Angiogenesis–Browning Interplay Mediated by Asprosin-Knockout Contributes to Weight Loss in Mice with Obesity
Abstract
1. Introduction
2. Results
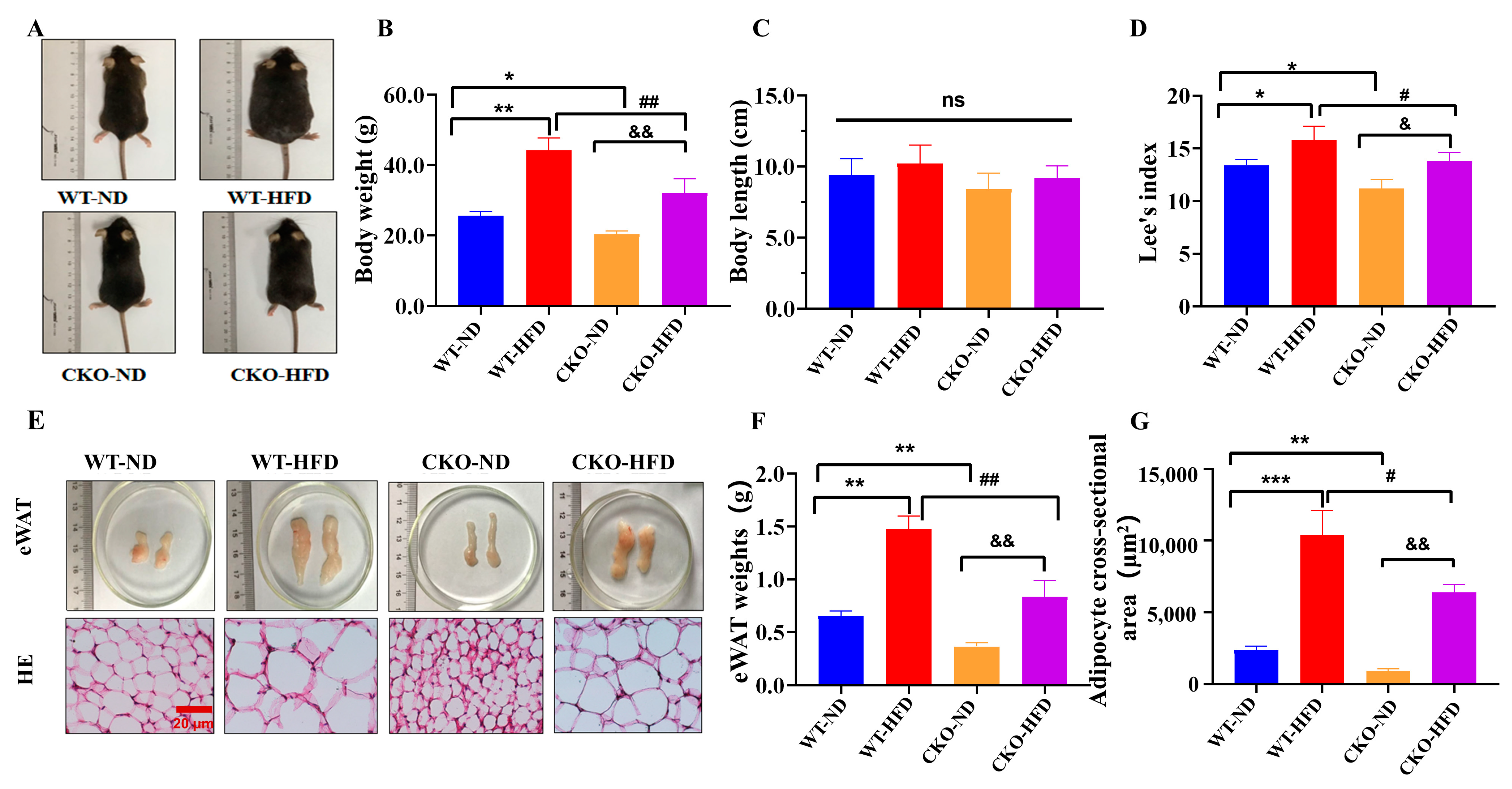
2.1. ASP-Deficiency Alleviates Mouse Obesity Induced by HFD
2.2. ASP-Deficiency Promotes the Angiogenesis and Browning of White Adipose In Vivo
2.3. ASP-Deficiency Promotes the Migration and Angiogenesis of Vascular Endothelial Cells In Vitro
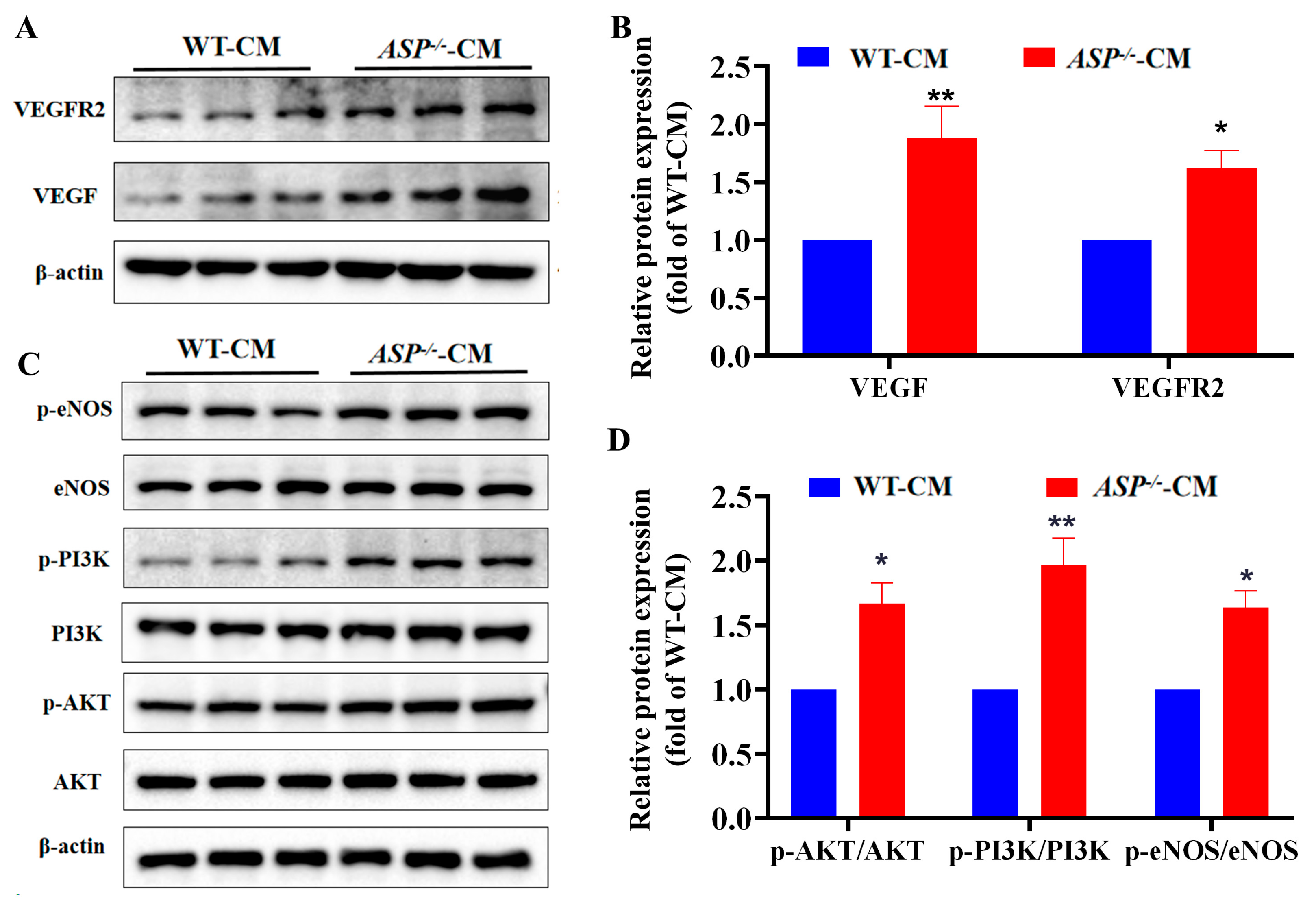
2.4. ASP-Deficiency Enhances VEGF/VEGFR2 and PI3K/AKT/eNOS Signaling In Vitro
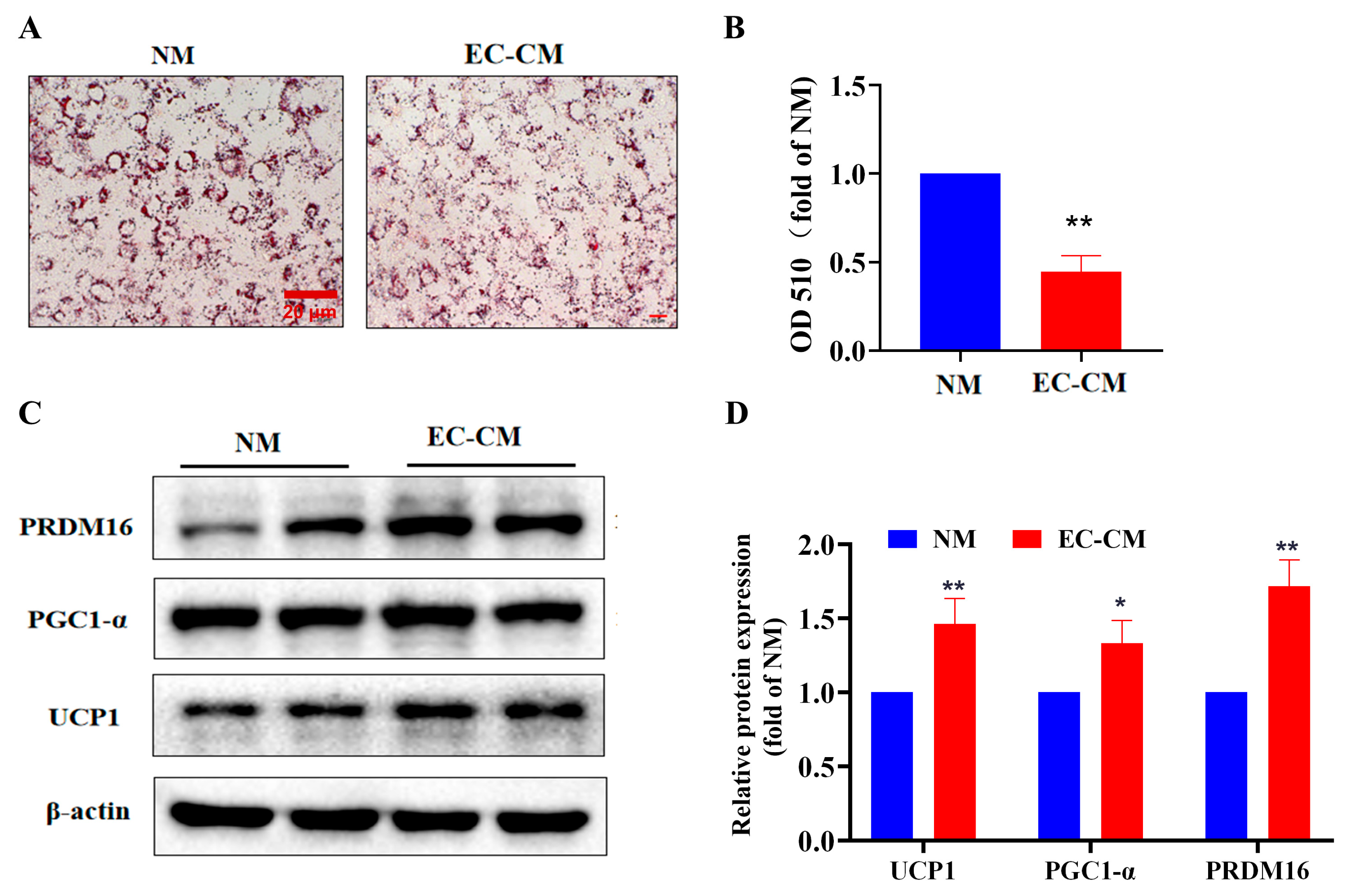
2.5. Effect of EC-CM on Lipid Aggregation and Browning In Vitro
2.6. EC-CM Treatment Enhances the Mitochondrial Biogenesis and Respiratory Function of Adipocytes In Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Experimental Protocols
4.2. Differentiation and Treatment of 3T3-L1 Preadipocytes
4.3. Histological Analysis
4.4. Wound-Healing Assay
4.5. Matrigel Tube Formation Assay for Angiogenesis
4.6. Oil Red O Staining and Quantification
4.7. Laser Confocal Microscopy
4.8. Detection of the Oxygen Consumption Rate (OCR)
4.9. Western Blotting
4.10. Preparation of Conditional Medium(CM)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacinska, M.; Blachnio-Zabielska, A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Alpert, M.A.; Milani, R.V.; Ventura, H.O. Management of cardiovascular diseases in patients with obesity. Nat. Rev. Cardiol. 2018, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, M.; Razafimanjato, F.; Ramalingam, L.; Kalupahana, N.S.; Moussa, H.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 2017, 39, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Kim, W.K.; Bae, K.H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem. Cells 2014, 6, 33–42. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Xu, M.; Gu, W.; Xi, Y.; Qi, L.; Li, B.; Wang, W. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS ONE 2015, 10, e0123795. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in adipose tissue: The interplay between adipose and endothelial cells. Front. Physiol. 2020, 11, 624903. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Lysaght, J.; Donohoe, C.L.; Reynolds, J.V. Obesity and gastrointestinal cancer: The interrelationship of adipose and tumour microenvironments. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 699–714. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Svenningsen, P. Adipocyte-endothelium crosstalk in obesity. Front. Endocrinol. 2021, 12, 681290. [Google Scholar] [CrossRef]
- Cao, Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell. Metab. 2013, 18, 478–489. [Google Scholar] [CrossRef]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Brakenhielm, E.; Veitonmaki, N.; Cao, R.; Kihara, S.; Matsuzawa, Y.; Zhivotovsky, B.; Funahashi, T.; Cao, Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P.K.; del Solar, M.; Zhu, B.; York, B.; et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell 2016, 165, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Duerrschmid, C.; He, Y.; Wang, C.; Li, C.; Bournat, J.C.; Romere, C.; Saha, P.K.; Lee, M.E.; Phillips, K.J.; Jain, M.; et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 2017, 23, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, C. Liver: Asprosin-new hormone involved in hepatic glucose release. Nat. Rev. Endocrinol. 2016, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, H.; Xiong, X.; Qiu, Y.; Liao, Y.; Chen, Y.; Zheng, Y.; Zheng, H. Plasma Asprosin concentrations are increased in individuals with glucose dysregulation and correlated with insulin resistance and first-phase insulin secretion. Mediators Inflamm. 2018, 2018, 9471583. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yin, C.; Wang, L.; Liu, Y.; Li, H.; Li, M.; Yi, X.; Xiao, Y. Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann. Nutr. Metab. 2019, 75, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, R.; Luo, X.Q.; Wang, G.; Zhang, Q.L.; Qiao, H.; Wang, N.; Yan, J.Q. Cold exposure differentially stimulates angiogenesis in BAT and WAT of mice: Implication in adrenergic ctivation. Cell Physiol. Biochem. 2017, 42, 974–986. [Google Scholar] [CrossRef]
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171. [Google Scholar] [CrossRef]
- Cheung, B.M.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 4, 171–181. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.J.; Huang, P.; Huang, T.L.; Xue, R.; McDougall, L.E.; Townsend, K.L.; Cypess, A.M.; Mishina, Y.; Gussoni, E.; Tseng, Y.H. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 2013, 495, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Honek, J.; Seki, T.; Iwamoto, H.; Fischer, C.; Li, J.; Lim, S.; Samani, N.J.; Zang, J.; Cao, Y. Modulation of age-related insulin sensitivity by VEGF-dependent vascular plasticity in adipose tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 14906–14911. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Henson, G.D.; Machin, D.R.; Bramwell, R.C.; Donato, A.J.; Lesniewski, L.A. Aging differentially impacts vasodilation and angiogenesis in arteries from the white and brown adipose tissues. Exp. Gerontol. 2020, 142, 111126. [Google Scholar] [CrossRef]
- Honek, J.; Lim, S.; Fischer, C.; Iwamoto, H.; Seki, T.; Cao, Y. Brown adipose tissue, thermogenesis, angiogenesis: Pathophysiological aspects. Horm. Mol. Biol. Clin. Investig. 2014, 19, 5–11. [Google Scholar] [CrossRef]
- Watanabe, E.; Wada, T.; Okekawa, A.; Kitamura, F.; Komatsu, G.; Onogi, Y.; Yamamoto, S.; Sasahara, M.; Kitada, M.; Koya, D.; et al. Stromal cell-derived factor 1 (SDF1) attenuates platelet-derived growth factor-B (PDGF-B)-induced vascular remodeling for adipose tissue expansion in obesity. Angiogenesis 2020, 23, 667–684. [Google Scholar] [CrossRef]
- Chakraborty, A.; Barajas, S.; Lammoglia, G.M.; Reyna, A.J.; Morley, T.S.; Johnson, J.A.; Scherer, P.E.; Rutkowski, J.M. Vascular endothelial growth factor-D (VEGF-D) overexpression and lymphatic expansion in murine adipose tissue improves metabolism in obesity. Am. J. Pathol. 2019, 189, 924–939. [Google Scholar] [CrossRef]
- Campbell, D.J.; Somaratne, J.B.; Prior, D.L.; Yii, M.; Kenny, J.F.; Newcomb, A.E.; Kelly, D.J.; Black, M.J. Obesity is associated with lower coronary microvascular density. PLoS ONE 2013, 8, e81798. [Google Scholar] [CrossRef]
- Shabir, K.; Brown, J.E.; Afzal, I.; Gharanei, S.; Weickert, M.O.; Barber, T.M.; Kyrou, I.; Randeva, H.S. Asprosin, a novel pleiotropic adipokine implicated in fasting and obesity-related cardio-metabolic disease: Comprehensive review of preclinical and clinical evidence. Cytokine Growth Factor Rev. 2021, 60, 120–132. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Pereira, N.B.; Pereira, L.X.; Oliveira, L.A.M.; Campos, P.P.; Andrade, S.P.; Moro, L. Double immunofluorescence labeling for CD31 and CD105 as a marker for polyether polyurethane-induced angiogenesis in mice. Histol. Histopathol. 2019, 34, 257–264. [Google Scholar] [CrossRef]
- Mahdaviani, K.; Chess, D.; Wu, Y.; Shirihai, O.; Aprahamian, T.R. Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism 2016, 65, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Yamada, T. UCP1 Dependent and independent thermogenesis in brown and beige adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.; Sun, K.; An, Y.A.; Gu, X.; Scherer, P.E. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-independent metabolic improvements. Diabetes 2017, 66, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hosaka, K.; Fischer, C.; Lim, S.; Andersson, P.; Abe, M.; Iwamoto, H.; Gao, Y.; Wang, X.; Fong, G.H.; et al. Ablation of endothelial VEGFR1 improves metabolic dysfunction by inducing adipose tissue browning. J. Exp. Med. 2018, 215, 611–626. [Google Scholar] [CrossRef]
- Li, F.; Huang, J.; Ji, D.; Meng, Q.; Wang, C.; Chen, S.; Wang, X.; Zhu, Z.; Jiang, C.; Shi, Y.; et al. Azithromycin effectively inhibits tumor angiogenesis by suppressing vascular endothelial growth factor receptor 2-mediated signaling pathways in lung cancer. Oncol. Lett. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Feliers, D.; Chen, X.; Akis, N.; Choudhury, G.G.; Madaio, M.; Kasinath, B.S. VEGF regulation of endothelial nitric oxide synthase in glomerular endothelial cells. Kidney Int. 2005, 68, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhu, H.; Jiang, W.; Zhang, S. Protocatechuic acid induces angiogenesis through PI3K-Akt-eNOS-VEGF signalling pathway. Basic Clin. Pharmacol. Toxicol. 2013, 113, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, S.; Zhao, J.Q.; Xiang, B.L.; Gu, X.; Zou, F.; Zhang, Z.H. ADAMTS-1 inhibits angiogenesis via the PI3K/Akt-eNOS-VEGF pathway in lung cancer cells. Transl. Cancer Res. 2019, 8, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Vijayan, V.; Singh, F.; Sudhakar, M.; Lakra, R.; Korrapati, P.S.; Kiran, M.S. White to brown adipocyte transition mediated by Apigenin via VEGF-PRDM16 signaling. J. Cell Biochem. 2022, 123, 1793–1807. [Google Scholar] [CrossRef]
- Fu, P.; Zhu, R.; Jia, J.; Hu, Y.; Wu, C.; Cieszczyk, P.; Holmberg, H.C.; Gong, L. Aerobic exercise promotes the functions of brown adipose tissue in obese mice via a mechanism involving COX2 in the VEGF signaling pathway. Nutr. Metab. 2021, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Czaja, A.M.; Kluger, P.J. Influence of epidermal growth factor (EGF) and hydrocortisone on the co-culture of mature adipocytes and endothelial cells for vascularized adipose tissue engineering. Cell Biol. Int. 2016, 40, 569–578. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Investig. 2014, 124, 2099–2112. [Google Scholar] [CrossRef]
- Macedo, A.P.A.; Cordeiro, G.S.; Santos, L.S.; Santo, D.A.E.; Perez, G.S.; Couto, R.D.; Machado, M.; Medeiros, J.M.B. Murinometric measurements and retroperitoneal adipose tissue in young rats exposed to the high-fat diet: Is there correlation? Braz. J. Biol. 2021, 81, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, Y.; Yuan, W.; Wang, M.; Zhou, Y.; Chen, K.; Huang, Q. Downregulation of osteopontin inhibits browning of white adipose tissues through PI3K-AKT pathway in C57BL/6 mice. Eur. J. Pharmacol. 2020, 866, 172822. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, T.; Chen, S.; Zeng, G.; Yuan, W.; Lu, Y.; Zhang, Y.; Huang, Q.; Xiong, X.; Xu, B.; Huang, Q. Angiogenesis–Browning Interplay Mediated by Asprosin-Knockout Contributes to Weight Loss in Mice with Obesity. Int. J. Mol. Sci. 2022, 23, 16166. https://doi.org/10.3390/ijms232416166
Yin T, Chen S, Zeng G, Yuan W, Lu Y, Zhang Y, Huang Q, Xiong X, Xu B, Huang Q. Angiogenesis–Browning Interplay Mediated by Asprosin-Knockout Contributes to Weight Loss in Mice with Obesity. International Journal of Molecular Sciences. 2022; 23(24):16166. https://doi.org/10.3390/ijms232416166
Chicago/Turabian StyleYin, Tingting, Sheng Chen, Guohua Zeng, Wanwan Yuan, Yanli Lu, Yanan Zhang, Qianqian Huang, Xiaowei Xiong, Baohua Xu, and Qiren Huang. 2022. "Angiogenesis–Browning Interplay Mediated by Asprosin-Knockout Contributes to Weight Loss in Mice with Obesity" International Journal of Molecular Sciences 23, no. 24: 16166. https://doi.org/10.3390/ijms232416166
APA StyleYin, T., Chen, S., Zeng, G., Yuan, W., Lu, Y., Zhang, Y., Huang, Q., Xiong, X., Xu, B., & Huang, Q. (2022). Angiogenesis–Browning Interplay Mediated by Asprosin-Knockout Contributes to Weight Loss in Mice with Obesity. International Journal of Molecular Sciences, 23(24), 16166. https://doi.org/10.3390/ijms232416166





