H.; Berger, K.; et al. Large-Scale Evidence for an Association between Low-Grade Peripheral Inflammation and Brain Structural Alterations in Major Depression in the BiDirect Study. J. Psychiatry Neurosci. 2019, 44, 423–431. [Google Scholar] [CrossRef]
- Lan, F.; Lin, G.; Cao, G.; Li, Z.; Ma, D.; Liu, F.; Duan, M.; Fu, H.; Xiao, W.; Qi, Z.; et al. Altered Intrinsic Brain Activity and Functional Connectivity Before and After Knee Arthroplasty in the Elderly: A Resting-State FMRI Study. Front. Neurol. 2020, 11, 556028. [Google Scholar] [CrossRef]
- Bang, M.; Kim, J.; An, S.K.; Youm, Y.; Chey, J.; Kim, H.C.; Park, K.; Namkoong, K.; Lee, E. Associations of Systemic Inflammation with Frontotemporal Functional Network Connectivity and Out-Degree Social-Network Size in Community-Dwelling Older Adults. Brain. Behav. Immun. 2019, 79, 309–313. [Google Scholar] [CrossRef]
- Alvarez, G.M.; Hackman, D.A.; Miller, A.B.; Muscatell, K.A. Systemic Inflammation Is Associated with Differential Neural Reactivity and Connectivity to Affective Images. Soc. Cogn. Affect. Neurosci. 2020, 15, 1024–1033. [Google Scholar] [CrossRef]
- Gwilym, S.E.; Filippini, N.; Douaud, G.; Carr, A.J.; Tracey, I. Thalamic Atrophy Associated with Painful Osteoarthritis of the Hip Is Reversible after Arthroplasty: A Longitudinal Voxel-Based Morphometric Study. Arthritis Rheum. 2010, 62, 2930–2940. [Google Scholar] [CrossRef]
- Smitha, K.; Akhil Raja, K.; Arun, K.; Rajesh, P.; Thomas, B.; Kapilamoorthy, T.; Kesavadas, C. Resting State FMRI: A Review on Methods in Resting State Connectivity Analysis and Resting State Networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Singh, K.K. A Review of Publicly Available Automatic Brain Segmentation Methodologies, Machine Learning Models, Recent Advancements, and Their Comparison. Ann. Neurosci. 2021, 28, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Vigotsky, A.D.; Branco, P.; Reis, A.M.; Schnitzer, T.J.; Galhardo, V.; Apkarian, A.V. Brain Gray Matter Abnormalities in Osteoarthritis Pain: A Cross-Sectional Evaluation. Pain 2020, 161, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, B.; Dehghan Nayyeri, M.; Pfleiderer, B.; Stahlberg, K.; Jünke, L.; Baie, L.; Dieckmann, R.; Liem, D.; Happe, T.; Burgmer, M. Subtle Changes of Gray Matter Volume in Fibromyalgia Reflect Chronic Musculoskeletal Pain Rather than Disease-specific Effects. Eur. J. Neurosci. 2019, 50, 3958–3967. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Ikeda, E.; Kobinata, H. Functional Connectivity Modulations during Offset Analgesia in Chronic Pain Patients: An FMRI Study. Brain Imaging Behav. 2022, 16, 1794–1802. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, H.; Zhang, M.; Wang, Y.; Li, D. Volumetric and Functional Connectivity Alterations in Patients with Chronic Cervical Spondylotic Pain. Neuroradiology 2020, 62, 995–1001. [Google Scholar] [CrossRef]
- Kitzbichler, M.G.; Aruldass, A.R.; Barker, G.J.; Wood, T.C.; Dowell, N.G.; Hurley, S.A.; McLean, J.; Correia, M.; Clarke, C.; Pointon, L.; et al. Peripheral Inflammation Is Associated with Micro-Structural and Functional Connectivity Changes in Depression-Related Brain Networks. Mol. Psychiatry 2021, 26, 7346–7354. [Google Scholar] [CrossRef]
- Aytur, S.A.; Ray, K.L.; Meier, S.K.; Campbell, J.; Gendron, B.; Waller, N.; Robin, D.A. Neural Mechanisms of Acceptance and Commitment Therapy for Chronic Pain: A Network-Based FMRI Approach. Front. Hum. Neurosci. 2021, 15, 587018. [Google Scholar] [CrossRef]
- Munshi, S.; Parrilli, V.; Rosenkranz, J.A. Peripheral Anti-Inflammatory Cytokine Interleukin-10 Treatment Mitigates Interleukin-1β-Induced Anxiety and Sickness Behaviors in Adult Male Rats. Behav. Brain Res. 2019, 372, 112024. [Google Scholar] [CrossRef]
- Konsman, J.P.; Parnet, P.; Dantzer, R. Cytokine-Induced Sickness Behaviour: Mechanisms and Implications. Trends Neurosci. 2002, 25, 154–159. [Google Scholar] [CrossRef]
- Borniger, J.C.; de Lecea, L. Peripheral Lipopolyssacharide Rapidly Silences REM-Active LHGABA Neurons. Front. Behav. Neurosci. 2021, 15, 649428. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Klawonn, A.M.; Jaarola, M.; Engblom, D. Interferon-γ Mediated Signaling in the Brain Endothelium Is Critical for Inflammation-Induced Aversion. Brain. Behav. Immun. 2018, 67, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Lasselin, J.; Sundelin, T.; Wayne, P.M.; Olsson, M.J.; Paues Göranson, S.; Axelsson, J.; Lekander, M. Biological Motion during Inflammation in Humans. Brain. Behav. Immun. 2020, 84, 147–153. [Google Scholar] [CrossRef]
- Lasselin, J.; Treadway, M.T.; Lacourt, T.E.; Soop, A.; Olsson, M.J.; Karshikoff, B.; Paues-Göranson, S.; Axelsson, J.; Dantzer, R.; Lekander, M. Lipopolysaccharide Alters Motivated Behavior in a Monetary Reward Task: A Randomized Trial. Neuropsychopharmacology 2017, 42, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.S.; Reichenberg, A.; Yirmiya, R.; Smed, A.; Pedersen, B.K.; Bruunsgaard, H. Low-Dose Endotoxemia and Human Neuropsychological Functions. Brain. Behav. Immun. 2005, 19, 453–460. [Google Scholar] [CrossRef]
- Odoj, K.; Brawek, B.; Asavapanumas, N.; Mojtahedi, N.; Heneka, M.T.; Garaschuk, O. In Vivo Mechanisms of Cortical Network Dysfunction Induced by Systemic Inflammation. Brain. Behav. Immun. 2021, 96, 113–126. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human Astrocytes: Secretome Profiles of Cytokines and Chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef]
- Sitcheran, R.; Gupta, P.; Fisher, P.B.; Baldwin, A.S. Positive and Negative Regulation of EAAT2 by NF-ΚB: A Role for N-Myc in TNFα-Controlled Repression. EMBO J. 2005, 24, 510–520. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhang, B.; Zhu, J.; Zhou, Z.; Cui, L. Regulation of Microglia by Glutamate and Its Signal Pathway in Neurodegenerative Diseases. Drug Discov. Today 2020, 25, 1074–1085. [Google Scholar] [CrossRef]
- Finsterwald, C.; Magistretti, P.; Lengacher, S. Astrocytes: New Targets for the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2015, 21, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-Induced Excitotoxicity in Parkinson’s Disease: The Role of Glial Cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Dejakaisaya, H.; Kwan, P.; Jones, N.C. Astrocyte and Glutamate Involvement in the Pathogenesis of Epilepsy in Alzheimer’s Disease. Epilepsia 2021, 62, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Henríquez, G.; Simon, M.S.; Burger, B.; Weidinger, E.; Wijkhuijs, A.; Arolt, V.; Birkenhager, T.K.; Musil, R.; Müller, N.; Drexhage, H.A. Low-Grade Inflammation as a Predictor of Antidepressant and Anti-Inflammatory Therapy Response in MDD Patients: A Systematic Review of the Literature in Combination With an Analysis of Experimental Data Collected in the EU-MOODINFLAME Consortium. Front. Psychiatry 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Bekhbat, M.; Treadway, M.T.; Felger, J.C. Inflammation as a Pathophysiologic Pathway to Anhedonia: Mechanisms and Therapeutic Implications. In Anhedonia: Preclinical, Translational, and Clinical Integration; Pizzagalli, D.A., Ed.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2022; Volume 58, pp. 397–419. ISBN 978-3-031-09682-2. [Google Scholar]
- Lucido, M.J.; Bekhbat, M.; Goldsmith, D.R.; Treadway, M.T.; Haroon, E.; Felger, J.C.; Miller, A.H. Aiding and Abetting Anhedonia: Impact of Inflammation on the Brain and Pharmacological Implications. Pharmacol. Rev. 2021, 73, 1084–1117. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Treadway, M.T. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 2017, 42, 216–241. [Google Scholar] [CrossRef]
- Ruscitti, P.; Cipriani, P.; Liakouli, V.; Carubbi, F.; Berardicurti, O.; Di Benedetto, P.; Ciccia, F.; Guggino, G.; Alvaro, S.; Triolo, G.; et al. The Emerging Role of IL-1 Inhibition in Patients Affected by Rheumatoid Arthritis and Diabetes. Rev. Recent Clin. Trials 2018, 13, 210–214. [Google Scholar] [CrossRef]
- Wu, Y.; Hsing, C.; Chiu, C.; Huang, H.; Hsu, Y. Roles of IL-1 and IL-10 Family Cytokines in the Progression of Systemic Lupus Erythematosus: Friends or Foes? IUBMB Life 2022, 74, 143–156. [Google Scholar] [CrossRef]
- Pope, J.E. Management of Fatigue in Rheumatoid Arthritis. RMD Open 2020, 6, e001084. [Google Scholar] [CrossRef]
- Kawka, L.; Schlencker, A.; Mertz, P.; Martin, T.; Arnaud, L. Fatigue in Systemic Lupus Erythematosus: An Update on Its Impact, Determinants and Therapeutic Management. J. Clin. Med. 2021, 10, 3996. [Google Scholar] [CrossRef]
- Dey, M.; Parodis, I.; Nikiphorou, E. Fatigue in Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Comparison of Mechanisms, Measures and Management. J. Clin. Med. 2021, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.J.; Klinedinst, N.J.; Resnick, B.; Renn, C.; Fiskum, G. A Review and Synthesis of Correlates of Fatigue in Osteoarthritis. Int. J. Orthop. Trauma Nurs. 2019, 33, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, X.; Cai, L.; Yan, C.; Yu, L.; Fan, J.; Zhang, R.; Huang, J.; Duan, X. Rheumatoid Arthritis and Risk of Anxiety: A Meta-Analysis of Cohort Studies. Clin. Rheumatol. 2019, 38, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Fakra, E.; Marotte, H. Rheumatoid Arthritis and Depression. Joint Bone Spine 2021, 88, 105200. [Google Scholar] [CrossRef]
- Singh, G.; Mahajan, N.; Abrol, S.; Raina, A. Anxiety and Depression Are Common in Rheumatoid Arthritis and Correlate with Poor Quality of Life in Indian Patients. Reumatol./Rheumatol. 2021, 59, 386–393. [Google Scholar] [CrossRef]
- Jung, J.H.; Seok, H.; Kim, J.-H.; Song, G.G.; Choi, S.J. Association between Osteoarthritis and Mental Health in a Korean Population: A Nationwide Study. Int. J. Rheum. Dis. 2018, 21, 611–619. [Google Scholar] [CrossRef]
- Sayre, E.C.; Esdaile, J.M.; Kopec, J.A.; Singer, J.; Wong, H.; Thorne, A.; Guermazi, A.; Nicolaou, S.; Cibere, J. Specific Manifestations of Knee Osteoarthritis Predict Depression and Anxiety Years in the Future: Vancouver Longitudinal Study of Early Knee Osteoarthritis. BMC Musculoskelet. Disord. 2020, 21, 467. [Google Scholar] [CrossRef]
- Xue, Y.-H.; Peng, Y.-S.; Ting, H.-F.; Hsieh, J.P.; Huang, Y.-K.; Wang, Y.-H.; Chiou, J.-Y.; Wei, J.C.-C. Etoricoxib and Diclofenac Might Reduce the Risk of Dementia in Patients with Osteoarthritis: A Nation-Wide, Population-Based Retrospective Cohort Study. Dement. Geriatr. Cogn. Disord. 2018, 45, 262–271. [Google Scholar] [CrossRef]
- Mayburd, A.L.; Baranova, A. Increased Lifespan, Decreased Mortality, and Delayed Cognitive Decline in Osteoarthritis. Sci. Rep. 2019, 9, 18639. [Google Scholar] [CrossRef]
- Sangha, P.S.; Thakur, M.; Akhtar, Z.; Ramani, S.; Gyamfi, R.S. The Link Between Rheumatoid Arthritis and Dementia: A Review. Cureus 2020, 12, e7855. [Google Scholar] [CrossRef]
- Mason, A.; Holmes, C.; Edwards, C.J. Inflammation and Dementia: Using Rheumatoid Arthritis as a Model to Develop Treatments? Autoimmun. Rev. 2018, 17, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Raji, M.A. Cognitive Impairment in Elderly Patients with Rheumatic Disease and the Effect of Disease-Modifying Anti-Rheumatic Drugs. Clin. Rheumatol. 2021, 40, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, N.; O’Keefe, J.H.; O’Keefe, E.L.; Isaacson, R.; Small, G. Therapeutic Potential of TNF-α Inhibition for Alzheimer’s Disease Prevention. J. Alzheimers Dis. 2020, 78, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Hashioka, S.; Wu, Z.; Klegeris, A. Glia-Driven Neuroinflammation and Systemic Inflammation in Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 19, 908–924. [Google Scholar] [CrossRef]
- Rossi, B.; Santos-Lima, B.; Terrabuio, E.; Zenaro, E.; Constantin, G. Common Peripheral Immunity Mechanisms in Multiple Sclerosis and Alzheimer’s Disease. Front. Immunol. 2021, 12, 639369. [Google Scholar] [CrossRef]
- La Vitola, P.; Balducci, C.; Baroni, M.; Artioli, L.; Santamaria, G.; Castiglioni, M.; Cerovic, M.; Colombo, L.; Caldinelli, L.; Pollegioni, L.; et al. Peripheral Inflammation Exacerbates α-Synuclein Toxicity and Neuropathology in Parkinson’s Models. Neuropathol. Appl. Neurobiol. 2021, 47, 43–60. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Chen, Z.-B.; Li, L.; Li, X.-Y.; Castellanos, F.X.; Bai, T.-J.; Bo, Q.-J.; Cao, J.; Chang, Z.-K.; et al. Disrupted Intrinsic Functional Brain Topology in Patients with Major Depressive Disorder. Mol. Psychiatry 2021, 26, 7363–7371. [Google Scholar] [CrossRef]
- Piguet, C.; Karahanoğlu, F.I.; Saccaro, L.F.; Van De Ville, D.; Vuilleumier, P. Mood Disorders Disrupt the Functional Dynamics, Not Spatial Organization of Brain Resting State Networks. NeuroImage Clin. 2021, 32, 102833. [Google Scholar] [CrossRef]
- Stange, J.P.; Jenkins, L.M.; Pocius, S.; Kreutzer, K.; Bessette, K.L.; DelDonno, S.R.; Kling, L.R.; Bhaumik, R.; Welsh, R.C.; Keilp, J.G.; et al. Using Resting-State Intrinsic Network Connectivity to Identify Suicide Risk in Mood Disorders. Psychol. Med. 2020, 50, 2324–2334. [Google Scholar] [CrossRef]
- Goodkind, M.; Eickhoff, S.B.; Oathes, D.J.; Jiang, Y.; Chang, A.; Jones-Hagata, L.B.; Ortega, B.N.; Zaiko, Y.V.; Roach, E.L.; Korgaonkar, M.S.; et al. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry 2015, 72, 305. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naumovs, V.; Groma, V.; Mednieks, J. From Low-Grade Inflammation in Osteoarthritis to Neuropsychiatric Sequelae: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 16031. https://doi.org/10.3390/ijms232416031
Naumovs V, Groma V, Mednieks J. From Low-Grade Inflammation in Osteoarthritis to Neuropsychiatric Sequelae: A Narrative Review. International Journal of Molecular Sciences. 2022; 23(24):16031. https://doi.org/10.3390/ijms232416031
Chicago/Turabian StyleNaumovs, Vladimirs, Valērija Groma, and Jānis Mednieks. 2022. "From Low-Grade Inflammation in Osteoarthritis to Neuropsychiatric Sequelae: A Narrative Review" International Journal of Molecular Sciences 23, no. 24: 16031. https://doi.org/10.3390/ijms232416031
APA StyleNaumovs, V., Groma, V., & Mednieks, J. (2022). From Low-Grade Inflammation in Osteoarthritis to Neuropsychiatric Sequelae: A Narrative Review. International Journal of Molecular Sciences, 23(24), 16031. https://doi.org/10.3390/ijms232416031





