Advances in Imaging of Inflammation, Fibrosis, and Cancer in the Gastrointestinal Tract
Abstract
:1. Introduction
2. Inflammation
2.1. Inflammatory Bowel Disease
2.2. Diverticulitis
2.3. Celiac Disease
2.4. Imaging Inflammation
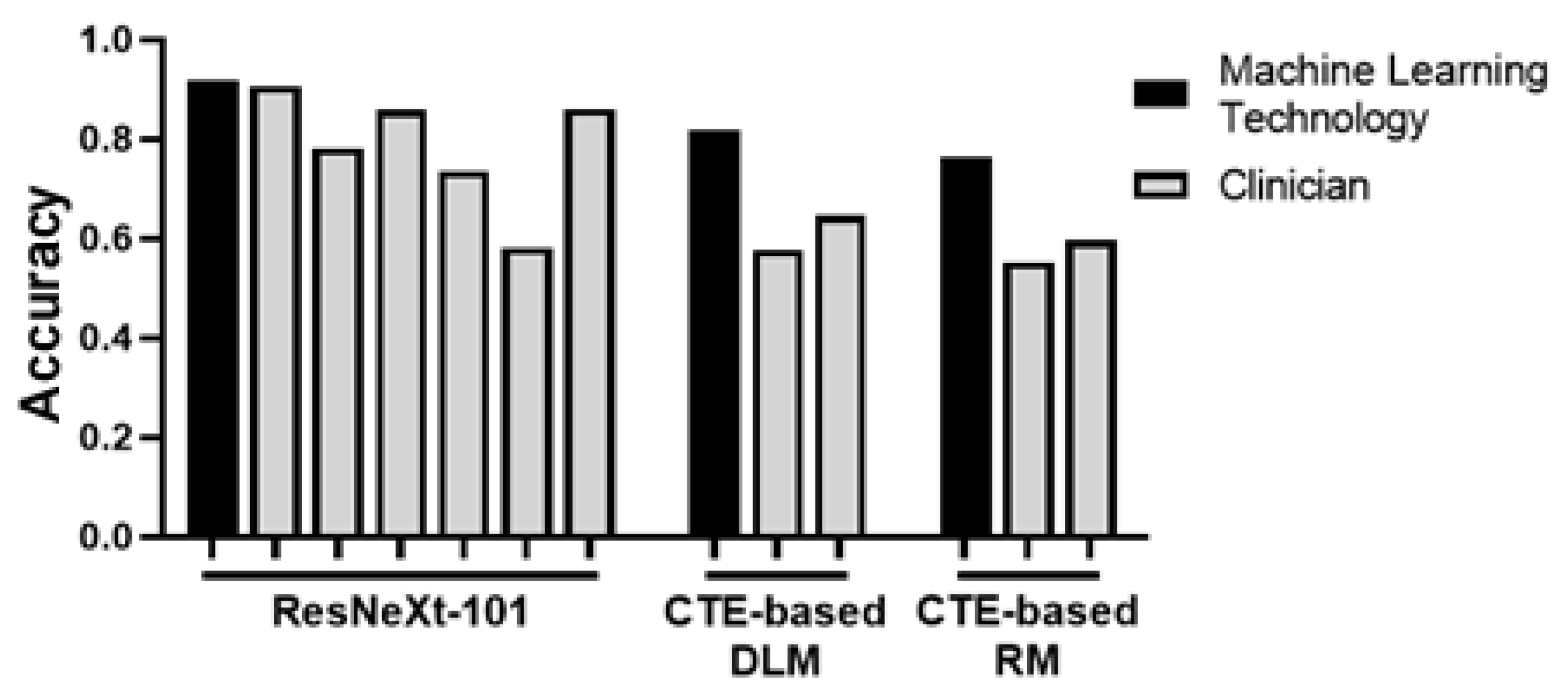
3. Fibrosis
3.1. Graft Versus Host Disease
3.2. Intestinal Fibrosis and Colorectal Stricture
3.3. Imaging Fibrosis
4. Cancer
4.1. Gastric and Colorectal Cancer
4.2. Imaging Gastrointestinal Tract Cancers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2021, 162, 621–644. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef] [Green Version]
- Lightdale, J.R.; Liu, Q.Y.; Sahn, B.; Troendle, D.M.; Thomson, M.; Fishman, D.S. Pediatric Endoscopy and High-risk Patients: A Clinical Report from the NASPGHAN Endoscopy Committee. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 595–606. [Google Scholar] [CrossRef]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Manne, S.; Treem, W.R.; Bennett, D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates from Large National Databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020, 26, 619–625. [Google Scholar] [CrossRef]
- Sýkora, J.; Pomahačová, R.; Kreslová, M.; Cvalínová, D.; Štych, P.; Schwarz, J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 2741–2763. [Google Scholar] [CrossRef]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative from the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2016, 389, 1756–1770. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis-a-Mon. 2019, 65, 100851. [Google Scholar] [CrossRef]
- Roberts-Thomson, I.C.; Bryant, R.V.; Costello, S.P. Uncovering the cause of ulcerative colitis. JGH Open 2019, 3, 274–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, M.; Klapproth, J.-M.A. The Role of Bacteria in the Pathogenesis of Ulcerative Colitis. J. Signal Transduct. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourad, F.H.; Hashash, J.G.; Kariyawasam, V.C.; Leong, R.W. Ulcerative Colitis and Cytomegalovirus Infection: From A to Z. J. Crohn’s Colitis 2020, 14, 1162–1171. [Google Scholar] [CrossRef]
- Wada, Y.; Matsui, T.; Matake, H.; Sakurai, T.; Yamamoto, J.; Kikuchi, Y.; Yorioka, M.; Tsuda, S.; Yao, T.; Yao, S.; et al. Intractable ulcerative colitis caused by cytomegalovirus infection: A prospective study on prevalence, diagnosis, and treatment. Dis. Colon Rectum 2003, 46. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Ulcerative Colitis: Epidemiology, diagnosis, and management. Mayo Clin. Proc. 2014, 89, 1553–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaenkumchorn, T.; Wahbeh, G. Ulcerative Colitis: Making the Diagnosis. Gastroenterol. Clin. N. Am. 2020, 49, 655–669. [Google Scholar] [CrossRef]
- Veauthier, B.; Hornecker, J.R. Crohn’s Disease: Diagnosis and Management. Am. Fam. Physician 2018, 98, 661–669. [Google Scholar]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis.-a-Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Kilcoyne, A.; Kaplan, J.L.; Gee, M.S. Inflammatory bowel disease imaging: Current practice and future directions. World J. Gastroenterol. 2016, 22, 917–932. [Google Scholar] [CrossRef]
- Geboes, K. Crohn’s disease, ulcerative colitis or indeterminate colitis—How important is it to differentiate? Acta Gastro-Enterol. Belg. 2001, 64, 197–200. [Google Scholar]
- Park, S.-K.; Kim, S.; Lee, G.-Y.; Kim, S.-Y.; Kim, W.; Lee, C.-W.; Park, J.-L.; Choi, C.-H.; Kang, S.-B.; Kim, T.-O.; et al. Development of a Machine Learning Model to Distinguish between Ulcerative Colitis and Crohn’s Disease Using RNA Sequencing Data. Diagnostics 2021, 11, 2365. [Google Scholar] [CrossRef] [PubMed]
- Alghoul, Z.; Yang, C.; Merlin, D. The Current Status of Molecular Biomarkers for Inflammatory Bowel Disease. Biomedicines 2022, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F. Management of colonic diverticulitis. BMJ 2021, 372, n72. [Google Scholar] [CrossRef]
- Simianu, V.V.; Flum, D.R. Rethinking elective colectomy for diverticulitis: A strategic approach to population health. World J. Gastroenterol. 2014, 20, 16609–16614. [Google Scholar] [CrossRef]
- Wilkins, T.; Embry, K.; George, R. Diagnosis and management of acute diverticulitis. Am. Fam. Physician 2013, 87, 612–620. [Google Scholar]
- Bailey, J.; Dattani, S.; Jennings, A. Diverticular Disease: Rapid Evidence Review. Am. Fam. Physician 2022, 106, 150–156. [Google Scholar] [PubMed]
- Frickenstein, A.N.; Jones, M.A.; Behkam, B.; McNally, L.R. Imaging Inflammation and Infection in the Gastrointestinal Tract. Int. J. Mol. Sci. 2019, 21, 243. [Google Scholar] [CrossRef] [Green Version]
- Laméris, W.; Van Randen, A.; Van Gulik, T.M.; Busch, O.R.C.; Winkelhagen, J.; Bossuyt, P.M.M.; Stoker, J.; Boermeester, M.A. A Clinical Decision Rule to Establish the Diagnosis of Acute Diverticulitis at the Emergency Department. Dis. Colon Rectum 2010, 53, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; MacCuaig, W.M.; Frickenstein, A.N.; Camalan, S.; Gurcan, M.N.; Holter-Chakrabarty, J.; Morris, K.T.; McNally, M.W.; Booth, K.K.; Carter, S.; et al. Molecular Imaging of Inflammatory Disease. Biomedicines 2021, 9, 152. [Google Scholar] [CrossRef]
- Gujral, N.; Freeman, H.J.; Thomson, A.B. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J. Gastroenterol. 2012, 18, 6036–6059. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Vanoli, A.; Leoncini, G.; Arpa, G.; Salviato, T.; Bonetti, L.R.; Baronchelli, C.; Saragoni, L.; Parente, P. Celiac disease: Histology-differential diagnosis-complications. A practical approach. Pathologica 2020, 112, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Beig, J.; Rostami, K.; Hayman, D.T.S.; Hassan, S.; Gerred, S.; Ogra, R. Is duodenal biopsy always necessary for the diagnosis of coeliac disease in adult patients with high anti-tissue transglutaminase (TTG) antibody titres? Front. Gastroenterol. 2021, 13, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Tye-Din, J.A.; Qiao, S.-W.; Russell, A.K.; Mayassi, T.; Ciszewski, C.; Sarna, V.K.; Wang, S.; Goldstein, K.E.; Dzuris, J.L.; et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv. 2019, 5, eaaw7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarna, V.K.; Lundin, K.E.; Mørkrid, L.; Qiao, S.-W.; Sollid, L.M.; Christophersen, A. HLA-DQ–Gluten Tetramer Blood Test Accurately Identifies Patients With and Without Celiac Disease in Absence of Gluten Consumption. Gastroenterology 2018, 154, 886–896.e6. [Google Scholar] [CrossRef] [Green Version]
- Hundorfean, G.; Pereira, S.P.; Karstensen, J.G.; Vilmann, P.; Saftoiu, A. Modern Endoscopic Imaging in Diagnosis and Surveillance of Inflammatory Bowel Disease Patients. Gastroenterol. Res. Pract. 2018, 2018, 5738068. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Wang, X.; Liu, K.; Li, T.; Yu, Y.; Han, J.; Xing, S.; Xu, J.; Tian, D.; et al. Development of a Convolutional Neural Network-Based Colonoscopy Image Assessment Model for Differentiating Crohn’s Disease and Ulcerative Colitis. Front. Med. 2022, 9, 980. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Angelucci, E.; Vetrano, S.; Pagano, N.; Rando, G.; Spinelli, A.; Malesci, A.; Repici, A. Narrow-band imaging endoscopy to assess mucosal angiogenesis in inflammatory bowel disease: A pilot study. World J. Gastroenterol. 2010, 16, 2396–2400. [Google Scholar] [CrossRef]
- Fan, Y.; Mu, R.; Xu, H.; Xie, C.; Zhang, Y.; Liu, L.; Wang, L.; Shi, H.; Hu, Y.; Ren, J.; et al. Novel deep learning–based computer-aided diagnosis system for predicting inflammatory activity in ulcerative colitis. Gastrointest. Endosc. 2022. [Google Scholar] [CrossRef]
- Baker, M.E.; Hara, A.K.; Platt, J.F.; Maglinte, D.D.T.; Fletcher, J.G. CT enterography for Crohn’s disease: Optimal technique and imaging issues. Abdom. Imaging 2015, 40, 938–952. [Google Scholar] [CrossRef]
- Khatri, G.; Coleman, J.; Leyendecker, J.R. Magnetic Resonance Enterography for Inflammatory and Noninflammatory Conditions of the Small Bowel. Radiol. Clin. N. Am. 2018, 56, 671–689. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, Y.; Park, S.H.; Kang, B.-K.; Seo, N.; Yang, S.-K.; Ye, B.D.; Park, S.H.; Kim, S.Y.; Baek, S.; et al. Diffusion-weighted MR Enterography for Evaluating Crohn’s Disease: How does it add diagnostically to conventional MR enterography? Inflamm. Bowel Dis. 2015, 21, 101–109. [Google Scholar] [CrossRef]
- Flynn, A.D.; Valentine, J.F. Chromoendoscopy for Dysplasia Surveillance in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 1440–1452. [Google Scholar] [CrossRef]
- Ziech, M.L.W.; Hummel, T.Z.; Smets, A.M.J.B.; Nievelstein, R.A.J.; Lavini, C.; Caan, M.W.; Nederveen, A.J.; Roelofs, J.J.T.H.; Bipat, S.; Benninga, M.A.; et al. Accuracy of abdominal ultrasound and MRI for detection of Crohn disease and ulcerative colitis in children. Pediatr. Radiol. 2014, 44, 1370–1378. [Google Scholar] [CrossRef]
- Langhorst, J.; Umutlu, L.; Schaarschmidt, B.M.; Grueneisen, J.; Demircioglu, A.; Forsting, M.; Beiderwellen, K.; Haubold, J.; Theysohn, J.M.; Koch, A.K.; et al. Diagnostic Performance of Simultaneous [18F]-FDG PET/MR for Assessing Endoscopically Active Inflammation in Patients with Ulcerative Colitis: A Prospective Study. J. Clin. Med. 2020, 9, 2474. [Google Scholar] [CrossRef]
- Bhutiani, N.; Grizzle, W.E.; Galandiuk, S.; Otali, D.; Dryden, G.W.; Egilmez, N.K.; McNally, L.R. Noninvasive Imaging of Colitis Using Multispectral Optoacoustic Tomography. J. Nucl. Med. 2017, 58, 1009–1012. [Google Scholar] [CrossRef] [Green Version]
- Knieling, F.; Neufert, C.; Hartmann, A.; Claussen, J.; Urich, A.; Egger, C.; Vetter, M.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography for Assessment of Crohn’s disease Activity. N. Engl. J. Med. 2017, 376, 1292–1294. [Google Scholar] [CrossRef]
- Nazerian, P.; Gigli, C.; Donnarumma, E.; de Curtis, E.; Bribani, A.; Lanzi, S.; Rovida, S.; Magazzini, S.; Grifoni, S.; Perani, C. Diagnostic Accuracy of Point-of-Care Ultrasound Integrated into Clinical Examination for Acute Diverticulitis: A Prospective Multicenter Study. Ultraschall Med. 2021, 42, 614–622. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, J.; Park, Y.Y.; Oh, S.T. Routine colonoscopy may be needed for uncomplicated acute right colonic diverticulitis. BMC Gastroenterol. 2021, 21, 91. [Google Scholar] [CrossRef]
- Schreyer, A.G.; Fürst, A.; Agha, A.; Kikinis, R.; Scheibl, K.; Schölmerich, J.; Feuerbach, S.; Herfarth, H.; Seitz, J. Magnetic resonance imaging based colonography for diagnosis and assessment of diverticulosis and diverticulitis. Int. J. Color. Dis. 2004, 19, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Rotert, H.; Nöldge, G.; Encke, J.; Richter, G.M.; Düx, M. The value of CT for the diagnosis of acute diverticulitis. Der Radiol. 2003, 43, 51–58. [Google Scholar] [CrossRef]
- Cammarota, G.; Cuoco, L.; Cesaro, P.; Santoro, L.; Cazzato, A.; Montalto, M.; La Mura, R.; Larocca, L.M.; Vecchio, F.M.; Gasbarrini, A.; et al. A highly accurate method for monitoring histological recovery in patients with celiac disease on a gluten-free diet using an endoscopic approach that avoids the need for biopsy: A double-center study. Endoscopy 2007, 39, 46–51. [Google Scholar] [CrossRef]
- Eid, M.; Abougabal, A.; Zeid, A. Celiac disease: Do not miss that diagnosis! Egypt. J. Radiol. Nucl. Med. 2022, 44, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Fraquelli, M.; Colli, A.; Colucci, A.; Bardella, M.T.; Trovato, C.; Pometta, R.; Pagliarulo, M.; Conte, D. Accuracy of Ultrasonography in Predicting Celiac Disease. Arch. Intern. Med. 2004, 164, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Stelljes, M.; Hermann, S.; Albring, J.; Köhler, G.; Löffler, M.; Franzius, C.; Poremba, C.; Schlösser, V.; Volkmann, S.; Opitz, C.; et al. Clinical molecular imaging in intestinal graft-versus-host disease: Mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood 2008, 111, 2909–2918. [Google Scholar] [CrossRef] [Green Version]
- Budjan, J.; Michaely, H.J.; Attenberger, U.; Haneder, S.; Heidenreich, D.; Kreil, S.; Nolte, F.; Hofmann, W.-K.; Schoenberg, S.O.; Klein, S.A. Assessment of acute intestinal graft versus host disease by abdominal magnetic resonance imaging at 3 Tesla. Eur. Radiol. 2014, 24, 1835–1844. [Google Scholar] [CrossRef]
- Roll, W.; Schindler, P.; Masthoff, M.; Strotmann, R.; Albring, J.; Reicherts, C.; Weckesser, M.; Noto, B.; Stelljes, M.; Schäfers, M.; et al. 18F-FDG-PET-MRI for the assessment of acute intestinal graft-versus-host-disease (GvHD). BMC Cancer 2021, 21, 1015. [Google Scholar] [CrossRef]
- Calabrese, E.; Zorzi, F.; Visconti, E.; De Angelis, G.; Cerretti, R.; Del Vecchio Blanco, G.; Picardi, A.; Cudillo, L.; Postorino, M.; Franceschini, L.; et al. Bowel ultrasonography as an aid for diagnosis of intestinal acute graft-versus-host-disease after allogeneic haematopoietic stem cell transplantation. Dig. Liver Dis. 2013, 45, 899–904. [Google Scholar] [CrossRef]
- Shimoni, A.; Rimon, U.; Hertz, M.; Yerushalmi, R.; Amitai, M.; Portnoy, O.; Guranda, L.; Nagler, A.; Apter, S. CT in the clinical and prognostic evaluation of acute graft-vs-host disease of the gastrointestinal tract. Br. J. Radiol. 2012, 85, e416–e423. [Google Scholar] [CrossRef] [Green Version]
- Du, J.-F.; Lu, B.-L.; Huang, S.-Y.; Mao, R.; Zhang, Z.-W.; Cao, Q.-H.; Chen, Z.-H.; Li, S.-Y.; Qin, Q.-L.; Sun, C.-H.; et al. A novel identification system combining diffusion kurtosis imaging with conventional magnetic resonance imaging to assess intestinal strictures in patients with Crohn’s disease. Abdom. Radiol. 2020, 46, 936–947. [Google Scholar] [CrossRef]
- Li, X.; Liang, D.; Meng, J.; Zhou, J.; Chen, Z.; Huang, S.; Lu, B.; Qiu, Y.; Baker, M.E.; Ye, Z.; et al. Development and Validation of a Novel Computed-Tomography Enterography Radiomic Approach for Characterization of Intestinal Fibrosis in Crohn’s Disease. Gastroenterology 2021, 160, 2303–2316.e11. [Google Scholar] [CrossRef]
- Meng, J.; Luo, Z.; Chen, Z.; Zhou, J.; Chen, Z.; Lu, B.; Zhang, M.; Wang, Y.; Yuan, C.; Shen, X.; et al. Intestinal fibrosis classification in patients with Crohn’s disease using CT enterography–based deep learning: Comparisons with radiomics and radiologists. Eur. Radiol. 2022, 1–14. [Google Scholar] [CrossRef]
- Lu, B.; Lin, J.; Du, J.; He, S.; Cao, Q.; Huang, L.; Mao, R.; Sun, C.; Li, Z.; Feng, S.; et al. Native T1 Mapping and Magnetization Transfer Imaging in Grading Bowel Fibrosis in Crohn’s Disease: A Comparative Animal Study. Biosensors 2021, 11, 302. [Google Scholar] [CrossRef]
- Chen, B.-B.; Liang, P.-C.; Liu, K.-L.; Hsiao, J.-K.; Huang, J.-C.; Wong, J.-M.; Lee, P.-H.; Shun, C.-T.; Ming-Tsang, Y. Preoperative Diagnosis of Gastric Tumors by Three-dimensional Multidetector Row CT and Double Contrast Barium Meal Study: Correlation with Surgical and Histologic Results. J. Formos. Med. Assoc. 2007, 106, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-K.; Kuo, Y.-T.; Liu, G.-C.; Tsai, K.-B.; Huang, Y.-S. Dynamic Contrast-Enhanced Subtraction and Delayed MRI of Gastric Tumors: Radiologic–Pathologic Correlation. J. Comput. Assist. Tomogr. 2000, 24, 872–877. [Google Scholar] [CrossRef]
- Redondo-Cerezo, E.; Martínez-Cara, J.G.; Jiménez-Rosales, R.; Valverde-López, F.; Caballero-Mateos, A.M.; Jérvez-Puente, P.; Ariza-Fernández, J.L.; Úbeda-Muñoz, M.; López-De-Hierro, M.; De Teresa, J. Endoscopic ultrasound in gastric cancer staging before and after neoadjuvant chemotherapy. A comparison with PET-CT in a clinical series. United Eur. Gastroenterol. J. 2017, 5, 641–647. [Google Scholar] [CrossRef]
- Gertsen, E.C.; Brenkman, H.J.F.; van Hillegersberg, R.; van Sandick, J.W.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Luyer, M.D.P.; Nieuwenhuijzen, G.A.P.; van Lanschot, J.J.B.; Lagarde, S.M.; et al. 18F-Fludeoxyglucose–Positron Emission Tomography/Computed Tomography and Laparoscopy for Staging of Locally Advanced Gastric Cancer: A Multicenter Prospective Dutch Cohort Study (PLASTIC). JAMA Surg. 2021, 156, e215340. [Google Scholar] [CrossRef]
- Smyth, E.; Schöder, H.; Strong, V.E.; Capanu, M.; Kelsen, D.P.; Coit, D.G.; Shah, M.A. A prospective evaluation of the utility of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 2012, 118, 5481–5488. [Google Scholar] [CrossRef]
- Ko, C.W.; Doria-Rose, V.P.; Barrett, M.J.; Kamineni, A.; Enewold, L.; Weiss, N.S. Screening colonoscopy and flexible sigmoidoscopy for reduction of colorectal cancer incidence: A case-control study. PLoS ONE 2019, 14, e0226027. [Google Scholar] [CrossRef] [Green Version]
- Gutman, F.; Alberini, J.-L.; Wartski, M.; Vilain, D.; Le Stanc, E.; Sarandi, F.; Corone, C.; Tainturier, C.; Pecking, A.P. Incidental Colonic Focal Lesions Detected by FDG PET/CT. Am. J. Roentgenol. 2005, 185, 495–500. [Google Scholar] [CrossRef]
- Callstrom, M.R.; Johnson, C.D.; Fletcher, J.G.; Reed, J.E.; Ahlquist, D.A.; Harmsen, W.S.; Tait, K.; Wilson, L.A.; Corcoran, K.E. CT Colonography without Cathartic Preparation: Feasibility Study. Radiology 2001, 219, 693–698. [Google Scholar] [CrossRef]
- Bedrikovetski, S.; Dudi-Venkata, N.N.; Kroon, H.M.; Seow, W.; Vather, R.; Carneiro, G.; Moore, J.W.; Sammour, T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 1058. [Google Scholar] [CrossRef]
- Rao, S.-X.; Zeng, M.S.; Xu, J.M.; Qin, X.Y.; Chen, C.Z.; Li, R.C.; Hou, Y.Y. Assessment of T staging and mesorectal fascia status using high-resolution MRI in rectal cancer with rectal distention. World J. Gastroenterol. 2007, 13, 4141–4146. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lemke, C.; Cox, B.F.; Newton, I.P.; Nathke, I.; Cochran, S. A Learning-Based Microultrasound System for the Detection of Inflammation of the Gastrointestinal Tract. IEEE Trans. Med. Imaging 2021, 40, 38–47. [Google Scholar] [CrossRef]
- Lalosevic, M.S.; Milutinovic, A.S.; Zaric, V.M.; Lolic, I.; Toplicanin, A.; Dragasevic, S.; Stojkovic, M.; Stojanovic, M.; Aleksic, M.; Stjepanovic, M.; et al. Intestinal Ultrasonography as a Tool for Monitoring Disease Activity in Patients with Ulcerative Colitis. Int. J. Clin. Pract. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Biernacka, K.B.; Barańska, D.; Matera, K.; Podgórski, M.; Czkwianianc, E.; Szabelska-Zakrzewska, K.; Dziembowska, I.; Grzelak, P. The value of magnetic resonance enterography in diagnostic difficulties associated with Crohn’s disease. Pol. J. Radiol. 2021, 86, 143–150. [Google Scholar] [CrossRef]
- Golusda, L.; Kühl, A.A.; Lehmann, M.; Dahlke, K.; Mueller, S.; Boehm-Sturm, P.; Saatz, J.; Traub, H.; Schnorr, J.; Freise, C.; et al. Visualization of Inflammation in Experimental Colitis by Magnetic Resonance Imaging Using Very Small Superparamagnetic Iron Oxide Particles. Front. Physiol. 2022, 13, 1369. [Google Scholar] [CrossRef]
- Wang, H.; Machtaler, S.; Bettinger, T.; Lutz, A.M.; Luong, R.; Bussat, P.; Gambhir, S.S.; Tranquart, F.; Tian, L.; Willmann, J.K. Molecular Imaging of Inflammation in Inflammatory Bowel Disease with a Clinically Translatable Dual-Selectin–targeted US Contrast Agent: Comparison with FDG PET/CT in a Mouse Model. Radiology 2013, 267, 818–829. [Google Scholar] [CrossRef] [Green Version]
- Aarntzen, E.H.; Hermsen, R.; Drenth, J.P.; Boerman, O.C.; Oyen, W.J. 99mTc-CXCL8 SPECT to Monitor Disease Activity in Inflammatory Bowel Disease. J. Nucl. Med. 2015, 57, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Dmochowska, N.; Tieu, W.; Keller, M.D.; Wardill, H.R.; Mavrangelos, C.; Campaniello, M.A.; Takhar, P.; Hughes, P.A. Immuno-PET of Innate Immune Markers CD11b and IL-1β Detects Inflammation in Murine Colitis. J. Nucl. Med. 2019, 60, 858–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCUAIG, W.M.; Jones, M.A.; Abeyakoon, O.; McNally, L.R. Development of Multispectral Optoacoustic Tomography as a Clinically Translatable Modality for Cancer Imaging. Radiol. Imaging Cancer 2020, 2, e200066. [Google Scholar] [CrossRef]
- Sun, L.; Ouyang, J.; Zeng, F.; Wu, S. An AIEgen-based oral-administration nanosystem for detection and therapy of ulcerative colitis via 3D-MSOT/NIR-II fluorescent imaging and inhibiting NLRP3 inflammasome. Biomaterials 2022, 283, 121468. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Samykutty, A.; McMasters, K.M.; Egilmez, N.K.; McNally, L.R. In vivo tracking of orally-administered particles within the gastrointestinal tract of murine models using multispectral optoacoustic tomography. Photoacoustics 2018, 13, 46–52. [Google Scholar] [CrossRef] [PubMed]
- McNally, L.R.; Mezera, M.; Morgan, D.E.; Frederick, P.J.; Yang, E.S.; Eltoum, I.-E.; Grizzle, W.E. Current and Emerging Clinical Applications of Multispectral Optoacoustic Tomography (MSOT) in Oncology. Clin. Cancer Res. 2016, 22, 3432–3439. [Google Scholar] [CrossRef] [Green Version]
- Waldner, M.J.; Knieling, F.; Egger, C.; Morscher, S.; Claussen, J.; Vetter, M.; Kielisch, C.; Fischer, S.; Pfeifer, L.; Hagel, A.; et al. Multispectral Optoacoustic Tomography in Crohn’s Disease: Noninvasive Imaging of Disease Activity. Gastroenterology 2016, 151, 238–240. [Google Scholar] [CrossRef] [Green Version]
- Knieling, F.; Hartmann, A.; Claussen, J.; Urich, A.; Atreya, R.; Rascher, W.; Waldner, M. Multispectral Optoacoustic Tomography in Ulcerative Colitis—A First-in-human Diagnostic Clinical Trial. J. Nucl. Med. 2017, 58, 1196. [Google Scholar]
- Rieder, F.; Bettenworth, D.; Ma, C.; Parker, C.E.; Williamson, L.A.; Nelson, S.A.; Van Assche, G.; Di Sabatino, A.; Bouhnik, Y.; Stidham, R.W.; et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 347–357. [Google Scholar] [CrossRef]
- Alfredsson, J.; Wick, M.J. Mechanism of fibrosis and stricture formation in Crohn’s disease. Scand. J. Immunol. 2020, 92, e12990. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef]
- Zeiser, R.; Blazar, B.R.; Longo, D.L. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef]
- Reddy, P. Pathophysiology of acute graft-versus-host disease. Hematol. Oncol. 2003, 21, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Reddy, P. Pathophysiology of Graft-Versus-Host Disease. Semin. Hematol. 2006, 43, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Socié, G. Treating chronic GVHD-induced fibrosis? Blood 2018, 131, 1396–1397. [Google Scholar] [CrossRef]
- Gergoudis, S.C.; DeFilipp, Z.; Özbek, U.; Sandhu, K.S.; Etra, A.M.; Choe, H.K.; Kitko, C.L.; Ayuk, F.; Aziz, M.; Baez, J.; et al. Biomarker-guided preemption of steroid-refractory graft-versus-host disease with α-1-antitrypsin. Blood Adv. 2020, 4, 6098–6105. [Google Scholar] [CrossRef]
- Srinagesh, H.K.; Ferrara, J.L. MAGIC biomarkers of acute graft-versus-host disease: Biology and clinical application. Best Pract. Res. Clin. Haematol. 2019, 32, 101111. [Google Scholar] [CrossRef]
- Rowlings, P.A.; Przepiorka, D.; Klein, J.P.; Gale, R.P.; Passweg, J.R.; Henslee-Downey, P.J.; Cahn, J.; Calderwood, S.; Gratwohl, A.; Socié, G.; et al. IBMTR Severity Index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. Br. J. Haematol. 1997, 97, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Martino, R.; Romero, P.; Subirá, M.; Bellido, M.; Altés, A.; Sureda, A.; Brunet, S.; Badell, I.; Cubells, J.; Sierra, J. Comparison of the classic Glucksberg criteria and the IBMTR Severity Index for grading acute graft-versus-host disease following HLA-identical sibling stem cell transplantation. International Bone Marrow Transplant Registry. Bone Marrow Transplant. 1999, 24, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Axelrad, J.E.; Faye, A.; Slaughter, J.C.; Harpaz, N.; Itzkowitz, S.H.; Shah, S.C. Colorectal Strictures in Patients with Inflammatory Bowel Disease Do Not Independently Predict Colorectal Neoplasia. Inflamm. Bowel Dis. 2021, 28, 855–861. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Jeon, S.W.; Lee, Y.K. Endoscopic Management of Refractory Benign Colorectal Strictures. Clin. Endosc. 2013, 46, 472–475. [Google Scholar] [CrossRef]
- Liu, Y.; He, S.; Zhang, Y.; Dou, L.; Liu, X.; Yu, X.; Lu, N.; Xue, L.; Wang, G. Comparing long-term outcomes between endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR) for type II esophagogastric junction neoplasm. Ann. Transl. Med. 2021, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.H.; Ge, J.; Yang, C.M.; Liu, J.Y.; Zhao, S.L. Endoscopic submucosal dissection vs. endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J. Gastroenterol. 2014, 20, 8282–8287. [Google Scholar] [CrossRef] [PubMed]
- Ohara, Y.; Toyonaga, T.; Tanaka, S.; Ishida, T.; Hoshi, N.; Yoshizaki, T.; Kawara, F.; Lui, K.L.; Tepmalai, K.; Damrongmanee, A.; et al. Risk of stricture after endoscopic submucosal dissection for large rectal neoplasms. Endoscopy 2016, 48, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Mao, Y.; Zhou, J.; Chen, Z.; Huang, S.; Wang, Y.; Huang, L.; Zhang, R.; Shen, X.; Lv, W.; et al. Mesenteric abnormalities play an important role in grading intestinal fibrosis in patients with Crohn’s disease: A computed tomography and clinical marker-based nomogram. Ther. Adv. Gastroenterol. 2022, 15. [Google Scholar] [CrossRef]
- Caruso, A.; Angriman, I.; Scarpa, M.; D’Incà, R.; Mescoli, C.; Rudatis, M.; Sturniolo, G.C.; Schifano, G.; Lacognata, C. Diffusion-weighted magnetic resonance for assessing fibrosis in Crohn’s disease. Abdom. Radiol. 2020, 45, 2327–2335. [Google Scholar] [CrossRef]
- Jensen, J.H.; Helpern, J.A.; Ramani, A.; Lu, H.; Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1432–1440. [Google Scholar] [CrossRef]
- Regensburger, A.P.; Fonteyne, L.M.; Jüngert, J.; Wagner, A.L.; Gerhalter, T.; Nagel, A.M.; Heiss, R.; Flenkenthaler, F.; Qurashi, M.; Neurath, M.F.; et al. Detection of collagens by multispectral optoacoustic tomography as an imaging biomarker for Duchenne muscular dystrophy. Nat. Med. 2019, 25, 1905–1915. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Mukaisho, K.-I.; Nakayama, T.; Hagiwara, T.; Hattori, T.; Sugihara, H. Two distinct etiologies of gastric cardia adenocarcinoma: Interactions among pH, Helicobacter pylori, and bile acids. Front. Microbiol. 2015, 6, 412. [Google Scholar] [CrossRef] [Green Version]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.; Vollset, S.E.; Derakhshan, M.; Fyfe, V.; Melby, K.K.; Aase, S.; Jellum, E.; McColl, K.E.L. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut 2007, 56, 918–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrumurthy, S.G.; Chaudry, M.A.; Hochhauser, D.; Mughal, M. The diagnosis and management of gastric cancer. BMJ 2013, 347, f6367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotfollahzadeh, S.; Recio-Boiles, A.; Cagir, B. Colon Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [Green Version]
- Holtedahl, K.; Borgquist, L.; Donker, G.A.; Buntinx, F.; Weller, D.; Campbell, C.; Månsson, J.; Hammersley, V.; Braaten, T.; Parajuli, R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: A prospective cohort study of diagnostic accuracy in primary care. BMC Fam. Pract. 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Shaukat, A.; Kahi, C.J.; Burke, C.A.; Rabeneck, L.; Sauer, B.G.; Rex, D.K. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am. J. Gastroenterol. 2021, 116, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Kwong, T.N.Y.; Chow, T.-C.; Luk, A.K.C.; Dai, R.Z.W.; Nakatsu, G.; Lam, T.Y.T.; Zhang, L.; Wu, J.C.Y.; Chan, F.K.L.; et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut 2017, 66, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Hayne, D.; Brown, R.; McCormack, M.; Quinn, M.; Payne, H.; Babb, P. Current Trends in Colorectal Cancer: Site, Incidence, Mortality and Survival in England and Wales. Clin. Oncol. 2001, 13, 448–452. [Google Scholar] [CrossRef]
- Horvat, N.; Carlos Tavares Rocha, C.; Clemente Oliveira, B.; Petkovska, I.; Gollub, M.J. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019, 39, 367–387. [Google Scholar] [CrossRef]
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.P.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.S.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA 2021, 325, 1998–2011. [Google Scholar] [CrossRef]
- Wright, A.P.; Piper, M.S.; Bishu, S.; Stidham, R.W. Systematic review and case series: Flexible sigmoidoscopy identifies most cases of checkpoint inhibitor-induced colitis. Aliment. Pharmacol. Ther. 2019, 49, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.; Lieberman, D.A.; McFarland, B.; Andrews, K.S.; Brooks, D.; Bond, J.; Dash, C.; Giardiello, F.M.; Glick, S.; Johnson, D.; et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 2008, 58, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmedovi, A.; Mesihovi, R.; Saray, A.; Vanis, N. Gastric Cancer Staging: EUS And CT. Med. Arch. 2014, 68, 34–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocellin, S.; Pasquali, S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst. Rev. 2015, 2015, CD009944. [Google Scholar] [CrossRef]
- Basij, M.; Yan, Y.; Alshahrani, S.S.; Helmi, H.; Burton, T.K.; Burmeister, J.W.; Dominello, M.M.; Winer, I.S.; Mehrmohammadi, M. Miniaturized phased-array ultrasound and photoacoustic endoscopic imaging system. Photoacoustics 2019, 15, 100139. [Google Scholar] [CrossRef]
- Chuah, S.Y.; Attia, A.B.E.; Long, V.; Ho, C.J.H.; Malempati, P.; Fu, C.Y.; Ford, S.J.; Lee, J.S.S.; Tan, W.P.; Razansky, D.; et al. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography. Ski. Res. Technol. 2016, 23, 221–226. [Google Scholar] [CrossRef]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef] [Green Version]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J.; et al. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology 2018, 287, 398–412. [Google Scholar] [CrossRef]
- Menezes, G.L.G.; Pijnappel, R.M.; Meeuwis, C.; Bisschops, R.; Veltman, J.; Lavin, P.T.; van de Vijver, M.; Mann, R. Downgrading of Breast Masses Suspicious for Cancer by Using Optoacoustic Breast Imaging. Radiology 2018, 288, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Krönke, M.; Karlas, A.; Fasoula, N.; Markwardt, N.; Kallmayer, M.; Eckstein, H.; Scheidhauer, K.; Weber, W.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT): A Novel Label-Free Imaging Technique for Thyroid Imaging. Nuklearmedizin 2019, 58, 142–143. [Google Scholar] [CrossRef]
- Borggreve, A.S.; Goense, L.; Brenkman, H.J.; Mook, S.; Meijer, G.J.; Wessels, F.J.; Verheij, M.; Jansen, E.P.; Van Hillegersberg, R.; Van Rossum, P.S.; et al. Imaging strategies in the management of gastric cancer: Current role and future potential of MRI. Br. J. Radiol. 2019, 92, 20181044. [Google Scholar] [CrossRef]
- Tham, E.; Sestito, M.; Markovich, B.; Garland-Kledzik, M. Current and future imaging modalities in gastric cancer. J. Surg. Oncol. 2022, 125, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Lee, D.H.; Lee, S.H.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Jung, S.H.; Kim, N.Y.; Kim, Y.H.; Lee, K.H.; et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J. Gastroenterol. Hepatol. 2010, 25, 512–518. [Google Scholar] [CrossRef]
- Ankersmit, M.; Hoekstra, O.S.; van Lingen, A.; Bloemena, E.; Jacobs, M.A.J.M.; Vugts, D.J.; Bonjer, H.J.; van Dongen, G.A.M.S.; Meijerink, W.J.H.J. Perioperative PET/CT lymphoscintigraphy and fluorescent real-time imaging for sentinel lymph node mapping in early staged colon cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, N.; Chen, Z.; Liu, T.; Xu, X.; Lei, X.; Shen, L.; Gao, J.; Yang, Z.; Zhu, H. Construction of 124I-trastuzumab for noninvasive PET imaging of HER2 expression: From patient-derived xenograft models to gastric cancer patients. Gastric Cancer 2020, 23, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.M.; Holter-Chakrabarty, J.; Lindenberg, L.; Duong, Q.; Vesely, S.K.; Nguyen, C.T.; Havlicek, J.P.; Kurdziel, K.; Gea-Banacloche, J.; Lin, F.I.; et al. Imaging of subclinical haemopoiesis after stem-cell transplantation in patients with haematological malignancies: A prospective pilot study. Lancet Haematol. 2018, 5, e44–e52. [Google Scholar] [CrossRef]
- Ribas, A.; Benz, M.R.; Allen-Auerbach, M.S.; Radu, C.; Chmielowski, B.; Seja, E.; Williams, J.L.; Gomez-Navarro, J.; McCarthy, T.; Czernin, J. Imaging of CTLA4 Blockade–Induced Cell Replication with 18F-FLT PET in Patients with Advanced Melanoma Treated with Tremelimumab. J. Nucl. Med. 2010, 51, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Williams, K.M.; Chakrabarty, J.H. Imaging haemopoietic stem cells and microenvironment dynamics through transplantation. Lancet Haematol. 2020, 7, e259–e269. [Google Scholar] [CrossRef]
- Iravani, A.; Hofman, M.; Hicks, R. Clinical impact of haematopoiesis imaging with 18F-Fluorothymidine (FLT) PET/CT in patients with hematologic disorders or bone marrow compartment involvement. J. Nucl. Med. 2017, 58, 186. [Google Scholar]
- Shields, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.-J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef]
- Volpe, A.; Adusumilli, P.S.; Schöder, H.; Ponomarev, V. Imaging cellular immunotherapies and immune cell biomarkers: From preclinical studies to patients. J. Immunother. Cancer 2022, 10, e004902. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.; Trager, M.H.; Rizk, E.M.; Finkel, G.G.; Barker, L.W.; Carvajal, R.D.; Geskin, L.J.; Schwartz, G.K.; Schwartz, L.; Dercle, L.; et al. FLT-PET At 6 Weeks Predicts Response Assessed by CT at 12 Weeks in Melanoma Patients Treated with Pembrolizumab. Clin. Nucl. Med. 2020, 45, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Aarntzen, E.H.J.G.; Srinivas, M.; De Wilt, J.H.W.; Jacobs, J.F.M.; Lesterhuis, W.J.; Windhorst, A.D.; Troost, E.G.; Bonenkamp, J.J.; van Rossum, M.M.; Blokx, W.A.M.; et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3′-fluoro-3′-deoxy-thymidine ([18F]FLT) PET imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18396–18399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.; Murray, W.K.; Link, E.; Hicks, R.J.; Duong, C. Improving Patient Selection for 18F-FDG PET Scanning in the Staging of Gastric Cancer. J. Nucl. Med. 2015, 56, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, K.S.; Hosseini-Nik, H. MRI of Rectal Cancer: An Overview and Update on Recent Advances. Am. J. Roentgenol. 2015, 205, W42–W55. [Google Scholar] [CrossRef]
- Ren, Y.; Ye, J.; Wang, Y.; Xiong, W.; Xu, J.; He, Y.; Cai, S.; Tan, M.; Yuan, Y. The Optimal Application of Transrectal Ultrasound in Staging of Rectal Cancer Following Neoadjuvant Therapy: A Pragmatic Study for Accuracy Investigation. J. Cancer 2018, 9, 784–791. [Google Scholar] [CrossRef] [Green Version]
- Samee, A.; Selvasekar, C.R. Current trends in staging rectal cancer. World J. Gastroenterol. 2011, 17, 828–834. [Google Scholar] [CrossRef]
- Cote, A.; Graur, F.; Lebovici, A.; Mois, E.; Al Hajjar, N.; Mare, C.; Badea, R.; Iancu, C. The accuracy of endorectal ultrasonography in rectal cancer staging. Clujul Med. 2015, 88, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Fowler, K.J.; Kaur, H.; Cash, B.D.; Feig, B.W.; Gage, K.L.; Garcia, E.M.; Hara, A.K.; Herman, J.M.; Kim, D.H.; Lambert, D.L.; et al. ACR Appropriateness Criteria ® Pretreatment Staging of Colorectal Cancer. J. Am. Coll. Radiol. 2017, 14, S234–S244. [Google Scholar] [CrossRef] [Green Version]
- Stanzione, A.; Verde, F.; Romeo, V.; Boccadifuoco, F.; Mainenti, P.P.; Maurea, S. Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World J. Gastroenterol. 2021, 27, 5306–5321. [Google Scholar] [CrossRef]
- Horvat, N.; Veeraraghavan, H.; Khan, M.; Blazic, I.; Zheng, J.; Capanu, M.; Sala, E.; Garcia-Aguilar, J.; Gollub, M.J.; Petkovska, I. MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy. Radiology 2018, 287, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, X.-Y.; Shi, Y.-J.; Wang, L.; Zhu, H.-T.; Tang, Z.; Wang, S.; Li, X.-T.; Tian, J.; Sun, Y.-S. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin. Cancer Res. 2017, 23, 7253–7262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Shen, F.; Jia, Y.; Xia, Y.; Li, Q.; Lu, J. MRI-based radiomics of rectal cancer: Preoperative assessment of the pathological features. BMC Med. Imaging 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]




| Disease | Modality | Citation | |
|---|---|---|---|
| Inflammation | IBD | Endoscopy and Convolution Neural Network (CNN) | [38] |
| Endoscopy | [39] | ||
| Endoscopy and Deep Learning CNN | [40] | ||
| Computed Tomography (CT) Enterography | [41] | ||
| Magnetic Resonance (MR) Enterography | [42] | ||
| MR Enterography: Diffusion Weighted Imaging | [43] | ||
| Chromoendoscopy | [44] | ||
| Transabdominal Ultrasound | [45] | ||
| 18F-FDG Positron Emission Tomography (PET)/MR Enterography | [46] | ||
| Multispectral Optoacoustic Tomography (MSOT) | [47] [48] | ||
| Diverticulitis | Ultrasound | [49] | |
| Colonoscopy | [50] | ||
| Magnetic Resonance Imaging (MRI) | [51] | ||
| CT | [52] | ||
| Celiac Disease | Endoscopy | [53] | |
| CT | [54] | ||
| Ultrasound | [55] | ||
| Fibrosis | Graft versus Host Disease | 18F-FDG PET | [56] |
| MRI | [57] | ||
| 18F-FDG PET and MRI | [58] | ||
| Ultrasound | [59] | ||
| CT | [60] | ||
| Intestinal Fibrosis | MRI: Diffusion Kurtosis Imaging | [61] | |
| CT Enterography: Radiomic Model | [62] | ||
| CT Enterography: Deep Learning Model | [63] | ||
| Magnetization Transfer Imaging and Native T1 Mapping | [64] | ||
| Cancer | Gastric Cancer | Multidetector Row Computed Tomography | [65] |
| MRI | [66] | ||
| Endoscopic Ultrasound | [67] | ||
| 18F-FDG-PET/CT and Laparoscopy | [68] | ||
| 18F-FDG-PET/CT | [69] | ||
| Colorectal Cancer | Colonoscopy and Sigmoidoscopy | [70] | |
| 18F-FDG-PET/CT | [71] | ||
| CT | [72] | ||
| CT + Artificial Intelligence (AI) and MRI + AI | [73] | ||
| MRI | [74] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harold, K.M.; MacCuaig, W.M.; Holter-Charkabarty, J.; Williams, K.; Hill, K.; Arreola, A.X.; Sekhri, M.; Carter, S.; Gomez-Gutierrez, J.; Salem, G.; et al. Advances in Imaging of Inflammation, Fibrosis, and Cancer in the Gastrointestinal Tract. Int. J. Mol. Sci. 2022, 23, 16109. https://doi.org/10.3390/ijms232416109
Harold KM, MacCuaig WM, Holter-Charkabarty J, Williams K, Hill K, Arreola AX, Sekhri M, Carter S, Gomez-Gutierrez J, Salem G, et al. Advances in Imaging of Inflammation, Fibrosis, and Cancer in the Gastrointestinal Tract. International Journal of Molecular Sciences. 2022; 23(24):16109. https://doi.org/10.3390/ijms232416109
Chicago/Turabian StyleHarold, Kylene M., William M. MacCuaig, Jennifer Holter-Charkabarty, Kirsten Williams, Kaitlyn Hill, Alex X. Arreola, Malika Sekhri, Steven Carter, Jorge Gomez-Gutierrez, George Salem, and et al. 2022. "Advances in Imaging of Inflammation, Fibrosis, and Cancer in the Gastrointestinal Tract" International Journal of Molecular Sciences 23, no. 24: 16109. https://doi.org/10.3390/ijms232416109
APA StyleHarold, K. M., MacCuaig, W. M., Holter-Charkabarty, J., Williams, K., Hill, K., Arreola, A. X., Sekhri, M., Carter, S., Gomez-Gutierrez, J., Salem, G., Mishra, G., & McNally, L. R. (2022). Advances in Imaging of Inflammation, Fibrosis, and Cancer in the Gastrointestinal Tract. International Journal of Molecular Sciences, 23(24), 16109. https://doi.org/10.3390/ijms232416109





