Gamma-Tocotrienol Modulates Total-Body Irradiation-Induced Hematopoietic Injury in a Nonhuman Primate Model
Abstract
:1. Introduction
2. Results
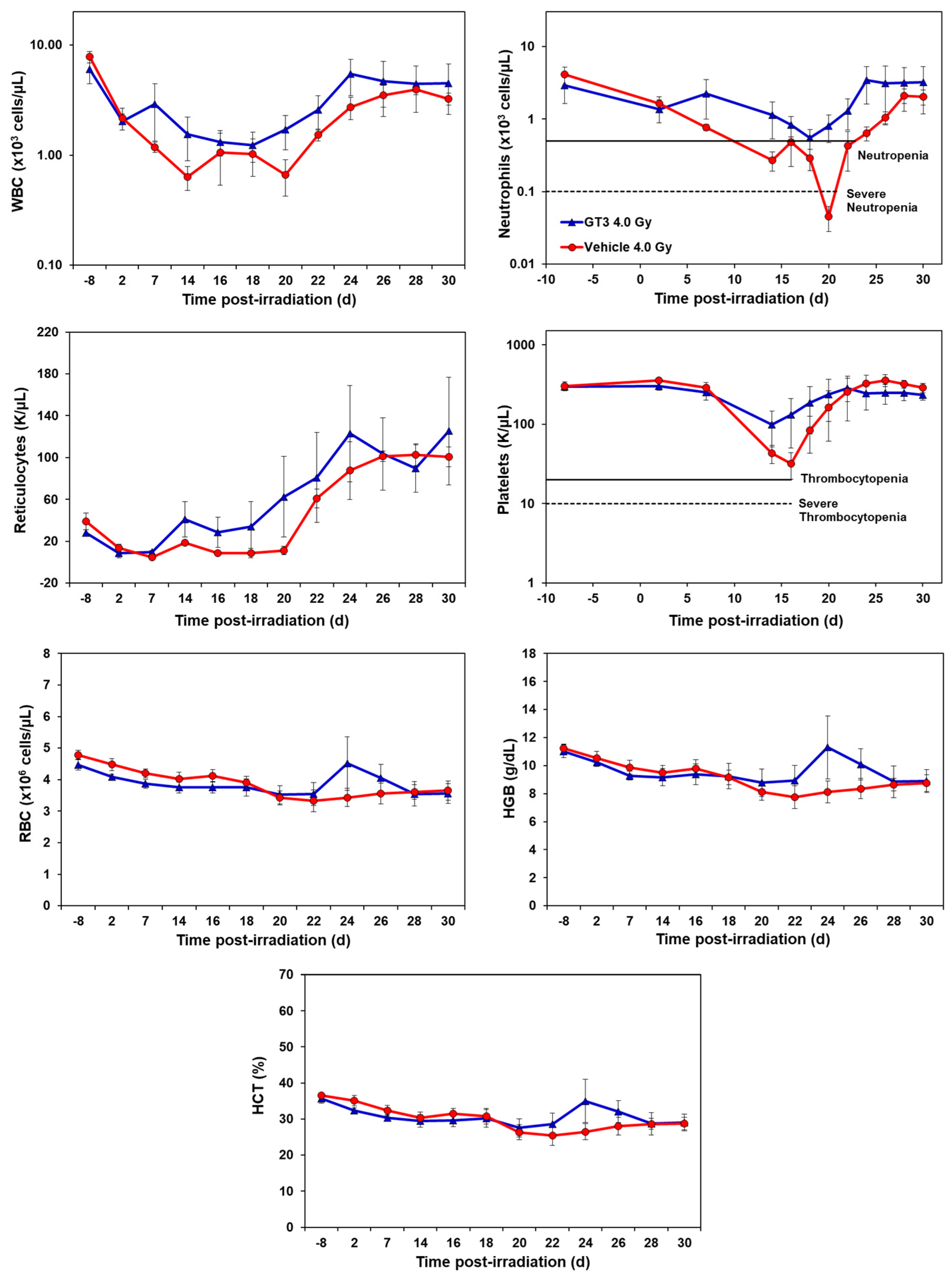
2.1. Effects of GT3 on Complete Blood Count (CBC) in NHPs Exposed to 4 and 5.8 Gy TBI
2.1.1. Effects of GT3 on CBC Post 4 Gy Radiation
2.1.2. Effects of GT3 on CBC Post 5.8 Gy Radiation
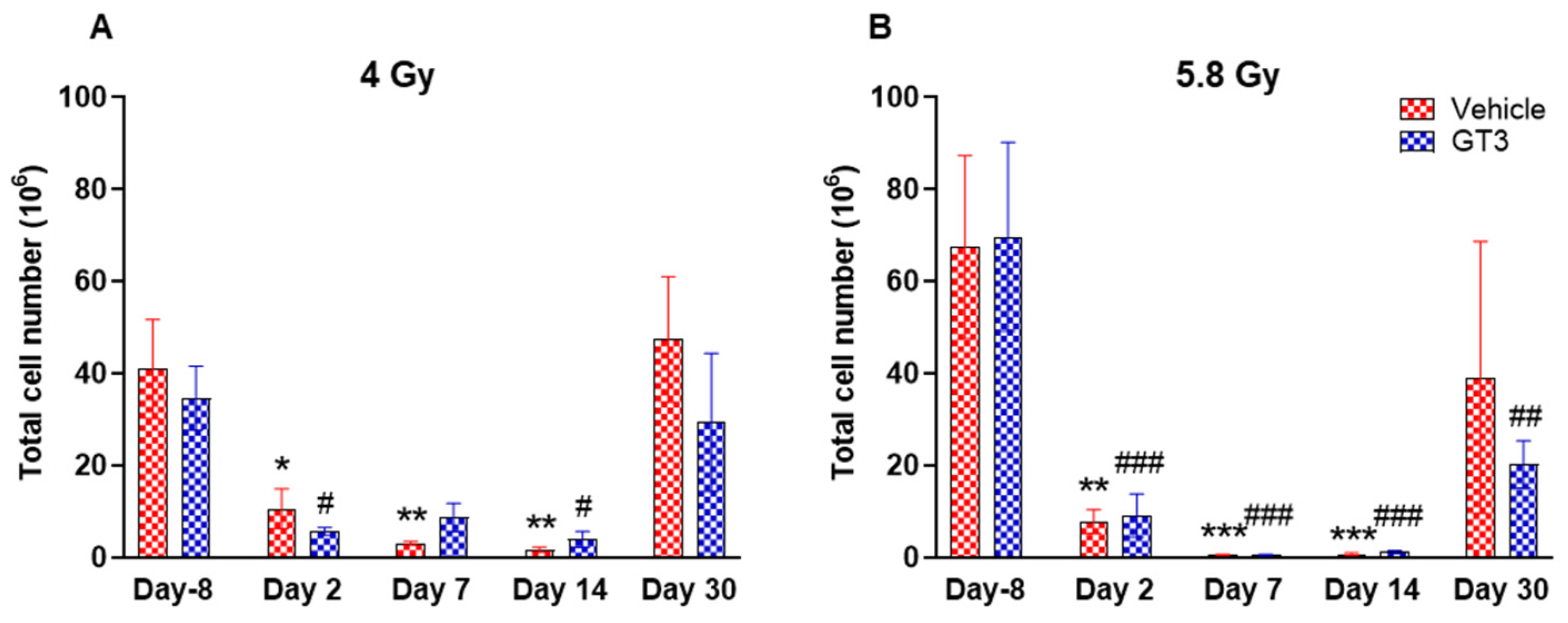
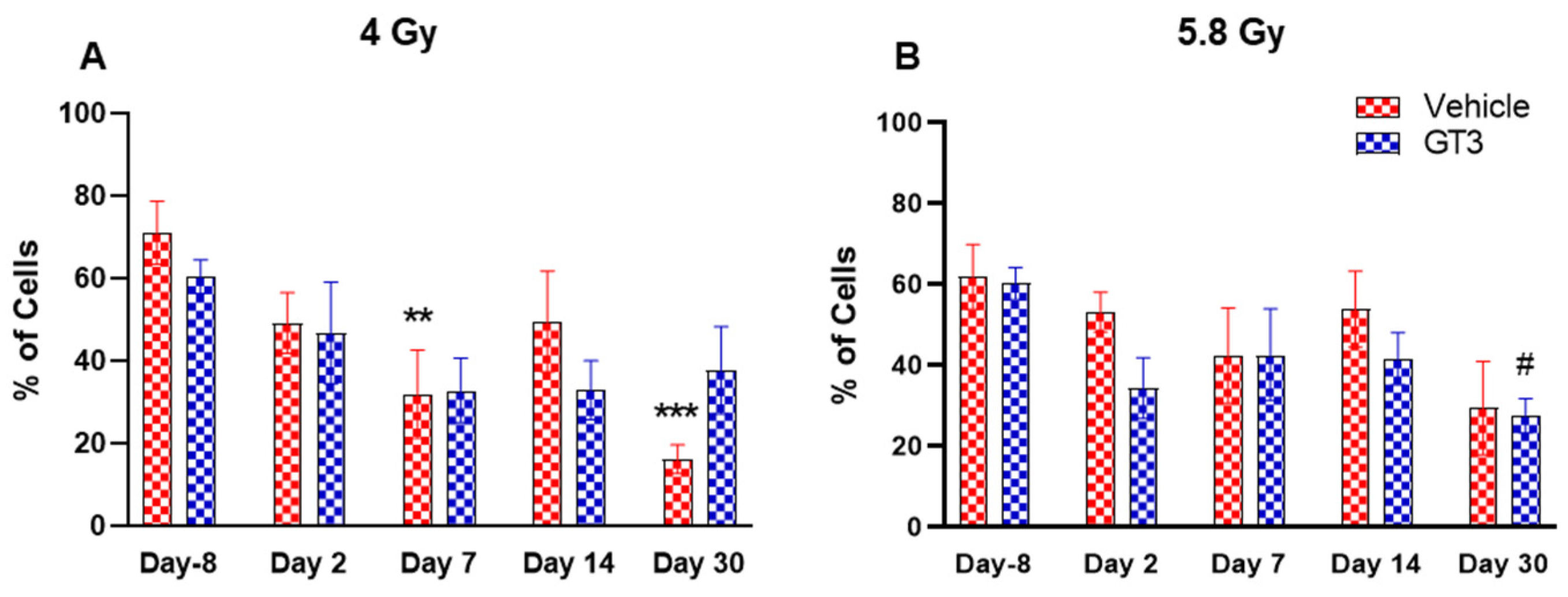
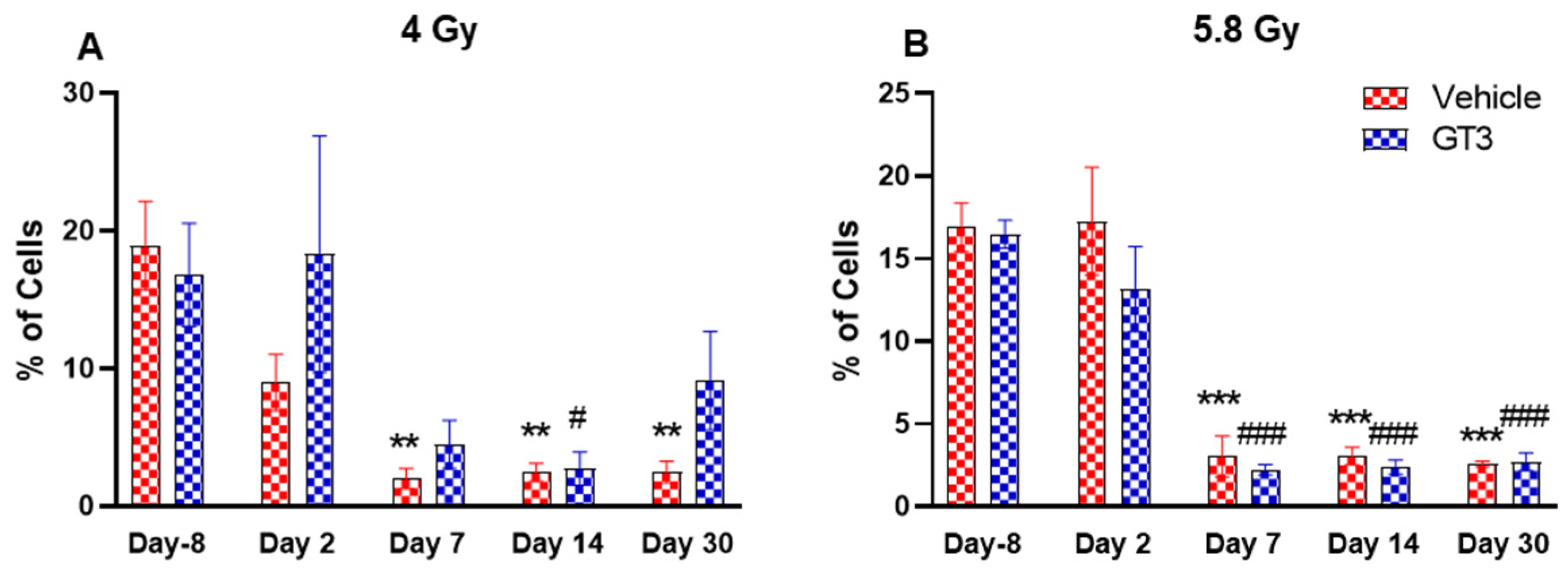
2.2. Effects of GT3 on Hematopoietic Injury in BM of NHPs Exposed to 4 and 5.8 Gy TBI
2.2.1. Leukocytes
2.2.2. Hematopoietic Cells (CD45+)
2.2.3. Hematopoietic Stem Cells (CD34+CD45+)
2.2.4. B Cells (CD3−CD20+)
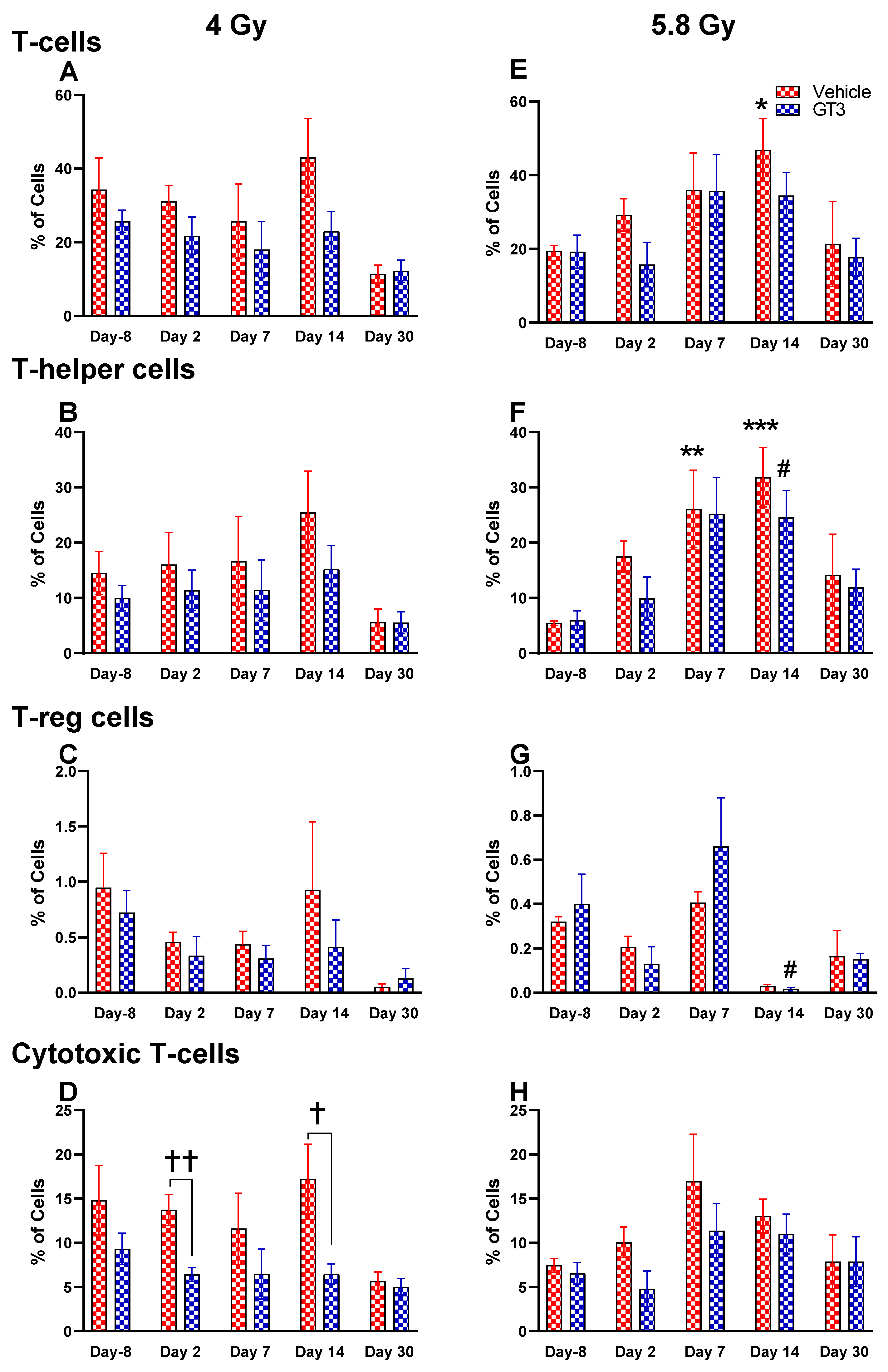
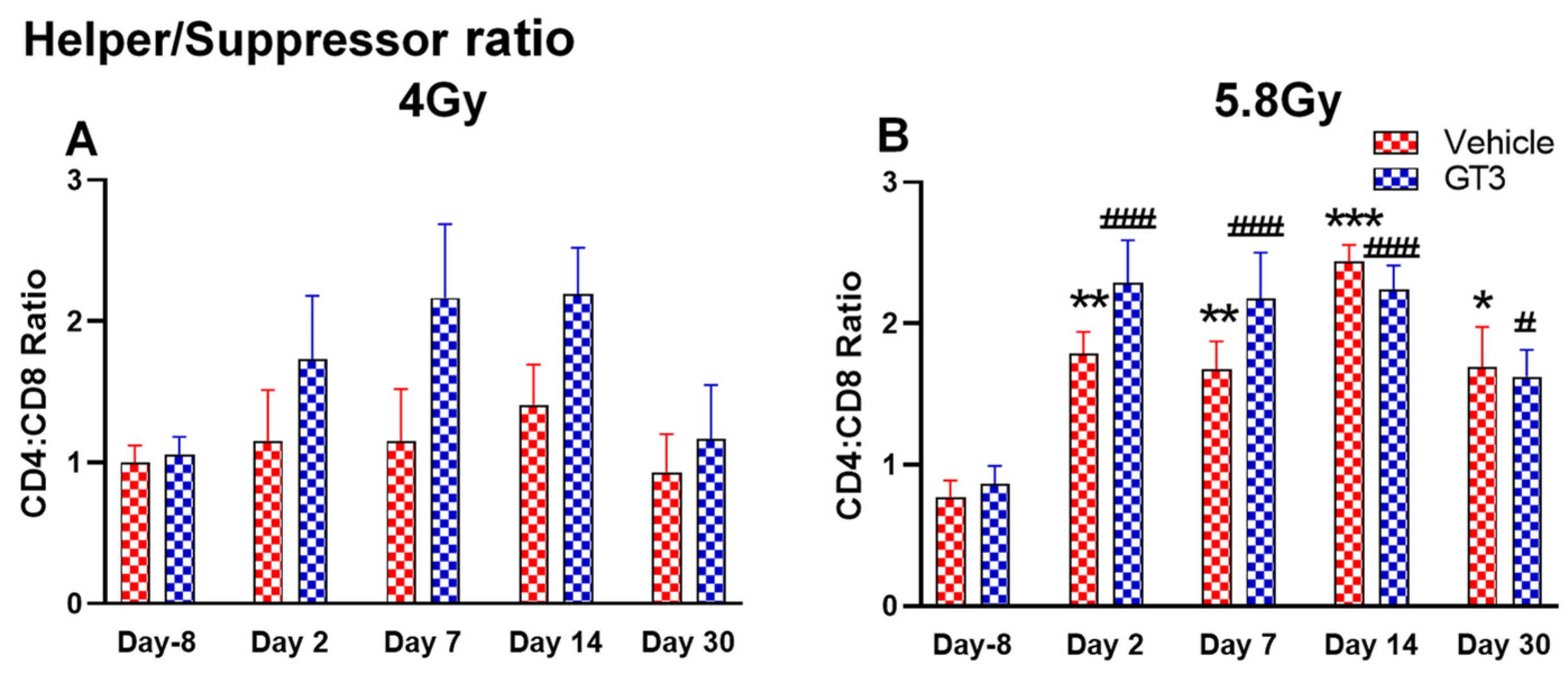
2.2.5. T Cells (CD3+) and Subsets
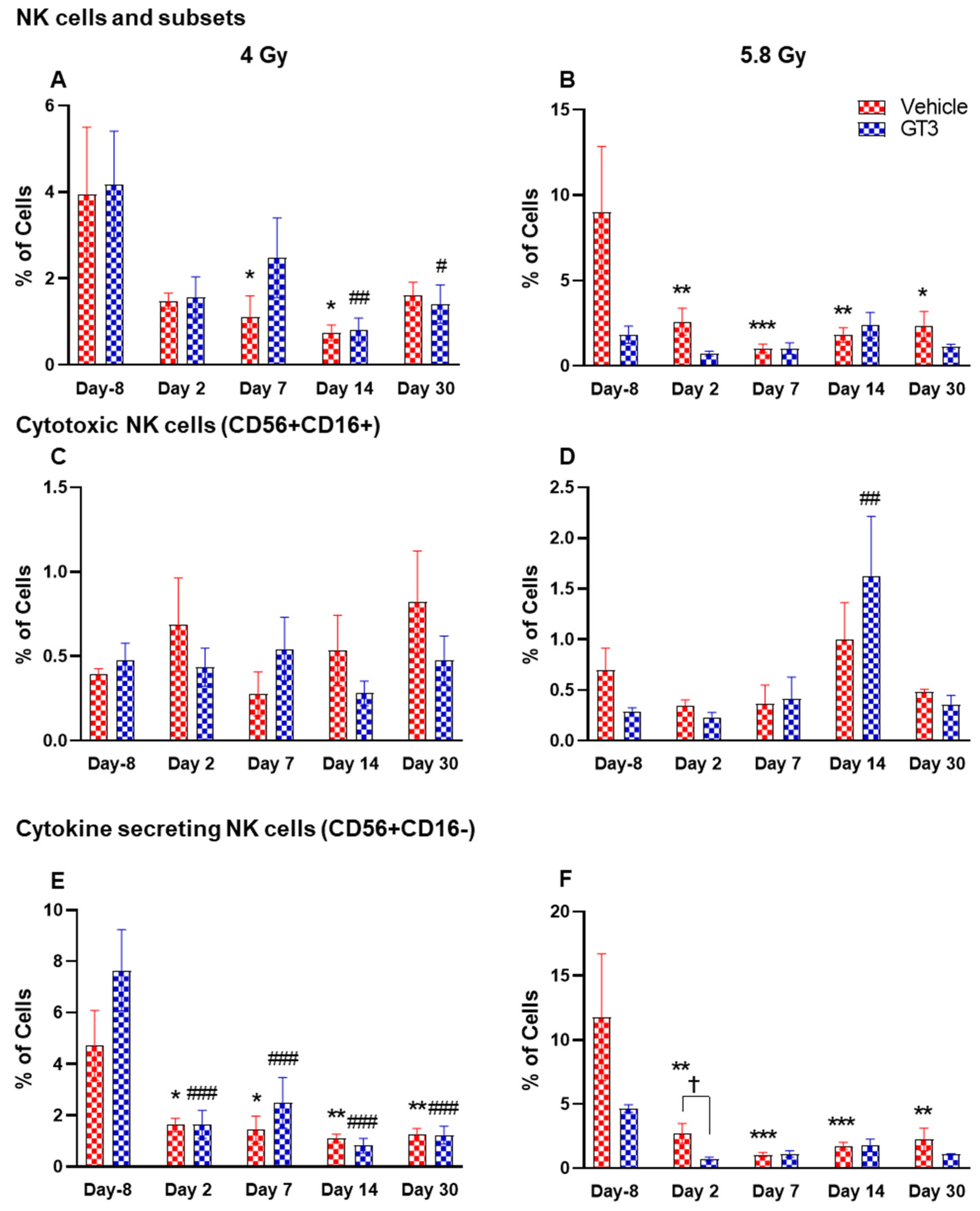
2.2.6. NK Cells and Subsets
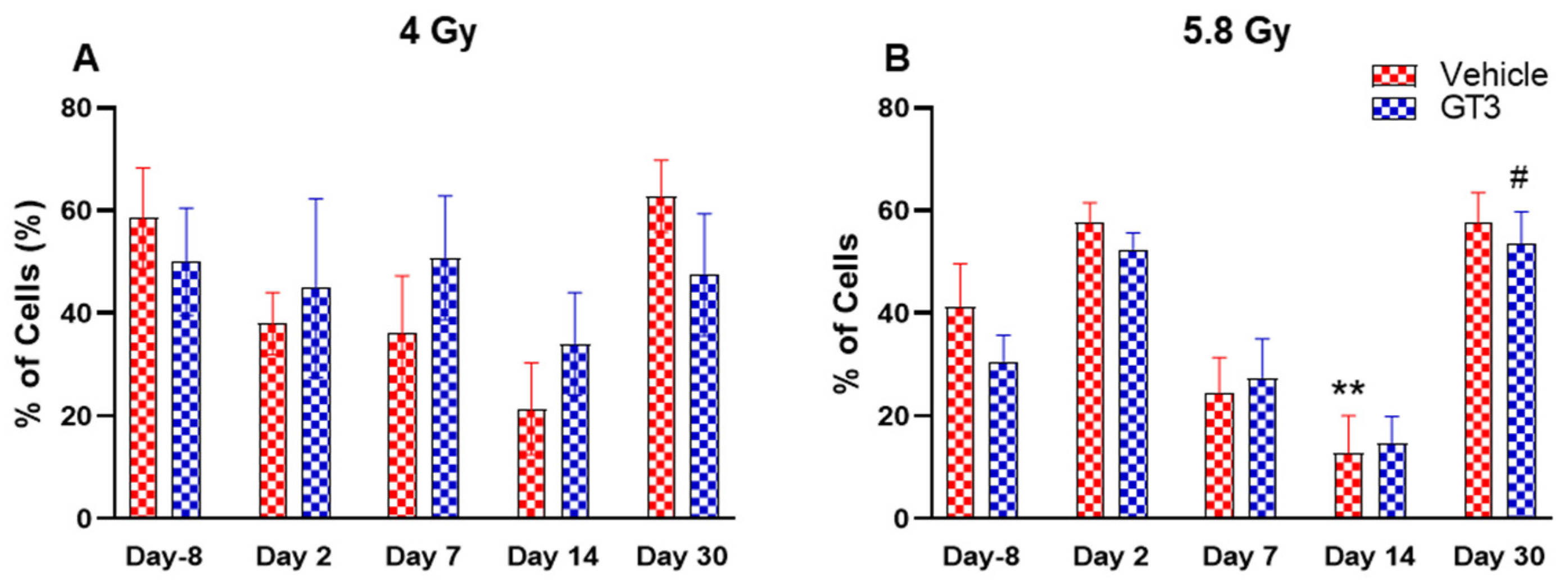
2.2.7. Granulocytes and Monocytes (CD11b+)
2.3. Effects of GT3 on Radiation-Induced Decrease in BM HSPCs Exposed to 4 and 5.8 Gy TBI
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Preparation and Administration of GT3 and Vehicle
4.4. Total-Body Irradiation
4.5. Peripheral Blood Collection and Complete Blood Counts
4.6. BM Collection
4.7. Flow Cytometry
Gating Strategy
4.8. Bone Marrow CFU Assay
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Mettler, F.A., Jr.; Voelz, G.L. Major radiation exposure—What to expect and how to respond. N. Engl. J. Med. 2002, 346, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Dorr, H.; Meineke, V. Acute radiation syndrome caused by accidental radiation exposure—Therapeutic principles. BMC Med. 2011, 9, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, A.B.; May, M.M.; Perry, W.J. The Day After: Action Following a Nuclear Blast in a U. S. City. The Washington Quarterly 2007, 30, 19–32. [Google Scholar] [CrossRef]
- Potten, C.S. A Comprehensive Study of the Radiobiological Response of the Murine (BDF1) Small Intestine. Int. J. Radiat. Biol. 1990, 58, 925–973. [Google Scholar] [CrossRef]
- Vigneulle, R.M.; Rao, S.; Fasano, A.; MacVittie, T.J. Structural and functional alterations of the gastrointestinal tract following radiation-induced injury in the rhesus monkey. Dig. Dis. Sci. 2002, 47, 1480–1491. [Google Scholar] [CrossRef]
- Green, D.E.; Rubin, C.T. Consequences of irradiation on bone and marrow phenotypes, and its relation to disruption of hematopoietic precursors. Bone 2014, 63, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.E.; Warner, N.L. Ionizing radiation and the immune response. Adv. Immunol. 1976, 24, 215–335. [Google Scholar] [CrossRef]
- Singh, V.K.; Newman, V.L.; Seed, T.M. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): A review. Cytokine 2015, 71, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Newman, V.L.; Berg, A.N.; MacVittie, T.J. Animal models for acute radiation syndrome drug discovery. Expert. Opin. Drug. Discov. 2015, 10, 497–517. [Google Scholar] [CrossRef]
- Dainiak, N. Hematologic consequences of exposure to ionizing radiation. Exp. Hematol. 2002, 30, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Wagemaker, G. Heterogeneity of radiation sensitivity of hemopoietic stem cell subsets. Stem Cells 1995, 13 (Suppl. S1), 257–260. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Candéias, S.M.; Gaipl, U.S.; Frey, B. Editorial: Radiation and the Immune System: Current Knowledge and Future Perspectives. Front. Immunol. 2017, 8, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, L.P.; Rajam, G.; Carlone, G.M.; Jiang, C.; Owzar, K.; Dugan, G.; Caudell, D.; Chao, N.; Cline, J.M.; Register, T.C.; et al. Late effects of total body irradiation on hematopoietic recovery and immune function in rhesus macaques. PLoS ONE 2019, 14, e0210663. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Ahn, G.; Yun, J.S.; Kim, M.J.; Bing, S.J.; Kim, D.S.; Lee, J.; Lee, N.H.; Park, J.W.; Jee, Y. Dieckol rescues mice from lethal irradiation by accelerating hemopoiesis and curtailing immunosuppression. Int. J. Radiat. Biol. 2010, 86, 848–859. [Google Scholar] [CrossRef]
- Charrier, S.; Michaud, A.; Badaoui, S.; Giroux, S.; Ezan, E.; Sainteny, F.; Corvol, P.; Vainchenker, W. Inhibition of angiotensin I-converting enzyme induces radioprotection by preserving murine hematopoietic short-term reconstituting cells. Blood 2004, 104, 978–985. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Seed, T.M. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today 2018, 54, 679–693. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. An update on romiplostim for treatment of acute radiation syndrome. Drugs Today 2022, 58, 133–145. [Google Scholar] [CrossRef]
- Farese, A.M.; MacVittie, T.J. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today 2015, 51, 537–548. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. Radiation countermeasures for hematopoietic acute radiation syndrome: Growth factors, cytokines and beyond. Int. J. Radiat. Biol. 2021, 97, 1526–1547. [Google Scholar] [CrossRef]
- Singh, V.K.; Beattie, L.A.; Seed, T.M. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. 2013, 54, 973–988. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P.; Simone, R.; Picci, N.; Buzzoni, L.; Ciliberti, N.; Natangelo, A.; Manfredini, S.; Vertuani, S. Design, synthesis, and antioxidant potency of novel alpha-tocopherol analogues in isolated membranes and intact cells. Free Radic. Biol. Med. 2008, 44, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Verdecchia, S.; Avanzi, L.; Vertuani, S.; Serini, S.; Iannone, A.; Manfredini, S. Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol. Cell Biochem. 2006, 287, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Scott, J.R.; Romaine, P.L.; Newman, V.L.; Fatanmi, O.O. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci. 2014, 98, 113–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Wise, S.Y.; Seed, T.M. Radiation countermeasure agents: An update (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1229–1255. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Hauer-Jensen, M. gamma-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. Int. J. Mol. Sci. 2016, 17, 663. [Google Scholar] [CrossRef] [Green Version]
- Baliarsingh, S.; Beg, Z.H.; Ahmad, J. The therapeutic impacts of tocotrienols in type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005, 182, 367–374. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Burger, W.C.; Peterson, D.M.; Elson, C.E. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J. Biol. Chem. 1986, 261, 10544–10550. [Google Scholar] [CrossRef]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef]
- Singh, V.K.; Kulkarni, S.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.; Hendrickson, H.; Gulani, J.; Ghosh, S.P.; Kumar, K.S.; et al. Radioprotective Efficacy of Gamma-Tocotrienol in Nonhuman Primates. Radiat. Res. 2016, 185, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ghosh, S.P.; Satyamitra, M.; Mog, S.; Hieber, K.; Romanyukha, L.; Gambles, K.; Toles, R.; Kao, T.C.; Hauer-Jensen, M.; et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat. Res. 2010, 173, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Cary, L.H.; Gambles, K.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int. Immunopharmacol. 2012, 14, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Singh, P.K.; Ghosh, S.P.; Posarac, A.; Singh, V.K. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine 2013, 62, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Fatanmi, O.O.; Scott, J.; Romaine, P.L.; Newman, V.L.; Verma, A.; Elliott, T.B.; Seed, T.M. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS ONE 2014, 9, e114078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.; Fatanmi, O.O.; Wise, S.Y.; Rao, V.A.; Singh, V.K. Tocol Prophylaxis for Total-body Irradiation: A Proteomic Analysis in Murine Model. Health Phys. 2020, 119, 12–20. [Google Scholar] [CrossRef]
- Rosen, E.; Fatanmi, O.O.; Wise, S.Y.; Rao, V.A.; Singh, V.K. Gamma-tocotrienol, a radiation countermeasure, reverses proteomic changes in serum following total-body gamma irradiation in mice. Sci. Rep. 2022, 12, 3387. [Google Scholar] [CrossRef]
- Garg, S.; Garg, T.K.; Wise, S.Y.; Fatanmi, O.O.; Miousse, I.R.; Savenka, A.V.; Basnakian, A.G.; Singh, V.K.; Hauer-Jensen, M. Effects of Gamma-Tocotrienol on Intestinal Injury in a GI-Specific Acute Radiation Syndrome Model in Nonhuman Primate. Int. J. Mol. Sci. 2022, 23, 4643. [Google Scholar] [CrossRef]
- Garg, S.; Garg, T.K.; Miousse, I.R.; Wise, S.Y.; Fatanmi, O.O.; Savenka, A.V.; Basnakian, A.G.; Singh, V.K.; Hauer-Jensen, M. Effects of Gamma-Tocotrienol on Partial-Body Irradiation-Induced Intestinal Injury in a Nonhuman Primate Model. Antioxidants 2022, 11, 1895. [Google Scholar] [CrossRef]
- Vellichirammal, N.N.; Sethi, S.; Pandey, S.; Singh, J.; Wise, S.Y.; Carpenter, A.D.; Fatanmi, O.O.; Guda, C.; Singh, V.K. Lung transcriptome of nonhuman primates exposed to total- and partial-body irradiation. Mol. Ther. Nucleic Acids 2022, 29, 584–598. [Google Scholar] [CrossRef]
- Zitsman, J.S.; Alonso-Guallart, P.; Ovanez, C.; Kato, Y.; Rosen, J.F.; Weiner, J.I.; Duran-Struuck, R. Distinctive Leukocyte Subpopulations According to Organ Type in Cynomolgus Macaques. Comp. Med. 2016, 66, 308–323. [Google Scholar] [PubMed]
- Karlsson, I.; Malleret, B.; Brochard, P.; Delache, B.; Calvo, J.; Le Grand, R.; Vaslin, B. Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus macaques. J. Virol. 2007, 81, 13456–13468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spits, H. Development of alphabeta T cells in the human thymus. Nat. Rev. Immunol. 2002, 2, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, L.; Hendrickson, H.P.; Liu, L.; Chang, J.; Luo, Y.; Seng, J.; Pouliot, M.; Authier, S.; Zhou, D.; et al. Total Body Irradiation in the “Hematopoietic” Dose Range Induces Substantial Intestinal Injury in Non-Human Primates. Radiat. Res. 2015, 184, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Radtke, S.; Adair, J.E.; Giese, M.A.; Chan, Y.Y.; Norgaard, Z.K.; Enstrom, M.; Haworth, K.G.; Schefter, L.E.; Kiem, H.P. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Sci. Transl. Med. 2017, 9, eaan1145. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.M.; Till, J.E.; Siminovitch, L.; McCulloch, E.A. Cytological evidence for a relationship between normal hematopoietic colony-forming cells and cells of the lymphoid system. J. Exp. Med. 1968, 127, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Knabe, D.A.; Flynn, N.E. Synthesis of citrulline from glutamine in pig enterocytes. Biochem. J. 1994, 299 Pt 1, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Sayles, P.C.; Gibson, G.W.; Johnson, L.L. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 2000, 68, 1026–1033. [Google Scholar] [CrossRef] [Green Version]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological decision-making: How does the immune system decide to mount a helper T-cell response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef]
- Foulds, K.E.; Donaldson, M.; Roederer, M. OMIP-005: Quality and phenotype of antigen-responsive rhesus macaque T cells. Cytom. A 2012, 81, 360–361. [Google Scholar] [CrossRef]
- Sun, J.C.; Lanier, L.L. NK cell development, homeostasis and function: Parallels with CD8(+) T cells. Nat. Rev. Immunol. 2011, 11, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Weisgrau, K.L.; Ries, M.; Pomplun, N.; Evans, D.T.; Rakasz, E.G. OMIP-035: Functional analysis of natural killer cell subsets in macaques. Cytom. A 2016, 89, 799–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkateswaran, K.; Shrivastava, A.; Agrawala, P.K.; Prasad, A.; Kalra, N.; Pandey, P.R.; Manda, K.; Raj, H.G.; Parmar, V.S.; Dwarakanath, B.S. Mitigation of radiation-induced hematopoietic injury by the polyphenolic acetate 7, 8-diacetoxy-4-methylthiocoumarin in mice. Sci. Rep. 2016, 6, 37305. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, D.M.; Case, C., Jr.; Bader, J.L.; Chao, N.J.; Coleman, C.N.; Hatchett, R.J.; Weisdorf, D.J.; Confer, D.L. Radiologic and nuclear events: Contingency planning for hematologists/oncologists. Blood 2008, 111, 5440–5445. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Ahn, G.N.; Lee, N.H.; Kim, J.M.; Yun, J.S.; Hyun, J.W.; Jeon, Y.J.; Wie, M.B.; Lee, Y.J.; Park, J.W.; et al. Radioprotective properties of eckol against ionizing radiation in mice. FEBS Lett. 2008, 582, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Macintyre, A.N.; French, M.J.; Sanders, B.R.; Riebe, K.J.; Shterev, I.D.; Wiehe, K.; Hora, B.; Evangelous, T.; Dugan, G.; Bourland, J.D.; et al. Long-Term Recovery of the Adaptive Immune System in Rhesus Macaques After Total Body Irradiation. Adv. Radiat. Oncol. 2021, 6, 100677. [Google Scholar] [CrossRef]
- Farese, A.M.; Hankey, K.G.; Cohen, M.V.; MacVittie, T.J. Lymphoid and Myeloid Recovery in Rhesus Macaques Following Total Body X-Irradiation. Health Phys. 2015, 109, 414–426. [Google Scholar] [CrossRef]
- Wang, Y.; Schulte, B.A.; Zhou, D. Hematopoietic stem cell senescence and long-term bone marrow injury. Cell Cycle 2006, 5, 35–38. [Google Scholar] [CrossRef]
- Gianni, A.M.; Bregni, M.; Siena, S.; Villa, S.; Sciorelli, G.A.; Ravagnani, F.; Pellegris, G.; Bonadonna, G. Rapid and complete hemopoietic reconstitution following combined transplantation of autologous blood and bone marrow cells. A changing role for high dose chemo-radiotherapy? Hematol. Oncol. 1989, 7, 139–148. [Google Scholar] [CrossRef]
- Laterveer, L.; Zijlmans, J.M.; Liehl, E.; Willemze, R.; Fibbe, W.E. Accelerated platelet reconstitution following transplantation of bone marrow cells derived from IL-6-treated donor mice. Ann. Hematol. 1996, 73, 239–245. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 2017, 93, 851–869. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Olabisi, A.O. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert. Opin. Drug. Discov. 2017, 12, 695–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacVittie, T.J.; Farese, A.M.; Jackson, W., 3rd. The Hematopoietic Syndrome of the Acute Radiation Syndrome in Rhesus Macaques: A Systematic Review of the Lethal Dose Response Relationship. Health Phys. 2015, 109, 342–366. [Google Scholar] [CrossRef] [PubMed]
- DeBo, R.J.; Register, T.C.; Caudell, D.L.; Sempowski, G.D.; Dugan, G.; Gray, S.; Owzar, K.; Jiang, C.; Bourland, J.D.; Chao, N.J.; et al. Molecular and cellular profiling of acute responses to total body radiation exposure in ovariectomized female cynomolgus macaques. Int. J. Radiat. Biol. 2015, 91, 510–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacVittie, T.J.; Bennett, A.W.; Cohen, M.V.; Farese, A.M.; Higgins, A.; Hankey, K.G. Immune cell reconstitution after exposure to potentially lethal doses of radiation in the nonhuman primate. Health Phys. 2014, 106, 84–96. [Google Scholar] [CrossRef]
- Monroy, R.L.; Skelly, R.R.; Taylor, P.; Dubois, A.; Donahue, R.E.; MacVittie, T.J. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp. Hematol. 1988, 16, 344–348. [Google Scholar]
- Farese, A.M.; Cohen, M.V.; Katz, B.P.; Smith, C.P.; Gibbs, A.; Cohen, D.M.; MacVittie, T.J. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat. Res. 2013, 179, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Farese, A.M.; Cohen, M.V.; Stead, R.B.; Jackson, W., 3rd; Macvittie, T.J. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat. Res. 2012, 178, 403–413. [Google Scholar] [CrossRef]
- Hankey, K.G.; Farese, A.M.; Blaauw, E.C.; Gibbs, A.M.; Smith, C.P.; Katz, B.P.; Tong, Y.; Prado, K.L.; MacVittie, T.J. Pegfilgrastim Improves Survival of Lethally Irradiated Nonhuman Primates. Radiat. Res. 2015, 183, 643–655. [Google Scholar] [CrossRef]
- Thrall, K.D.; Love, R.; O’Donnell, K.C.; Farese, A.M.; Manning, R.; MacVittie, T.J. An Interlaboratory Validation of the Radiation Dose Response Relationship (DRR) for H-ARS in the Rhesus Macaque. Health Phys. 2015, 109, 502–510. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J. Med. Primatol. 1997, 26, 113–119. [Google Scholar] [CrossRef]
- Uno, Y.; Uehara, S.; Yamazaki, H. Utility of non-human primates in drug development: Comparison of non-human primate and human drug-metabolizing cytochrome P450 enzymes. Biochem. Pharmacol. 2016, 121, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Boerma, M.; Wang, J.; Kumar, K.S.; Hauer-Jensen, M. gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat. Res. 2009, 171, 596–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuks, Z.; Strober, S.; Bobrove, A.; Sasazuki, T.; McMichael, A.; Kaplan, H. Long term effects of radiation of T and B lymphocytes in peripheral blood of patients with Hodgkin’s disease. J. Clin. Investig. 1976, 58, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Lyons, C. Homogeneous sensitivity of human peripheral blood lymphocytes to radiation-induced chromosome damage. Nature 1979, 278, 756–758. [Google Scholar] [CrossRef]
- Till, J.E.; McCulloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 213–222. [Google Scholar] [CrossRef]
- Gridley, D.S.; Pecaut, M.J.; Dutta-Roy, R.; Nelson, G.A. Dose and dose rate effects of whole-body proton irradiation on leukocyte populations and lymphoid organs: Part I. Immunol. Lett. 2002, 80, 55–66. [Google Scholar] [CrossRef]
- Wu, A.M.; Till, J.E.; Siminovitch, L.; McCulloch, E.A. A cytological study of the capacity for differentiation of normal hemopoietic colony-forming cells. J. Cell. Physiol. 1967, 69, 177–184. [Google Scholar] [CrossRef]
- Kapoor, V.; Collins, A.; Griffith, K.; Ghosh, S.; Wong, N.; Wang, X.; Challen, G.A.; Krambs, J.; Link, D.; Hallahan, D.E.; et al. Radiation induces iatrogenic immunosuppression by indirectly affecting hematopoiesis in bone marrow. Oncotarget 2020, 11, 1681–1690. [Google Scholar] [CrossRef]
- Wang, X.-B.; Wu, D.-J.; Chen, W.-P.; Liu, J.; Ju, Y.-J. Impact of radiotherapy on immunological parameters, levels of inflammatory factors, and clinical prognosis in patients with esophageal cancer. J. Radiat. Res. 2019, 60, 353–363. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Y. Overview of orchestration of CD4+ T cell subsets in immune responses. Adv. Exp. Med. Biol. 2014, 841, 1–13. [Google Scholar] [CrossRef]
- Hu, S.; Rotschafer, J.H.; Lokensgard, J.R.; Cheeran, M.C. Activated CD8+ T lymphocytes inhibit neural stem/progenitor cell proliferation: Role of interferon-gamma. PLoS ONE 2014, 9, e105219. [Google Scholar] [CrossRef] [PubMed]
- Bigley, A.B.; Simpson, R.J. NK cells and exercise: Implications for cancer immunotherapy and survivorship. Discov. Med. 2015, 19, 433–445. [Google Scholar] [PubMed]
- Chen, J.; Liu, X.; Zeng, Z.; Li, J.; Luo, Y.; Sun, W.; Gong, Y.; Zhang, J.; Wu, Q.; Xie, C. Immunomodulation of NK Cells by Ionizing Radiation. Front. Oncol. 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Zarcone, D.; Tilden, A.B.; Lane, V.G.; Grossi, C.E. Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood 1989, 73, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Beach, T.; Authier, S.; Javitz, H.S.; Wong, K.; Bakke, J.; Gahagen, J.; Bunin, D.I.; Chang, P.Y. Total body irradiation models in NHPs—consideration of animal sex and provision of supportive care to advance model development. Int. J. Radiat. Biol. 2021, 97, 126–130. [Google Scholar] [CrossRef]
- National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Li, Y.; Singh, J.; Varghese, R.; Zhang, Y.; Fatanmi, O.O.; Cheema, A.K.; Singh, V.K. Transcriptome of rhesus macaque (Macaca mulatta) exposed to total-body irradiation. Sci. Rep. 2021, 11, 6295. [Google Scholar] [CrossRef]



| Study Design with 16 NHPs | |||||
|---|---|---|---|---|---|
| Hematopoietic Study (Total-Body Irradiation, 60Co γ-Radiation, 0.6 Gy/min) | |||||
| NHP | Drug | Route | Dose | Frequency | Irradiation Dose (Gy) |
| 4 | GT3 | sc | 37.5 mg/kg | 24 h prior to irradiation | 4 |
| 4 | Veh | sc | 37.5 mg/kg | 24 h prior to irradiation | 4 |
| 4 | GT3 | sc | 37.5 mg/kg | 24 h prior to irradiation | 5.8 |
| 4 | Veh | sc | 37.5 mg/kg | 24 h prior to irradiation | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, T.K.; Garg, S.; Miousse, I.R.; Wise, S.Y.; Carpenter, A.D.; Fatanmi, O.O.; van Rhee, F.; Singh, V.K.; Hauer-Jensen, M. Gamma-Tocotrienol Modulates Total-Body Irradiation-Induced Hematopoietic Injury in a Nonhuman Primate Model. Int. J. Mol. Sci. 2022, 23, 16170. https://doi.org/10.3390/ijms232416170
Garg TK, Garg S, Miousse IR, Wise SY, Carpenter AD, Fatanmi OO, van Rhee F, Singh VK, Hauer-Jensen M. Gamma-Tocotrienol Modulates Total-Body Irradiation-Induced Hematopoietic Injury in a Nonhuman Primate Model. International Journal of Molecular Sciences. 2022; 23(24):16170. https://doi.org/10.3390/ijms232416170
Chicago/Turabian StyleGarg, Tarun K., Sarita Garg, Isabelle R. Miousse, Stephen Y. Wise, Alana D. Carpenter, Oluseyi O. Fatanmi, Frits van Rhee, Vijay K. Singh, and Martin Hauer-Jensen. 2022. "Gamma-Tocotrienol Modulates Total-Body Irradiation-Induced Hematopoietic Injury in a Nonhuman Primate Model" International Journal of Molecular Sciences 23, no. 24: 16170. https://doi.org/10.3390/ijms232416170
APA StyleGarg, T. K., Garg, S., Miousse, I. R., Wise, S. Y., Carpenter, A. D., Fatanmi, O. O., van Rhee, F., Singh, V. K., & Hauer-Jensen, M. (2022). Gamma-Tocotrienol Modulates Total-Body Irradiation-Induced Hematopoietic Injury in a Nonhuman Primate Model. International Journal of Molecular Sciences, 23(24), 16170. https://doi.org/10.3390/ijms232416170






