1. Introduction
Female breast cancer is one of the scourges of our time, becoming the most common cancer globally in 2020 by surpassing lung cancer as the leading cause of global cancer incidence. With an estimated 2.3 million new cases, representing 11.7% of all cancer cases worldwide in both sexes, and 685,000 deaths, breast cancer is the fifth leading cause of cancer mortality [
1,
2]. Among women, it accounts for 1 in 4 cancer cases and 1 in 6 cancer deaths, making it first in ranking for incidence globally [
1]. In 2022, an estimated 287,850 new cases of invasive breast cancer are expected to be diagnosed in women in the U.S. alone, along with 51,400 new cases of non-invasive (in situ) breast cancer [
2]. Causes and risk factors for the development of breast cancer are numerous and include genetic predisposition, reproductive and hormonal risk factors (early age at menarche, later age at menopause, advanced age at first birth, fewer number of children, less breastfeeding, menopausal hormone therapy, oral contraceptives) and lifestyle risk factors such as alcohol intake, excess body weight, and physical inactivity [
3]. Among hereditary cases, almost a fourth result from a mutation in one of a few rare but highly penetrant genes, including breast cancer 1 (
BRCA1), breast cancer 2 (
BRCA2), phosphatase and tensin homolog (
PTEN), tumor protein P53 (
TP53), E-cadherin (
CDH1) and serine/threonine kinase 11 (
STK11), which present up to an 80% lifetime risk of developing breast cancer [
4]. The relationship between breast cancer development and outcomes and certain lifestyle factors is an area that has been researched extensively [
5].
Breast cancer is the most invasive cancer in women, and the immense tumor heterogeneity is the major issue limiting the efficacy of targeted cancer therapies. Seven subtypes of female breast cancer can be differentiated, depending on their molecular characteristics, and stratification into these subtypes, along with staging, directs the course of treatment and is paramount to better clinical outcomes [
6,
7,
8]. A key factor for a good prognosis and high survival rate is—besides the elimination of lifestyle risk factors—the early diagnosis of breast cancer. The improvement of current diagnostic tools, the development of new methods, and regular screenings have reduced cancer mortality dramatically, in combination with the overall survival rate and quality of life for patients [
9,
10].
In the pursuit of alternative and “outside-the-box” approaches for this and other detrimental diseases, medical research has begun to develop an interest in microgravity (μg) research [
11,
12,
13]. The strain on the human body caused by the lack of gravitational force became apparent very early on in the age of spaceflight, as the technical challenges were overcome and astronauts started exploring the orbit and beyond and continued to do so [
14,
15,
16,
17]. Prolonged exposure of the human body to μg leads to multiple impairments in various physiological systems, among them the musculoskeletal system and bone metabolism, cardiovascular physiology, metabolic processes, and the microbiome [
17,
18,
19,
20,
21,
22,
23,
24,
25]. These effects are observable not only on an organic but also on a cellular level, displaying changes in proteomic, genomic, and metabolomic profiles [
26,
27]. Studies of the angiovascular system in μg lead, among others, to advances in the understanding of wound healing and the three-dimensional growth of tumors [
28,
29,
30,
31].
Any kind of tissue growth is dependent on cell-cell communication, healthy or otherwise. Until roughly a decade ago, the only known methods of cell communication in multi-cellular systems were via direct cell contact or receptor-based signaling [
32]. Since then, extracellular vesicles (EVs) have been discovered as an alternative way of cellular information exchange and have quickly gained attention. EVs comprise a family of membrane vesicles secreted from the majority, if not all, cells into the extracellular environment and functionally mediate cell-cell communication [
33,
34]. Three major types of extracellular vesicles can be distinguished: microvesicles, apoptotic bodies, and the so-called small EVs or exosomes. Exosomes are found in essentially all circulating body fluids, including blood, saliva, and urine, and can be distinguished from other EVs by their endosomal biogenesis, size, as well as several surfaces and internal markers [
33,
34,
35,
36,
37,
38,
39]. The vesicles reflect their cell of origin in regard to their cargo and surface markers and, therefore, present as potential clinical biomarkers for a vast number of diseases [
34,
40,
41]. With their properties to deliver their cargo and influence virtually any cell type, exosomes also promise a wide range of therapeutical applications [
42]. Overcoming the technical challenges, starting with isolation and enrichment as well as proper characterization of these vesicles, the recent developments of very specific methods for small EV analysis enable the research community to address many questions about these fascinating cellular tools [
43,
44].
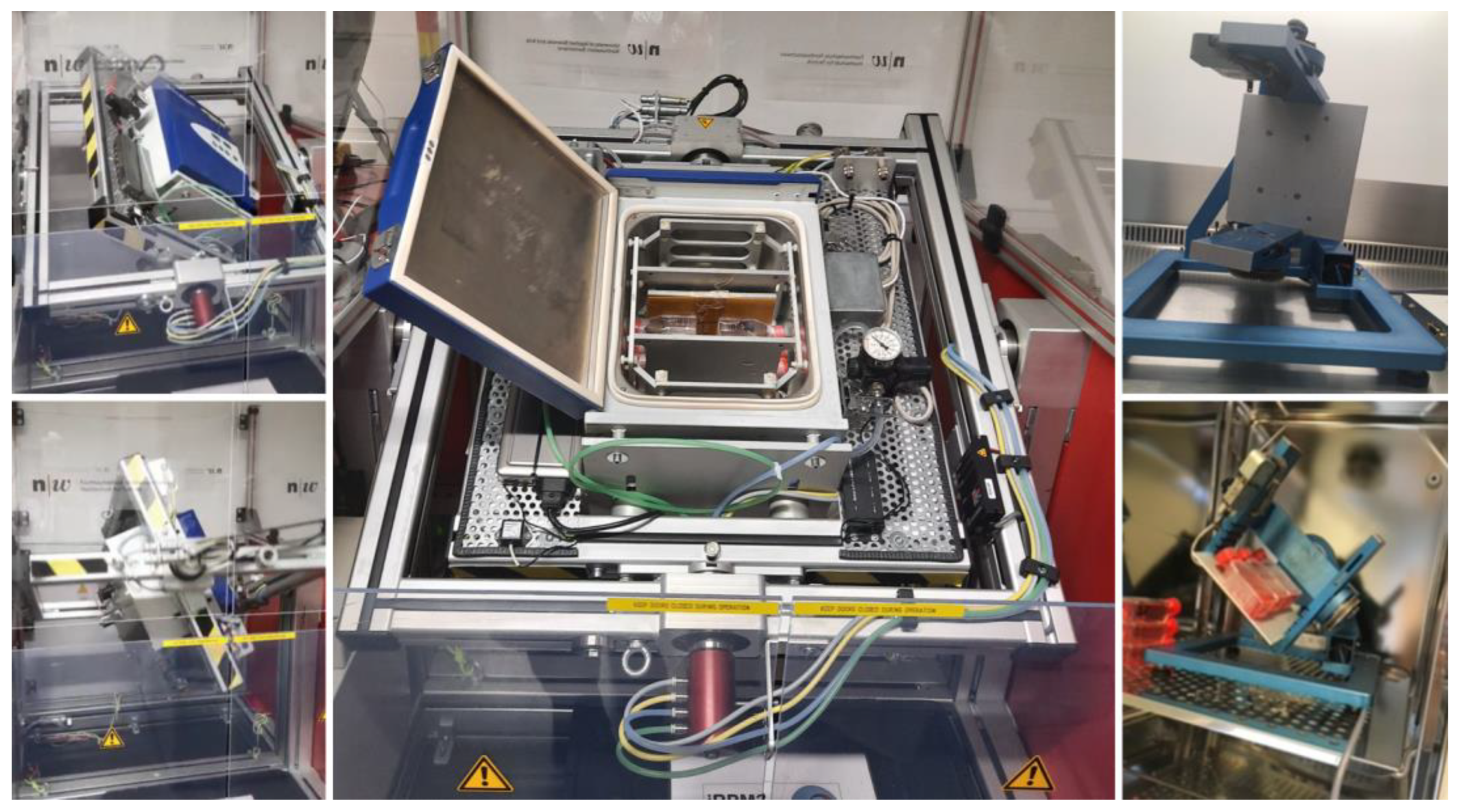
Space travel has developed considerably in recent years in technology and the volume of spaceflights. The International Space Station (ISS) harbors research resources and supports multiple projects [
45]. Nonetheless, with the apparent limitations in available space on the ISS, the still small amount of shuttle flights, and last but not least, the considerable costs, the opportunities for experiments in real microgravity (r-μg) are scarce. Therefore, a majority of experiments are being conducted on ground-based facilities under simulated microgravity (s-μg). To replicate μg satisfactorily, various devices have been developed, such as the random positioning machine (RPM,
Figure 1) and clinostats [
46,
47,
48,
49,
50]. These instruments are valuable resources for studying the influence of μg on a variety of cells under changing conditions and multiple time points. The experimental setup can be easily adapted and fairly rapidly, and the changes can be documented regularly during the time course of the experiment, which is a major advantage to experiments in r-μg [
49]. The observed changes in cells following incubation within these devices are generally comparable to the cellular adaptations in r-μg, and even though they are not identical, they can often predict results gained during spaceflights [
51].
Over the course of our investigations with several breast cancer cells exposed to both s-μg and r-μg, we have found that the tumor cells, incubated in s-μg, grow either adherently as a monolayer or as a 3D spheroid [
52,
53,
54,
55]. The spheroids model is a better in vitro representation of tumor growth in vivo; therefore, the cellular changes during the spheroid formation promise a deeper insight into tumor development and progression than ground-based cell culture experiments [
54]. With cell-crosstalk in mind and the knowledge of the impact of small EVs on the cell-cell communication not only between tumor cells but also the tumor microenvironment and the preparation of the metastatic niche [
34,
56,
57,
58,
59], the role of small EVs in the cellular response to μg is a factor that should not be underestimated. This research topic may provide a different angle and new findings worth exploring, also in the processes triggering 3D spheroid development.
Here we are investigating the changes in exosomal release and population in the breast cancer cell line MCF-7 following exposure to s-μg. We are aiming to learn whether the adaptive changes we observed in the CellBox-1 study will be mirrored. Ultimately, we would like to expand our knowledge on the three-dimensional growth of these and other tumor cells and how the communication between cells can be influenced to reduce tumorigenicity.
2. Results
The number of studies on EVs, and small EVs in particular, has risen quite rapidly following the first description of their importance in cell crosstalk [
60,
61]. The earliest experimental methods for EV isolation, namely differential centrifugation, filtration, and precipitation protocols, meanwhile proved to produce highly variable results in regard to yield, purity, and functionality [
62,
63,
64]. To ensure reproducible experimental outcomes as much as to accommodate reliable research on the wide variety of source materials and investigational requirements, multiple methods have been developed to improve the isolation and characterization of EVs. In line with the volume of different source materials and cell types small and other EVs can be harvested from, the definitions and descriptions of exosomes were manifold, overlapping, and confusing. The International Society of Extracellular Vesicles (ISEV), therefore, divulged guidelines for the minimal information of studies in extracellular vesicles (MISEV), which includes a list of transmembrane proteins and cytosolic proteins with membrane-binding capacity anticipated to be present or absent in the various EV groups and recommended investigators to determine the expression of three or more of these proteins at least semi-quantitatively. Transmembrane proteins expected to be present in exosomal preparations include, among others, tetraspanins (CD9, CD63, CD81), integrins, and growth factor receptors. The group of expected or enriched cytosolic proteins includes endosome or membrane-binding proteins (TSG101) and signal transduction or scaffolding proteins (syntenin). Intracellular proteins that are absent or under-represented in exosomes but present in other types of EVs are proteins found in the endoplasmic reticulum (ER) (calnexin) or the Golgi apparatus (GM130).
As in our previous study on cell supernatants from the CellBox-1 experiment, the method of choice to investigate the supernatants of MCF-7 cells subjected to s-μg was SP-IRIS, specifically the ExoView™ system (Unchained Labs, Pleasanton, CA, USA). This way, human tetraspanin-positive exosomes are selectively captured on an antibody-coated chip directly from biofluids without a prior need to isolate and concentrate EVs. Additionally, the method is suitable for very small sample sizes, as high as 20 µL for unprocessed, not concentrated samples. As described previously, we used EV-TETRA-C ExoView Tetraspanin chip plates, which capture exosomes on spots that are separately pre-loaded with antibodies (Abs) to one of the tetraspanins CD9, CD63, and CD81, to assess the amount and population distribution of exosomes in the cell supernatants harvested following incubation of the cells on RPM for 5 and 10 d, as well as the corresponding 1 g controls. Via interferometric analysis, we were able to investigate the particle count and the size distribution of the given samples [
43,
65], and counter-staining the captured exosomes with fluorescent Abs to CD9, CD63, and CD81, we characterized the small EVs according to their transmembrane protein expression and identified the different populations present in the sample. Here, we describe the results we gained from these analyses.
2.1. Interferometric Analysis
2.1.1. Particle Concentration
The absolute number of captured particles in the size range of 50–200 nm was measured via interferometric analyses by scanning the tetraspanin spots (CD9, CD63, and CD81) as well as the IgG control, all in triplicates from three samples each of the following experimental condition: 5 d 1 g, 5 d RPM, 10 d 1 g and 10 d RPM.
Figure 2 shows an overview of the particles captured in the size range from 50–200 nm. An increase in particle number following exposure to s-μg is found in both time points, with a considerably more pronounced increase at the 5 d timepoint.
Table 1 details the average of all measured triplicates, their means, and standard deviation (SD). The number of small EVs captured at 5 d 1 g and 10 d 1 g is fairly constant at all three Tetraspanin spots (CD81: 5 d 1 g = 683.7; 10 d 1 g = 777.7; CD63: 5 d 1 g = 1151.3; 10 d 1 g = 169.3; CD9: 5 d 1 g = 1611.3; 10 d 1 g = 1625.0). The largest increase between the 1 g and RPM condition was seen at the 5 d time point: CD81: 5 d 1 g = 683.7; 5 d RPM = 1726.3; CD63: 5 d 1 g = 1151.3; 5 d RPM = 2511.0; CD9: 5 d 1 g = 1611.3; 5 d RPM = 2296.0, with none of the changes being significant. After 10 d incubation, there was still a slight increase in exosome particles after RPM exposure, but less distinct than after 5 d (CD81: 10 d 1 g = 777.7; 10 d RPM = 852.7; CD63: 10 d 1 g = 1169.3; 10 d RPM = 1667.7; CD9: 10 d 1 g = 1625.0; 10 d RPM = 1840.0). It is noteworthy that the amount of captured small EVs after 10 d on the RPM decreased considerably compared to 5 d exposure (
Figure 2,
Table 1).
2.1.2. Particle Size Distribution
Measurement of the size distribution of the exosomes captured was equally done by interferometry. As this technique is limited to a range of 50–200 nm, it should be noted that a large amount of smaller particles, identified by the presence of a fluorescent signal and the absence of interferometric-based size measurement, are not included in these data. The number of these <50 nm particles will be included in the fluorimetric analysis. The majority of particles can be found within the range of 50–120 nm in all conditions and time points, as could be expected, and is consistent with the defined size range of exosomes (
Figure 3,
Supplemental Table S1) [
62]. As described above, the difference between the particle number increase in this size range following RPM exposure vs. the 1 g control is more pointed after 5 d than 10 d (5 d 1 g = 1136.6; 5 d RPM = 2127.9; 10 d 1 g = 1173.1; 10 d RPM = 1433.2).
The mode of particles is at around 55 nm with an average of 519.9 particles at 5 d 1 g, 768.7 at 5 d RPM, 459.2 at 10 d 1 g, and 585.1 at 10 d RPM.
2.2. Fluorescent Analysis
2.2.1. Total Fluorescent Particle Counts
As noted above, the interferometric analysis of the ExoView method is limited to the detection of particles sized 50 nm or larger, thus excluding a substantial part of exosomes. The results are still valuable to determine size distribution, but counterstaining the small EVs bound on the chip plate with fluorescent antibodies to the tetraspanins completes this initial analysis. Therefore, the combination of interferometric sizing and fluorometric detection allows the analysis of the entire EV content by detecting the size and evaluating the distribution of subpopulations defined by the presence of one or multiple exosomal markers.
The results of our examination of the total fluorescent particle counts with all three ABs on the three capture spots can be seen in
Figure 4. Panel
a displays the combined values of all three fluorescent tetraspanin ABs, and panels
b–d displays the number of fluorescently stained particles on each capture spot. Comparing this to the analysis of the particles captured by interferometry, it can be stated that there is an increase in particle number after exposure of the BC cells to the RPM. The increase after 10 d RPM is on all three tetraspanin spots similar to the increase after 5 d RPM, suggesting that small EVs < 50 nm not only make up a large part of the total particle number but their expression is also much more influenced by exposure to s-μg. Statistically, the exposure of the MCF-7 cells to s-μg via RPM vs. the 1 g control shows significance in all comparisons and on all tetraspanin spots. The means of all samples (
n = 3), the standard error of the means as well as the corresponding
p-value are listed in
Table 2 There is also a small but not significant increase in particle count after 10 d exposure vs. 5 d, in both the 1 g condition as well as after incubation of the cells on the RPM.
Looking at separate capture spots on the chip following the counterstain with the AB for CD81, CD63, and CD9, it becomes apparent that CD9 is the most dominantly expressed of the three tetraspanins (
Figure 4a).
On the CD81 spot particularly, we observed that the EV count increased after s-μg at both time points; similarly, the surface protein expression of all three tetraspanins increased (
Figure 4b,
Table 3).
This pattern holds true for the CD63 spot as well, CD9 was the most expressed of the three surface proteins, but in contrast to the CD81 spot, the number of particles expressing the remaining two proteins showed a comparable value (
Table 4). As prior, the particle count was elevated after incubation on the RPM compared to 1 g in both time points (
Figure 4c).
On the CD9 spot again, EVs with CD9 expression displayed the highest particle count in all conditions and time points (
Figure 4d,
Table 5). This spot, though, has a considerably larger number expressing CD81 on its surface. All in all, the total exosome number captured on the CD9 spot is far greater than on either the CD81 or the CD63 spot (CD81 = 7823.1; CD63 = 7000.5; CD9 = 13,710.6).
2.2.2. Colocalization Analysis
Single Tetraspanin Surface Expression—CD9, CD63, and CD81
The number of small EVs with an expression of one tetraspanin only, the previously described trend of an increased particle number post-exposure to s-μg continues apart from CD9 at the 10 d timepoint. Here the count of bound exosomes on the CD9 spot is slightly lower than after 10 d at 1 g with a mean of 912 (RPM) vs. 936.7 (1 g) (
Figure 5,
Table 6).
The count of CD81 only expressing exosomes is by far the lowest, the increase after 5 d RPM is more than double to 5 d 1 g (2.3-fold), whereas the increase following 10 d RPM is much more modest and even lower than after day 5 (1.4-fold).
CD63-only expression vesicles show a stark increase between 5 d 1 g and 10 d 1 g; exposure of the MCF-7 cells to s-μg for 5 d increases the small EV number almost 7-fold, which is the only significant change in all populations (
p = 0.0256). After the 10 d time point, the increase in the RPM sample is 2.8-fold (
Figure 6,
Table 7).
EVs captured on the CD9 slot with single tetraspanin expression comprise the largest number of all. Overall, their number decreased after 10 d in both experimental conditions compared to 5 d; after 5 d, we recorded a 1.3-fold increase in particle number but a decrease of 0.03 after 10 d (
Figure 7,
Table 8).
Co-Expression of Two Tetraspanins—CD9/CD63, CD9/CD81, and CD63/CD81
When it comes to co-expression of CD9/CD63, the number of exosomes found on the CD63 spot compared to CD9 is slightly higher, and the EV numbers increase on both card spots after RPM incubation, spanning over a range of 1.3- to 1.9-fold (
Table 7 and
Table 8).
Tetraspanin co-expression of CD9/CD81 on the CD81 spot shows lower counts than on the CD9 spot, the fold-increase following the incubation under s-μg condition varies between 1.4 and 1.8 (
Table 6 and
Table 8).
Lastly, the number of EVs expressing CD63 and CD81 is low compared to the other tetraspanin combinations. Numbers on the CD63 spot are higher than on the CD81 card spot, with mean counts ranging from 46.3 to 254.3 and the fold increase varying from 1.55 to 2.95 (
Table 6 and
Table 7).
Co-Expression of All Three Tetraspanins—CD9/CD63/CD81
The population expressing all three tetraspanins is the largest in all conditions over both time points. Comparing the three card spots, this set of exosomes captured on the CD63 spots provide the smallest group (average counts from 620.7–1049.7) with almost the same fold increases in the RPM group: 1.54 at 5 d and 1.52 at 10 d (
Table 8,
Figure 8). The small EVs with triple protein expression on the CD81 spot show a higher count; the average numbers range from 720.3 to 1325.3 with a fold change of 1.7 at 5 d and 1.44 at 10 d (
Table 7,
Figure 8). The largest group of exosomes is found on the CD9 spot, with values between 763.7 and 1554.0 on average and a fold change of 1.68 on 5 d and 1.53 on 10 d of s-μg (
Table 7,
Figure 8).
3. Discussion
Female breast cancer is a vast burden for patients and caregivers alike. The incidence rates are higher than ever, despite the considerable advances made in treatments and early discovery. The reasons why breast cancer cases are still on the rise are multifarious, be it the heterogeneity of the disease, which calls for special targeted or even personalized treatment options, the rise in lifestyle and environmental risk factors, genetic predisposition, or in some instances the lack of access to care [
1,
3,
5,
10,
66]. The fast-paced development of new detection methods as well as new approaches to treatment though, have better clinical outcomes for breast cancer patients and improved their quality of life [
66]. Despite these improvements, there is still a need to look further to discover new ways to better understand the development of this cancer and to find new opportunities for a cure.
Cancer cells and tumors, even more than every other living tissue, depend on cell-cell communication [
56,
67,
68]. Besides the exchange of information via direct cell contact or receptor-based transmission, extracellular vesicles are the alternate option for cell communication [
56,
57,
69,
70]. In recent years, these EVs have emerged as the major player in information transfer during tumor growth, immune escape, TME education, progression, metastasis, and the preparation of the metastatic niche [
56,
57,
70,
71,
72]. With their presence in virtually all biofluids, EVs are easily accessible biomarkers for cancers and other physiological maladies [
42,
69,
73]. As biomolecules, they are very stable and thus a great resource to research changes in the number of secreted vesicles as well as in the contained cargo following varying physiological or experimental conditions [
70,
74,
75,
76,
77]. Lastly, by altering the cargo and/or the expression of surface proteins of the EVs, they present themselves as ideal vesicles for personalized, targeted treatments for the majority of malignancies and other clinical pictures.
Our group has worked with various tumor cell lines, including breast cancer, and explored their genomic and proteomic changes after subjection to s- or r-μg. s-μg is a particularly useful tool in tumor research as several malignant cell lines will form 3-dimensional spheroids when incubated under μg conditions, which is a much better representation of a tumorigenic growth than in regular 2D cell culture [
31,
78,
79]. To find supporting evidence of the results gained in previous experiments, we have also examined the changes in EV release in thyroid cancer cells following exposure to r-μg on the ISS [
80].
In this current study, we examined the changes in exosome release in MCF-7 breast cancer cells following the subjection to s-μg for either 5 d or 10 d compared to the respective 1 g controls. As we did previously, we analyzed the small EV number, their size distribution, and the expression of the surface tetraspanins CD81, CD63, and CD9. Similar to the results we described in the study using r-μg, here we find an increased secretion of EVs following incubation to the RPM. The experimental time points were 5 d and 10 d; our results show a merely minimal increase in exosome numbers, if at all. This leads us to believe that the adaptive changes in the cells leading to a change in exosomal release occur fairly quickly upon the onset of s-μg. As mentioned previously, the interferometric analysis of small EVs is due to the limitation to vesicles larger than 50 nm lacking a large part of the entire exosome population. Still, through the size distribution we have shown that the particles captured on the card spots fall into the defined size range of exosomes with a mode of around 55 nm. Looking at the total particle counts after the fluorescent analysis of the bound vesicles, we can see that a very large part of small EVs is found in the group smaller than 50 nm. Additionally, the variance between the different samples is much lower, most likely due to the higher vesicle number. The fluorescence analysis shows significant changes in the release of exosomes following the cultivation on the RPM at both time points on every capture spot, whereas we have no significant changes to report when we analyze the populations in the 50–200 nm size range. The changes in the various populations expressing one, two, or all three tetraspanins seem to follow the release pattern of exosomes with increases in most populations following the exposure to s-μg. It seems noteworthy to point out that compared to our previous study, our current results are somewhat comparable regardless of the exposure to s-μg vs. r-μg and the use of breast cancer vs. thyroid cancer cells.
Of the three analyzed tetraspanins, small EVs with surface expression of CD9 are, compared to CD81 and CD63 populations, the largest group, both within single or coexpression. CD9 has been described as determining invasiveness and tumorigenicity in breast cancer cells [
81]. Similarly, CD9 overexpression is implied to play a role in breast cancer chemoresistance and is considered a biomarker for various cancers [
41,
82,
83,
84]. CD63 expression is the second highest overall; this tetraspanin has been described as carrying both tumor suppressor as well as protumorigenic properties and is a predictor of poor prognosis in several cancers [
85]. Just like CD9, CD63 plays a part in the regulation of breast cancer cell malignancy and the therapy resistance to tamoxifen [
86,
87]. Lastly, CD81 is the tetraspanin expressed in the lowest number of exosomes in the entire pool of populations. It does seem to hold the same properties, though, in breast cancer in regard to cell migration and proliferation as CD9 and CD63, and suppression of this surface protein is reported to reduce cancer invasion and metastasis [
88,
89].
All in all, these results support our previous findings of the samples gained from the CellBox-1 experiment. The demonstrated increases in small EV release (
Figure 9) suggest that the adaptive changes following μg conditions, either simulated or real, that we have described in previous studies on both the genomic or proteomic level, are somewhat mirrored in the cellular EV release and setup even though we cannot make any conclusions on possible adaptations to the cargo composition in the released vesicles. Whether the information transferred between cancer cells and the surrounding tumor microenvironment is restricted to the modification in the number of released vesicles or whether there is an actual variation in cargo needs to be determined. This study does demonstrate that the adaptive alterations within the cancer cells following changes in μg conditions are not only internal cellular events but that these adaptations are transmitted by vesicle-based cell-cell communication, which, therefore, may be a determining factor for the speed of the adaptive variations within the entire organism. This elevates the need for further investigations on the proteomic changes to the small EV cargo following exposure to μg. A recent proteomic analysis of both EVs and cells revealed a significant correlation with GTPases and proliferation of MDA-MB-231 cells in μg, and the authors conclude that EVs may be superior to cells in analyzing differentially expressed proteins, especially those that are down-regulated ones and usually unidentified or neglected in the analysis of intact cellular contents [
90]. These are interesting new facets to this research area, which may aid in answering the question of how variations in the cargo setup add to the overall cellular response. We will attempt to address a variety of these questions in future follow-up studies.
4. Materials and Method
4.1. Cell Cultures
MCF-7 human breast adenocarcinoma cells were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultivated in RPMI 1640 medium (Life Technologies, Naerum, Denmark), supplemented with 10% fetal calf serum (FCS) (Biochrom, Berlin, Germany) and 1% penicillin/streptomycin (Biochrom) at 37 °C and 5% CO
2. 1 × 10
6 cells were seeded into T25 vented cell culture flasks (Sarstedt, Nümbrecht, Germany) and incubated ON to ensure proper attachment of the cells. Prior to the installation onto the RPM start and the experimental run, the flasks were filled completely with medium, air bubble-free. After 5 d, half of the flasks were removed, and cells and supernatant were harvested. The remaining flasks underwent a media change as described earlier [
91], were incubated for another 5 d, and subsequently harvested. For the corresponding 1 g-controls, the flasks were placed and incubated adjacent to the RPM in the same incubator.
4.2. Random Positioning Machine
The desktop RPM (Airbus Defense and Space (ADS), Leiden, The Netherlands) was installed inside a standard incubator at 37 °C, 5% CO
2. The RPM was operated in real random mode with random direction and interval and at a maximum speed of 12.5 revolutions per minute. Sample flasks to be tested were placed onto the middle frame with a maximal distance of 7 cm to the center of rotation, allowing a μg quality between 10
−4 and 10
−2 g, which is reached over time [
49,
67]. Corresponding static 1 g-controls, which were completely filled with medium, were placed next to the RPM in the same incubator (
n = 15 samples for each group/run).
4.3. Exosome Harvest and Isolation
After the harvest, the cell supernatants of the RPM and control samples were subjected to an adjusted differential centrifugation protocol [
92] using a swinging bucket rotor, consisting of two consecutive centrifugations, 300×
g (10 min, 4 °C) followed by 2500×
g (15 min, 4 °C, twice) to pellet cells, cell debris, and large vesicles. High-speed centrifugation was not necessary as the analysis via ExoView
® does not require upstream particle isolation. The collected supernatants were divided into 2 mL aliquots and stored at −80 °C until further analysis.
4.4. ExoView® Kit Assay Procedure
For the capture and analysis of the CellBox-1 supernatants, the EV-TETRA-C ExoView Tetraspanin Kit (Unchained Labs, Pleasanton, CA, USA) was used, with all samples processed and stained according to the manufacturer’s protocol. The spots on the chip plates were coated with antibodies for the tetraspanins CD9, CD81, and CD63, as well as a negative IgG control, all in triplicates. In short, the samples, 0.5 uL, were diluted to 10 uL in PBS and incubated overnight, in a 1:2 dilution with the provided buffer (Solution A), on the sealed chip plate in order to capture the exosomes present. The incubation was followed by three wash steps prior to the surface membrane immunofluorescence staining. An antibody mixture containing fluorescently labeled anti-CD9 (CF® 488A), anti-CD81 (CF® 555), and anti-CD63 (CF® 647) was pipetted onto the chip and incubated for 1 h on an orbital shaker, blocked from light exposure. Three wash steps with the supplied wash buffer, and two washes with DI water for 3 min each (RT, shaking, light omitted) followed this incubation. Subsequently, the chip plates were dried and scanned as described below.
4.5. Digital Detection of Exosomes
The chip plates were scanned using the ExoView R100 (Unchained labs, Pleasanton, CA, USA) in combination with the ExoViewer software. All the chip plates were pre-scanned prior to the exosome capture to obtain a baseline signal; then, the exosome-laden chips were scanned identically positioned on the stage platform. The analysis was visualized with the ExoViewer software, resulting in the EV count, size distribution, and colocalization of EV subpopulations.
4.6. Statistical Analysis
The total counts of numbers were analyzed via the unpaired t-test, comparing all the samples from either the GM or FM group respective to the capture spot on the chip plate, CD9, CD63, or CD81. The analysis was conducted using the GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA).