An ABCG-Type Transporter Facilitates ABA Influx and Regulates Camptothecin Biosynthesis in Camptotheca acuminata
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of CaABAT
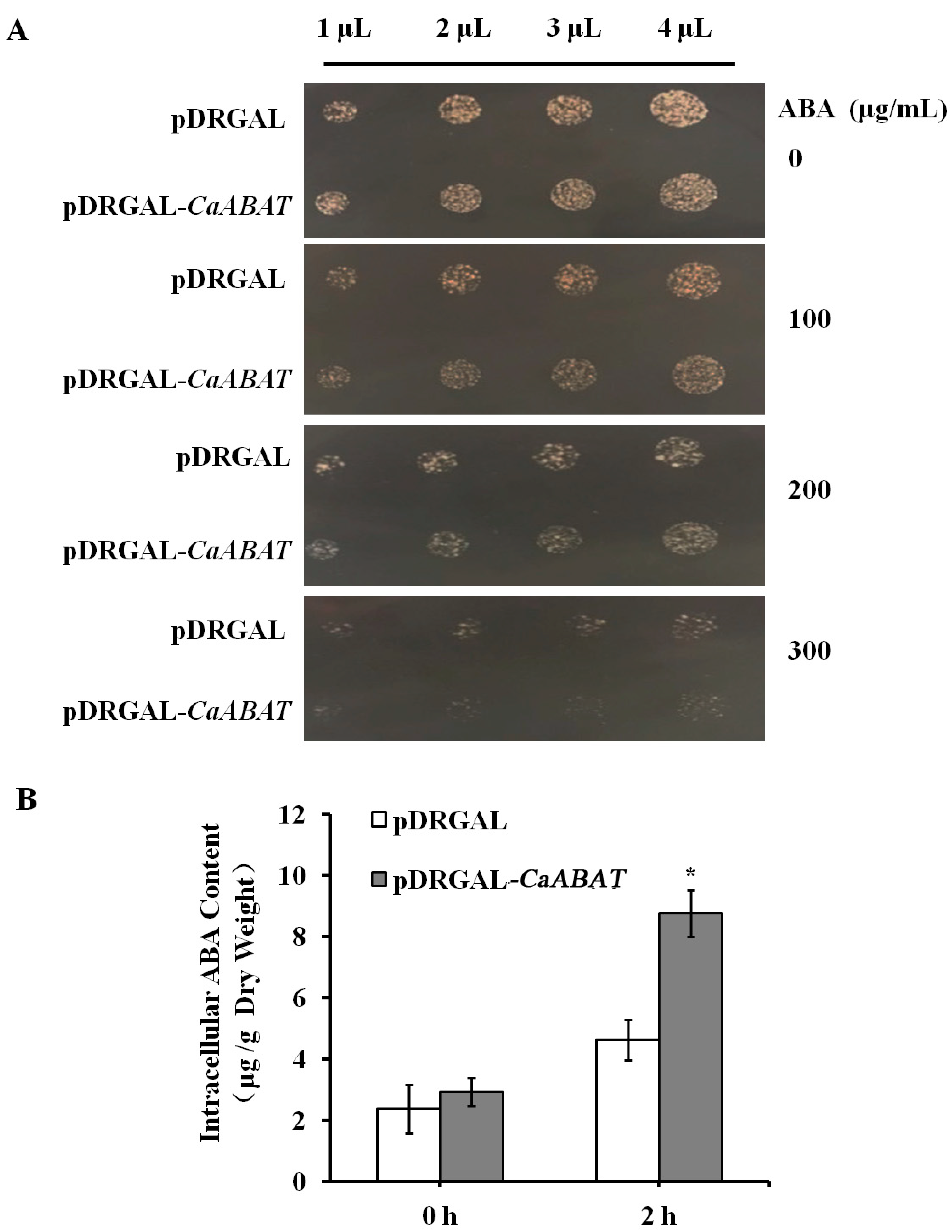
2.2. Functional Identification of CaABAT in Yeast Cells
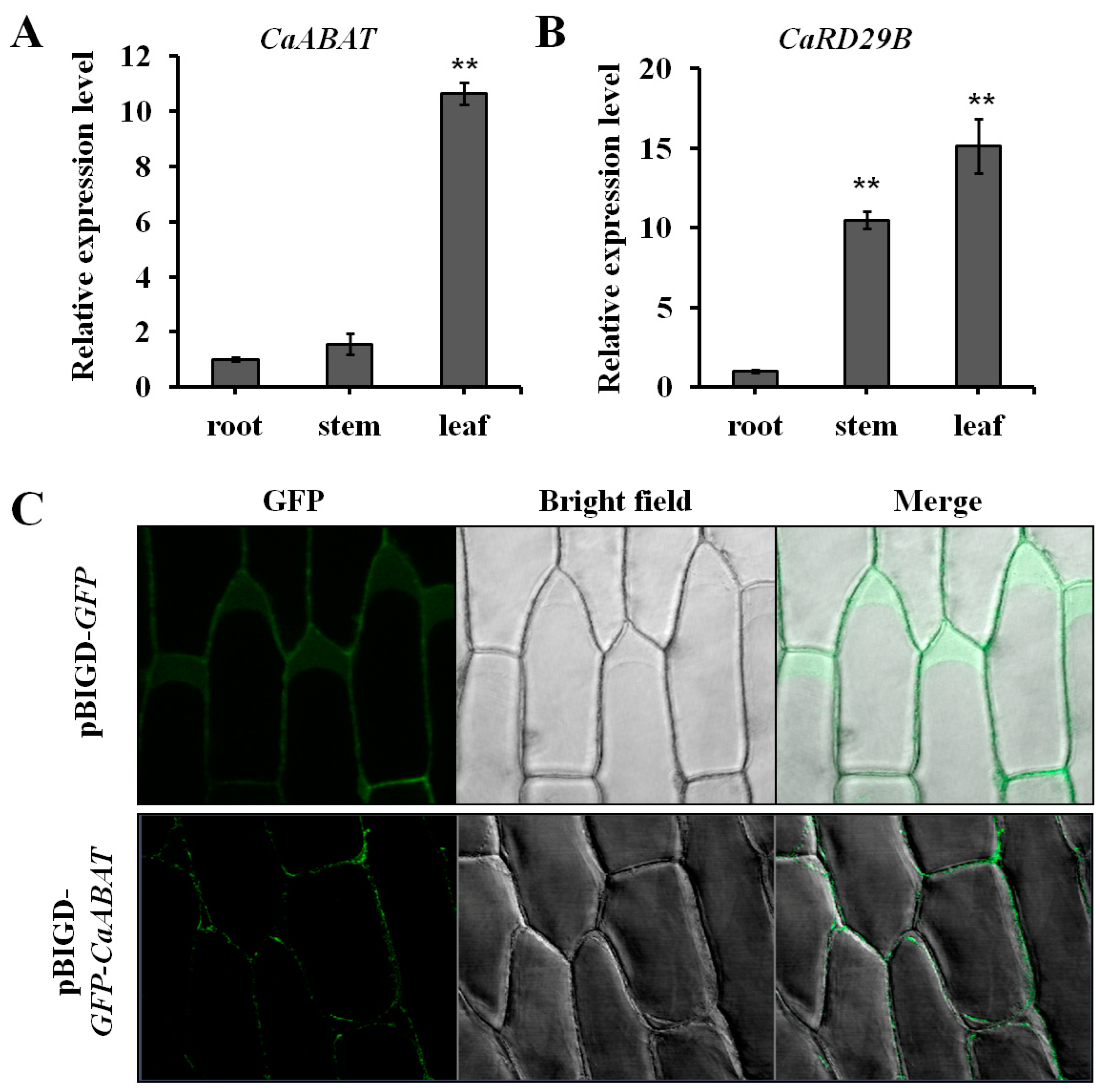
2.3. Expression Analysis and Subcellular Localization of CaABAT
2.4. Exogenous Plant Hormone Treatments on C. acuminata Seedlings
2.5. Silencing and Overexpression of CaABAT in C. acuminata
3. Discussion
3.1. CaABAT Belongs to the ABCG Subfamily and Is Involved in ABA Transport
3.2. Effect of CaABAT on ABA-Regulated Camptothecin Biosynthesis
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Isolation and Characterization of CaABAT
4.3. RNA Extraction and Real-Time PCR Analysis
4.4. Transient Overexpression and Virus-Induced Gene Silencing (VIGS) of CaABAT in C. acuminata
4.5. Functional Identification of CaABAT in Yeast Cells
4.6. Subcellular Localization of CaABAT
4.7. Exogenous Plant Hormone Treatment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, A.H.; Tempero, M.A.; Shan, Y.S.; Su, W.C.; Lin, Y.L.; Dito, E.; Ong, A.; Wang, Y.W.; Yeh, C.G.; Chen, L.T. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br. J. Cancer 2013, 109, 920–925. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Doi, T.; Eguchi-Nakajima, T.; Kato, K.; Yamada, Y.; Shimada, Y.; Fuse, N.; Ohtsu, A. Phase I study of NK012, a novel SN-38- incorporating micellar nanoparticle, in adult patients with solid Tumors. Clin. Cancer Res. 2010, 16, 5058–5066. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Wang, Y.; Liu, X.; Zhang, W.; Xiong, X.; Han, Z.; Liang, X. Camptothecin based nanodrug delivery systems. Cancer Biol. Med. 2017, 14, 363–370. [Google Scholar]
- Lorence, A.; Nessler, C.L. Camptothecin, over four decades of surprising findings. Phytochemistry 2004, 65, 2735–2749. [Google Scholar] [CrossRef]
- Curaba, J.; Singh, M.B.; Bhalla, P.L. miRNAs in the crosstalk between phytohormone signalling pathways. J. Exp. Bot. 2014, 65, 1425–1438. [Google Scholar] [CrossRef]
- El-Sayed, M.; Verpoorte, R. Methyl jasmonate accelerates catabolism of monoterpenoid indole alkaloids in Catharanthus roseus during leaf processing. Fitoterapia 2005, 76, 83–90. [Google Scholar] [CrossRef]
- Amini, A.; Glevarec, G.; Andreu, F.; Rideau, M.; Crèche, J. Low levels of gibberellic acid control the biosynthesis of ajmalicine in Catharanthus roseus cell suspension cultures. Planta Med. 2009, 75, 187–191. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Tchernoded, G.K.; Mischenko, N.P.; Khodakovskaya, M.V.; Glazunov, V.P.; Radchenko, S.V.; Zvereva, E.V.; Fedoreyev, S.A.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Kai, G.; Teng, X.; Cui, L.; Li, S.; Hao, X.; Shi, M.; Yan, B. Effect on three plant hormone elicitors on the Camptothecin accumulation and gene transcript profiling in Camptptheca Acuminata Seelings. Int. J. Sci. Res. 2014, 3, 86–95. [Google Scholar]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA transport and transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol. 2014, 164, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Kuromorim, T.; Shinozaki, K. ABA transport factors found in Arabidopsis ABC transporters. Plant Signal. Behav. 2010, 5, 1124–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuromori, T.; Miyaji, T.; Yabuuchi, H.; Shimizu, H.; Sugimoto, E.; Kamiya, A.; Moriyama, Y.; Shinozaki, K. ABC transporter AtABCG25 is involved in abscisic acid transport and responses. Proc. Natl. Acad. Sci. USA 2010, 107, 2361–2366. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhu, H.; Pan, Y.; Yu, Y.; Luan, S.; Li, L. A DTX/MATE type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol. Plant 2014, 7, 1522–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merilo, E.; Jalakas, P.; Laanemets, K.; Mohammadi, O.; Hõrak, H.; Kollist, H.; Brosché, M. Abscisic acid transport and homeostasis in the context of stomatal regulation. Mol. Plant 2015, 8, 1321–1333. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Kanno, Y.; Watanabe, S.; Seo, M. Arabidopsis NPF5.1 regulates ABA homeostasis and seed germination by mediating ABA uptake into the seed coat. Plant Signal. Behav. 2022, 17, 2095488. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.U.; Lee, M.; Kim, Y.Y.; Assmann, S.M.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [Green Version]
- Valletta, A.; Trainotti, L.; Santamaria, A.R.; Pasqua, G. Cell-specific expression of tryptophan decarboxylase and 10-hydroxygeraniol oxidoreductase, key genes involved in camptothecin biosynthesis in Camptotheca acuminata Decne (Nyssaceae). BMC Plant Biol. 2010, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; De Luca, V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2013, 110, 15830–15835. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, K.; Fujita, Y.; Katsura, K.; Maruyama, K.; Narusaka, Y.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulation of ABI3- and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol. Biol. 2006, 60, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Liu, Z.; Wang, Y.; Tang, Z.; Yu, F. A bZIP transcription factor, CaLMF, mediated light-regulated camptothecin biosynthesis in Camptotheca acuminata. Tree Physiol. 2019, 39, 372–380. [Google Scholar] [CrossRef]
- Fu, X.; Liu, H.; Hassani, D.; Peng, B.; Yan, X.; Wang, Y.; Wang, C.; Li, L.; Liu, P.; Pan, Q.; et al. AaABCG40 Enhances Artemisinin Content and Modulates Drought Tolerance in Artemisia annua. Front. Plant Sci. 2020, 11, 950. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J.; Sauter, A.; Wichert, K.; Messdaghi, D.; Hartung, W. Extracellular beta-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J. Exp. Bot. 2000, 51, 937–944. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381e5. [Google Scholar] [CrossRef] [Green Version]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195e201. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Cong, Y.; Zhu, S.; Xing, R.; Zhang, D.; Yao, X.; Wan, R.; Wang, Y.; Yu, F. Two classes of cytochrome P450 reductase genes and their divergent functions in Camptotheca acuminate Decne. Int. J. Biol. Macromol. 2019, 138, 1098–1108. [Google Scholar] [CrossRef]
- Jin, Z.; Yan, T.; Chang, C.; Liu, Z.; Wang, Y.; Tang, Z.; Yu, F. Application of virus-induced gene silencing approach in Camptotheca acuminata. Plant Cell Tiss. Organ Cult. 2016, 126, 533–540. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Bai, H.; Han, Y.; Yu, F. An ABCG-Type Transporter Facilitates ABA Influx and Regulates Camptothecin Biosynthesis in Camptotheca acuminata. Int. J. Mol. Sci. 2022, 23, 16120. https://doi.org/10.3390/ijms232416120
Wang Y, Wang Y, Bai H, Han Y, Yu F. An ABCG-Type Transporter Facilitates ABA Influx and Regulates Camptothecin Biosynthesis in Camptotheca acuminata. International Journal of Molecular Sciences. 2022; 23(24):16120. https://doi.org/10.3390/ijms232416120
Chicago/Turabian StyleWang, Yanyan, Yang Wang, Hefei Bai, Yuqian Han, and Fang Yu. 2022. "An ABCG-Type Transporter Facilitates ABA Influx and Regulates Camptothecin Biosynthesis in Camptotheca acuminata" International Journal of Molecular Sciences 23, no. 24: 16120. https://doi.org/10.3390/ijms232416120



