Acylcarnitines in Ophthalmology: Promising Emerging Biomarkers
Abstract
:1. Introduction
2. Methodology
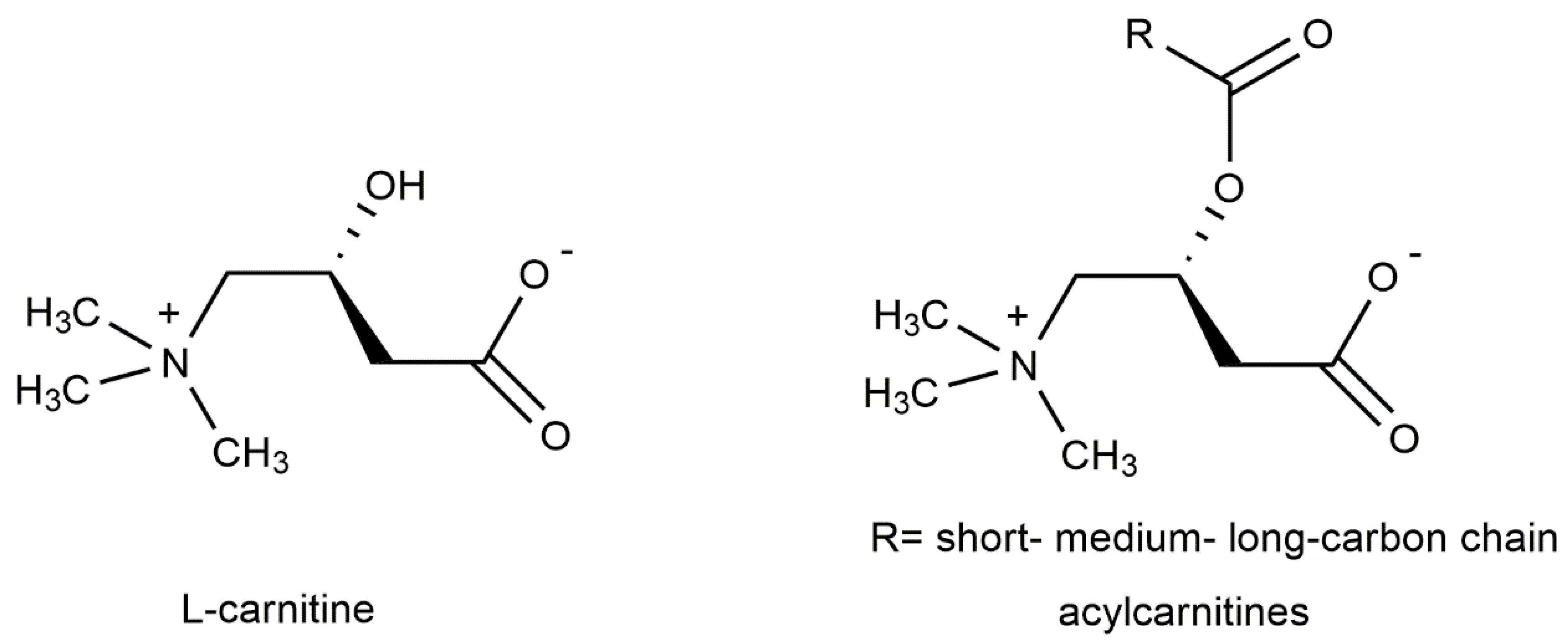
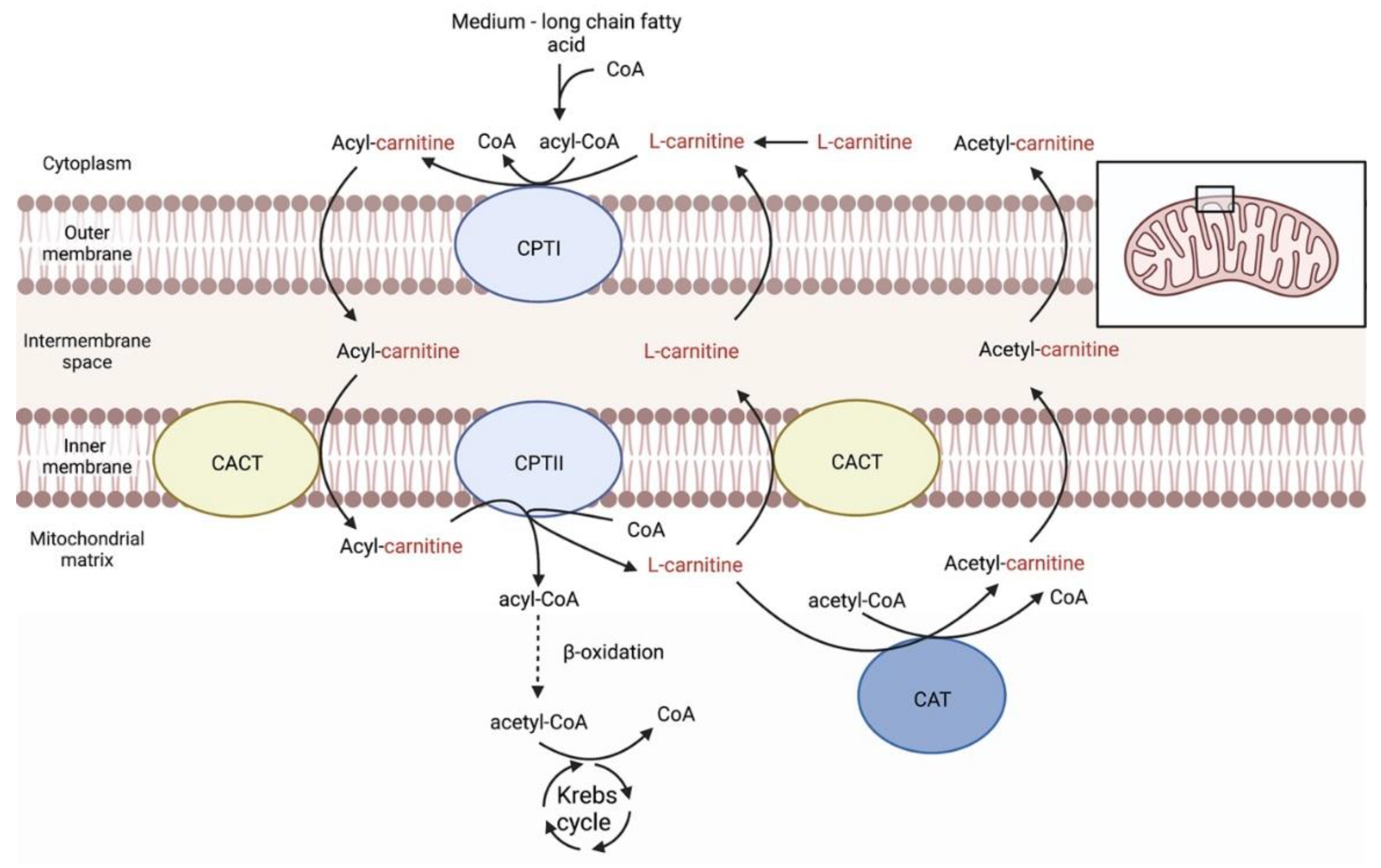
3. Carnitines Biological Role
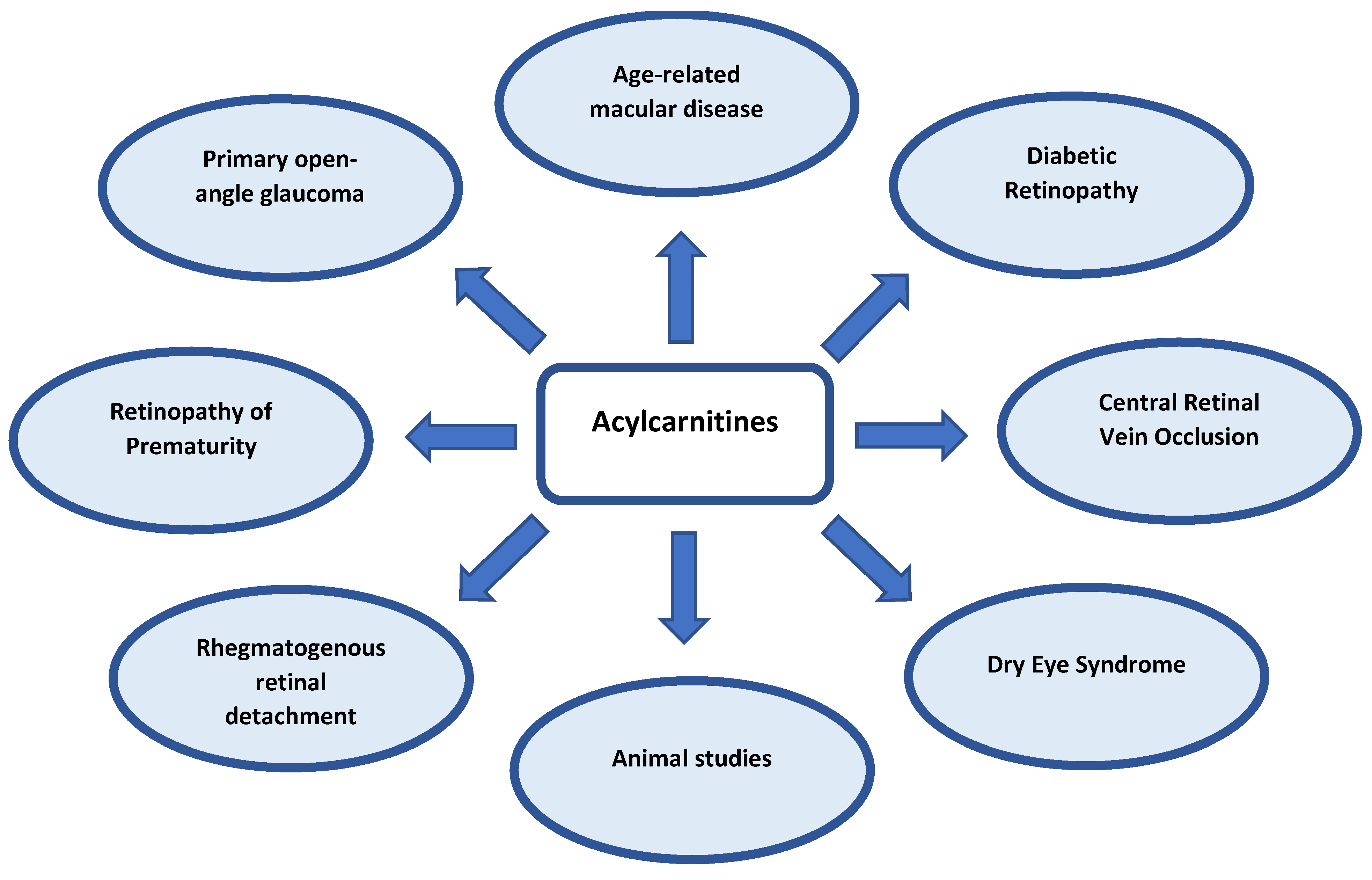
4. Carnitines in Ocular Diseases
4.1. Age-Related Macular Degeneration (AMD)
4.2. Diabetic Retinopathy (DR)
4.3. Retinopathy of Prematurity (ROP)
4.4. Central Retinal Vein Occlusion (CRVO)
4.5. Primary Open-Angle Glaucoma (POAG)
4.6. Rhegmatogenous Retinal Detachment (RRD)
4.7. Dry Eye Syndrome (DES)
4.8. Animal Studies
5. Discussion
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blindness and Vision Impairment, World Health Organization. 2021. Available online: https://www.who.int/en/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 6 December 2022).
- Cheng, K.J.; Hsieh, C.M.; Nepali, K.; Liou, J.P. Ocular Disease Therapeutics: Design and Delivery of Drugs for Diseases of the Eye. J. Med. Chem. 2020, 63, 10533–10593. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.; Virgiliou, C.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Untargeted LC/MS-based metabolic phenotyping (metabonomics/metabolomics): The state of the art. J. Chromatogr. B 2019, 1117, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.G.; Theodoridis, G.A.; Wilson, I.D. Metabolic Profiling: Status, Challenges, and Perspective. Methods Mol. Biol. 2018, 1738, 3–13. [Google Scholar] [PubMed]
- Laíns, I.; Gantner, M.; Murinello, S.; Lasky-Su, J.A.; Miller, J.W.; Friedlander, M.; Husain, D. Metabolomics in the study of retinal health and disease. Prog. Retin. Eye Res. 2019, 69, 57–79. [Google Scholar] [CrossRef] [PubMed]
- Nazifova-Tasinova, N.; Radeva, M.; Galunska, B.; Grupcheva, C. Metabolomic analysis in ophthalmology. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2020, 164, 236–246. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, H.P.; Liu, Y.; Chen, L. Metabolomics and biomarkers in ocular matrix: Beyond ocular diseases. Int. J. Ophthalmol. 2020, 13, 991–1003. [Google Scholar] [CrossRef]
- Li, X.; Cai, S.; He, Z.; Reilly, J.; Zeng, Z.; Strang, N.; Shu, X. Metabolomics in Retinal Diseases: An Update. Biology 2021, 10, 944. [Google Scholar] [CrossRef]
- Homma, K.; Toda, E.; Osada, H.; Nagai, N.; Era, T.; Tsubota, K.; Okano, H.; Ozawa, Y. Taurine rescues mitochondria-related metabolic impairments in the patient-derived induced pluripotent stem cells and epithelial-mesenchymal transition in the retinal pigment epithelium. Redox Biol. 2021, 41, 101921. [Google Scholar] [CrossRef]
- Tribble, J.R.; Otmani, A.; Sun, S.; Ellis, S.A.; Cimaglia, G.; Vohra, R.; Jöe, M.; Lardner, E.; Venkataraman, A.P.; Domínguez-Vicent, A.; et al. Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction. Redox Biol. 2021, 43, 101988. [Google Scholar] [CrossRef]
- Luo, D.; Deng, T.; Yuan, W.; Deng, H.; Jin, M. Plasma metabolomic study in Chinese patients with wet age-related macular degeneration. BMC Ophthalmol. 2017, 17, 165. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Uppal, K.; Williamson, S.M.; Liu, K.; Burgess, L.G.; Tran, V.; Umfress, A.C.; Jarrell, K.L.; Cooke Bailey, J.N.; Agarwal, A.; et al. The carnitine shuttle pathway is altered in patients with neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4978–4985. [Google Scholar] [CrossRef] [Green Version]
- Chao de la Barca, J.M.; Rondet-Courbis, B.; Ferré, M.; Muller, J.; Buisset, A.; Leruez, S.; Plubeau, G.; Macé, T.; Moureauzeau, L.; Chupin, S.; et al. A plasma metabolomic profiling of exudative age-related macular degeneration showing carnosine and mitochondrial deficiencies. J. Clin. Med. 2020, 9, 631. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Wei, P.; He, M.; Teng, H.; Chu, Y. Metabolomic profiling of the aqueous humor in patients with wet age-related macular degeneration using UHPLC-MS/MS. J. Proteome Res. 2020, 19, 2358–2366. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Ma, C.; Scott, W.K.; Agarwal, A.; Pericak-Vance, M.A.; Haines, J.L.; Jones, D.P.; Uppal, K.; Brantley, M.A., Jr. Plasma Metabolomics of Intermediate and Neovascular Age-Related Macular Degeneration Patients. Cells 2021, 10, 3141. [Google Scholar] [CrossRef]
- Sumarriva, K.; Uppal, K.; Ma, C.; Herren, D.J.; Wang, Y.; Chocron, I.M.; Warden, C.; Mitchell, S.L.; Burgess, L.G.; Goodale, M.P.; et al. Arginine and carnitine metabolites are altered in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3119–3126. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.H.; Kim, J.M.; Jeon, H.J.; Oh, T.; Choi, H.J.; Kim, B.J. Metabolomics profiles associated with diabetic retinopathy in type 2 diabetes patients. PLoS ONE 2020, 15, e0241365. [Google Scholar] [CrossRef]
- Paris, L.P.; Johnson, C.H.; Aguilar, E.; Usui, Y.; Cho, K.; Hoang, L.T.; Feitelberg, D.; Benton, H.P.; Westenskow, P.D.; Kurihara, T.; et al. Global metabolomics reveals metabolic dysregulation in ischemic retinopathy. Metabolomics 2016, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wu, Z.; Li, S.; Yang, M.; Xiao, X.; Lian, C.; Wen, W.; He, H.; Zeng, J.; Wang, J.; et al. Targeted Blood Metabolomic Study on Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef] [Green Version]
- Burgess, L.G.; Uppal, K.; Walker, D.I.; Roberson, R.M.; Tran, V.; Parks, M.B.; Wade, E.A.; May, A.T.; Umfress, A.C.; Jarrell, K.L.; et al. Metabolome-wide association study of primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5020–5028. [Google Scholar] [CrossRef] [Green Version]
- Leruez, S.; Marill, A.; Bresson, T.; De Saint Martin, G.; Buisset, A.; Muller, J.; Tessier, L.; Gadras, C.; Verny, C.; Gohier, P.; et al. A metabolomics profiling of glaucoma points to mitochondrial dysfunction, senescence, and polyamines deficiency. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4355–4361. [Google Scholar] [CrossRef]
- Buisset, A.; Gohier, P.; Leruez, S.; Muller, J.; Amati-Bonneau, P.; Lenaers, G.; Bonneau, D.; Simard, G.; Procaccio, V.; Annweiler, C.; et al. Metabolomic profiling of aqueous humor in glaucoma points to taurine and spermine deficiency: Findings from the Eye-D study. J. Proteome Res. 2019, 18, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Lillo, A.; Marin, S.; Serrano-Marín, J.; Binetti, N.; Navarro, G.; Cascante, M.; Sánchez-Navés, J.; Franco, R. Targeted Metabolomics Shows That the Level of Glutamine, Kynurenine, Acyl-Carnitines and Lysophosphatidylcholines Is Significantly Increased in the Aqueous Humor of Glaucoma Patients. Front. Med. 2022, 9, 935084. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Agnifili, L.; Mastropasqua, L.; Lanuti, P.; Marchisio, M.; De Laurenzi, V.; Del Boccio, P.; Pieragostino, D. Multi-omics approach for studying tears in treatment-naive glaucoma patients. Int. J. Mol. Sci. 2019, 20, 4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Li, H.; Jiang, P.; Liu, X.; Xu, D.; Wang, F. Investigating the pathological processes of rhegmatogenous retinal detachment and proliferative vitreoretinopathy with metabolomics analysis. Mol. Biosyst. 2014, 10, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; He, M.; Teng, H.; Han, G. Metabolomic analysis of the aqueous humor from patients with central retinal vein occlusion using UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 188, 113448. [Google Scholar] [CrossRef]
- Pescosolido, N.; Imperatrice, B.; Koverech, A.; Messano, M. L-carnitine and short chain ester in tears from patients with dry eye. Optom. Vis. Sci. 2009, 86, E132–E138. [Google Scholar] [CrossRef]
- Fu, Z.; Kern, T.S.; Hellström, A.; Smith, L.E.H. Fatty acid oxidation and photoreceptor metabolic needs. J Lipid Res. 2021, 62, 100035. [Google Scholar] [CrossRef] [Green Version]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines: Pharmacokinetic, Pharmacological and Clinical Aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of L-Carnitine for Human Health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of Carnitine in Disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- Latham, L.E.; Wang, C.; Patterson, T.A.; Slikker, W., Jr.; Liu, F. Neuroprotective Effects of Carnitine and Its Potential Application to Ameliorate Neurotoxicity. Chem. Res. Toxicol. 2021, 34, 1208–1222. [Google Scholar] [CrossRef]
- Deda, O.; Panteris, E.; Meikopoulos, T.; Begou, O.; Mouskeftara, T.; Karagiannidis, E.; Papazoglou, A.S.; Sianos, G.; Theodoridis, G.; Gika, H. Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules 2022, 12, 354. [Google Scholar] [CrossRef]
- Gong, L.L.; Yang, S.; Zhang, W.; Han, F.F.; Xuan, L.L.; Lv, Y.L.; Liu, H.; Liu, L.H. Targeted Metabolomics for Plasma Amino Acids and Carnitines in Patients with Metabolic Syndrome Using HPLC-MS/MS. Dis. Markers 2020, 2020, 8842320. [Google Scholar] [CrossRef]
- Sampey, B.P.; Freemerman, A.J.; Zhang, J.; Kuan, P.F.; Galanko, J.A.; O’Connell, T.M.; Ilkayeva, O.R.; Muehlbauer, M.J.; Stevens, R.D.; Newgard, C.B.; et al. Metabolomic Profiling Reveals Mitochondrial-Derived Lipid Biomarkers That Drive Obesity-Associated Inflammation. PLoS ONE 2012, 7, e38812. [Google Scholar] [CrossRef]
- Tang, Y.M.; Wang, J.P.; Bao, W.M.; Yang, J.H.; Ma, L.K.; Yang, J.; Chen, H.; Xu, Y.; Yang, L.H.; Li, W.; et al. Urine and serum metabolomic profiling reveals that bile acids and carnitine may be potential biomarkers of primary biliary cirrhosis. Int. J. Mol. Med. 2015, 36, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- García-Layana, A.; Cabrera-López, F.; Garcia-Arumi, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and Intermediate Age-Related Macular Degeneration: Update and Clinical Review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Bayer, A.J.; Girling, A.J.; Woodhouse, K.W. Older adults, diabetes mellitus and visual acuity: A community-based case-control study. Age Ageing 2000, 29, 335–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Stroud, S.; Mehta, A.; Rangasamy, S. New treatments for diabetic retinopathy. Diabetes Obes. Metab. 2015, 17, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Hartnett, M.E.; Penn, J.S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2012, 367, 2515–2526. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, Q.; Luo, S.; Zhang, Y.; Lian, C.; He, H.; Zeng, J.; Zhang, G. Comparative Analysis Reveals Novel Changes in Plasma Metabolites and Metabolomic Networks of Infants With Retinopathy of Prematurity. Investig. Ophthalmol. Vis. Sci. 2022, 63, 28. [Google Scholar] [CrossRef]
- Brown, J.M.; Campbell, J.P.; Beers, A.; Chang, K.; Ostmo, S.; Chan, R.V.P.; Dy, J.; Erdogmus, D.; Ioannidis, S.; Kalpathy-Cramer, J.; et al. Imaging and Informatics in Retinopathy of Prematurity (i-ROP) Research Consortium. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018, 136, 803–810. [Google Scholar] [CrossRef]
- Soares, R.R.; Cai, L.Z.; Bowe, T.; Samuelson, A.G.; Liu, C.K.; Parikh, D.; Patel, S.N.; Hinkle, J.W.; Yonekawa, Y. Geographic access disparities to clinical trials in retinopathy of prematurity in the United States. Retina 2021, 41, 2253–2260. [Google Scholar] [CrossRef]
- McAllister, I.L. Central retinal vein occlusion: A review. Clin. Exp. Ophthalmol. 2012, 40, 48–58. [Google Scholar] [CrossRef]
- Chen, T.Y.; Uppuluri, A.; Zarbin, M.A.; Bhagat, N. Risk factors for central retinal vein occlusion in young adults. Eur. J. Ophthalmol. 2021, 31, 2546–2555. [Google Scholar] [CrossRef]
- Pournaras, C.J.; Rungger-Brandle, E.; Riva, C.E.; Hardarson, S.H.; Stefansson, E. Regulation of retinal blood flow in health and disease. Prog. Retin. Eye Res. 2008, 27, 284–330. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Heijl, A.; Bengtsson, B.; Hyman, L.; Leske, M.C.; Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology 2009, 116, 2271–2276. [Google Scholar] [CrossRef] [Green Version]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, R.; Montfort, J.; Jeffries, M.; Agar, A.; Bank, A.; McNaught, P.; Francis, I.C. Carnitine palmitoyl transferase II deficiency: A possible association with progression of normal pressure glaucoma. Clin. Exp. Ophthalmol. 2012, 40, e237–e238. [Google Scholar] [CrossRef]
- Go, Y.M.; Roede, J.R.; Orr, M.; Liang, Y.; Jones, D.P. Integrated redox proteomics and metabolomics of mitochondria to identify mechanisms of cd toxicity. Toxicol. Sci. 2014, 139, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Rong, S.; Li, Y.; Guan, Y.; Zhu, L.; Zhou, Q.; Gao, M.; Pan, H.; Zou, L.; Chang, D. Long-chain unsaturated fatty acids as possible important metabolites for primary angle-closure glaucoma based on targeted metabolomic analysis. Biomed. Chromatogr. 2017, 31, e3963. [Google Scholar] [CrossRef]
- Mayordomo-Febrer, A.; López-Murcia, M.; Morales-Tatay, J.M.; Monleón-Salvado, D.; Pinazo-Durán, M.D. Metabolomics of the aqueous humor in the rat glaucoma model induced by a series of intracamerular sodium hyaluronate injection. Exp. Eye Res. 2015, 131, 84–92. [Google Scholar] [CrossRef]
- Rinaldo, P.; Cowan, T.M.; Matern, D. Acylcarnitine profile analysis. Genet. Med. 2008, 10, 151–156. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calandrella, N.; De Seta, C.; Scarsella, G.; Risuleo, G. Carnitine reduces the lipoperoxidative damage of the membrane and apoptosis after induction of cell stress in experimental glaucoma. Cell Death Dis. 2010, 1, e62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idrees, S.; Sridhar, J.; Kuriyan, A.E. Proliferative Vitreoretinopathy: A Review. Int. Ophthalmol. Clin. 2019, 59, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Fortin, G. L-Carnitine and intestinal inflammation. Vitam. Horm. 2011, 86, 353–366. [Google Scholar] [PubMed]
- Corrales, R.M.; Luo, L.; Chang, E.Y.; Pflugfelder, S.C. Effects of osmoprotectants on hyperosmolar stress in cultured human corneal epithelial cells. Cornea 2008, 27, 574–579. [Google Scholar] [CrossRef]
- Xie, H.; Yang, B.; Zhou, X.M.; Song, F.L.; Li, J.M.; Zhou, K.; Hu, W.; Peng, Y.Q.; Tang, S.Y.; Yuan, L.Q.; et al. L-carnitine and taurine synergistically inhibit the proliferation and osteoblastic differentiation of vascular smooth muscle cells. Acta Pharmacol. Sin. 2010, 31, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Khanna, R.K.; Catanese, S.; Emond, P.; Corcia, P.; Blasco, H.; Pisella, P.J. Metabolomics and lipidomics approaches in human tears: A systematic review. Surv. Ophthalmol. 2022, 67, 1229–1243. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Snytnikova, O.A.; Kiryutin, A.S.; Yanshole, L.V.; Sagdeev, R.Z.; Tsentalovich, Y.P. Metabolomics of the rat lens: A combined LC-MS and NMR study. Exp. Eye Res. 2014, 125, 71–78. [Google Scholar] [CrossRef]
- Kurihara, T.; Westenskow, P.D.; Gantner, M.L.; Usui, Y.; Schultz, A.; Bravo, S.; Aguilar, E.; Wittgrove, C.; Friedlander, M.S.; Paris, L.P.; et al. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife 2016, 5, e14319. [Google Scholar] [CrossRef]
- Rowan, S.; Jiang, S.; Korem, T.; Szymanski, J.; Chang, M.L.; Szelog, J.; Cassalman, C.; Dasuri, K.; McGuire, C.; Nagai, R.; et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E4472–E4481. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 2009, 6, e31–e39. [Google Scholar] [CrossRef] [Green Version]
- Schoors, S.; Bruning, U.; Missiaen, R.; Queiroz, K.C.; Borgers, G.; Elia, I.; Zecchin, A.; Cantelmo, A.R.; Christen, S.; Goveia, J.; et al. Fatty Acid Carbon Is Essential for DNTP Synthesis in Endothelial Cells. Nature 2015, 520, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Kalucka, J.; Bierhansl, L.; Conchinha, N.V.; Missiaen, R.; Elia, I.; Brüning, U.; Scheinok, S.; Treps, L.; Cantelmo, A.R.; Dubois, C.; et al. Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018, 28, 881–894. [Google Scholar] [CrossRef] [Green Version]
- Alagoz, G.; Celiker, U.; Ilhan, N.; Yekeler, H.; Demir, T.; Celiker, H. L-carnitine in experimental retinal ischemia-reperfusion injury. Ophthalmologica 2002, 216, 144–150. [Google Scholar] [CrossRef]
- Fletcher, A.L.; Pennesi, M.E.; Harding, C.O.; Weleber, R.G.; Gillingham, M.B. Observations regarding retinopathy in mitochondrial trifunctional protein deficiencies. Mol. Genet. Metab. 2012, 106, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.C.; McKenna, M.C. L-carnitine and Acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Peluso, G.; Barbarisi, A.; Savica, V.; Reda, E.; Nicolai, R.; Benatti, P.; Calvani, M. Carnitine: An osmolyte that plays a metabolic role. J. Cell. Biochem. 2000, 80, 1–10. [Google Scholar] [CrossRef]
- Yazdani, M.; Elgstøen, K.B.P.; Rootwelt, H.; Shahdadfar, A.; Utheim, Ø.A.; Utheim, T.P. Tear Metabolomics in Dry Eye Disease: A Review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Transport of l-carnitine in human corneal and conjunctival epithelial cells. Mol. Vis. 2010, 16, 1823. [Google Scholar]
- Tang, Z.; Cao, T.; Lin, S.; Fu, L.; Li, S.; Guan, X.Y.; Cai, Z. Characterization of oncogene-induced metabolic alterations in hepatic cells by using ultrahigh performance liquid chromatography-tandem mass spectrometry. Talanta 2016, 152, 119–126. [Google Scholar] [CrossRef] [PubMed]
| Eye Disease | Study Participants | Carnitine Biomarker | Change | Bio- Specimen | Study Method/ Total Metabolites /Number (Type) of Carnitines | Analytical Technique | Ref. |
|---|---|---|---|---|---|---|---|
| AMD (wet) | AMD: 20 (27 eyes) Controls: 20 | Palmitoylcarnitine (C16) | ↓ | Plasma | Untargeted | UHPLC-Q-TOF MS | [11] |
| AMD (wet) | NVAMD: 100* Controls: 192** | 9-Hexadecenoylcarnitine (C16:1) Heptadecanoylcarnitine (C17) 11Z-octadecenylcarnitine (18:1) Palmitoylcarnitine (C16) Stearoylcarnitine (C18) | ↑ | Plasma | Untargeted | LC-MS/MS | [12] |
| AMD (wet) | Patients: 40 Controls: 40 | L-carnitine (C0) Valerylcarnitine (C5) | ↑ | Plasma | Targeted/116/ 40 (C0 & 39 ACs) | FIA- MS /MS | [13] |
| AMD (wet) | Wet AMD patients: 26 (26 eyes) Controls: 20 (20 eyes) | L-carnitine (C0) Deoxycarnitine | ↓ ↑ | Aqueous humor | Untargeted | UHPLC-MS/MS | [14] |
| AMD (wet) vs. control NVAMD vs IAMD | IAMD: 91 NVAMD: 100 Controls: 195 | Linoleylcarnitine (C18:2) Linolenylcarnitine (C18:3) Glutaconylcarnitine (C5:2) Heptadecanoylcarnitine (C17) 11Z-octadecenylcarnitine (C18:1) Stearoylcarnitine (C18) | ↑ ↑ ↑ ↑ ↑ ↑ | Plasma | Untargeted | LC-MS/MS | [15] |
| DR vs. DM PDR vs. NPDR | DR patients: 83 Controls: 90*** | Dehydroxycarnitine L-carnitine (C0) | ↑ ↑ | Plasma | Untargeted | LC-MS | [16] |
| DR vs. NDR | NPDR: 123 PDR: 51 Controls: 143**** | Propionylcarnitine (C3) Butyrylcarnitine (C4) | ↑ ↑ | Serum | Targeted/80/ 11 (C0 & ACs) | LC-MS, FIA-MS | [17] |
| Dodecanoylcarnitine (C12) Tetradecenoylcarnitine(C14:1) Tetradecadienylcarnitine (C14:2) Hexadecanoylcarnitine (C16) Octadecenoylcarnitine(C18:1) Octadecadienylcarnitine (C18:2) | ↓ ↓ ↓ ↓ ↓ ↓ | ||||||
| NPDR vs. NDR | L-carnitine (C0) Tetradecenoylcarnitine(C14:1) Hexadecanoylcarnitine (C16) | ↓ ↓ ↓ | |||||
| Propionylcarnitine (C3) Butyrylcarnitine (C4) | ↑ ↑ | ||||||
| PDR vs. NDR | Valerycarnitine (C5) Pimelycarnitine (C7 DC) | ↓ ↓ | |||||
| Tetradecenoylcarnitine(C14:1) Hexadecanoylcarnitine (C16) Octadecanoylcarnitine (C18) Octadecenoylcarnitine(C18:1) Octadecadienylcarnitine(C18:2) | ↓ ↓ ↓ ↓ ↓ | ||||||
| PDR and NPDR vs. NDR | Tetradecenoylcarnitine (C14:1) Hexadecanoylcarnitine (C16) | ↓ ↓ | |||||
| PDR vs. controls | PDR patients: 20 Controls: 31***** | Octanoylcarnitine (C8) Decanoylcarnitine (C10) | ↑ ↑ | Vitreous humor | Targeted/17/ 4 (C0 & 3ACs) | LC-MS/MS | [18] |
| ROP | ROP: 40 Controls: 41****** | Malonylcarnitine (C3DC) | ↑ | Blood | Targeted/10/ 1 (ACs) | UPLC-MS | [19] |
| POAG | POAG: 72 Controls: 72 | Palmitoylcarnitine (C16) | ↑ | Plasma | Untargeted | LC-MS | [20] |
| POAG | POAG: 36 Controls: 27******* | Octadecenoylcarnitine (C18:1) Propionylcarnitine (C3) Butyrylcarnitine (C4) Decanoylcarnitine (C10:1) Dodecanoylcarnitine (C12:1) Octadecadienylcarnitine (C18:2) | ↓ ↑ ↑ ↑ ↑ ↑ | Plasma | Targeted/151/ 14 (C0 & 13ACs) | FIA-MS | [21] |
| POAG | POAG: 26 Controls: 26******* | L-carnitine (C0) Acetylcarnitine (C2) Propionylcarnitine (C3) Butyrylcarnitine (C4) | ↑ ↑ ↑ ↑ | Aqueous humor | Targeted/54/ 4 (C0 & 3ACs) | FIA-MS/MS | [22] |
| POAG | POAG: 8 Controls: 16 | L-carnitine (C0) Acetylcarnitine (C2) Propionylcarnitine (C3) Malonylcarnitine (C3-DC (C4-OH)) Butyrylcarnitine (C4) Butenoylcarnitine (C4:1) Valerylcarnitine (C5) Glutarylcarnine (C5-DC) Hydroxyhexanoylcarnitine (C6-OH) Hydroxypentanoylcarnitine (C5-OH) Malonylcarnitine (C3-DC-M) Pentenoylcarnitine (C5:1) Dodecanoylcarnitine (C12:1) Decatranoylcarnitine (C14:2-OH) Decanoylcarnitine (C10) | ↑ ↑ ↑ ↑ ↑ - ↑ ↑ ↑ ↑ ↑ ↑ ↑ - ↓ | Aqueous humor | Targeted/ 80/13(ACs) | [23] | |
| POAG | POAG:16 Controls: 17 | Acetylcarnitine (C2) | ↓ | Tears | Targeted/57/ 36 (C0 & 35As) | UPLC-MS/MS | [24] |
| RRD vs. RRD and PVR RRD or RRD and PVR vs. controls | RRD: 8 PVR: 9 Controls: 6 | L-carnitine (C0) | ↓ | Vitreous humor | Targeted/31/ 1 (C0) | LC-Q-TOF-MS | [25] |
| CRVO vs. controls | CRVO: 15 (15 eyes) Controls: 20******* | L-carnitine (C0) Butyrylcarnitine (C4) Deoxycarnitine | ↑ ↑ ↑ | Aqueous humor | Untargeted | UHPLC-MS/MS HILIC | [26] |
| DES vs. controls | DES: 10 Controls: 10 | Carnitine (C0) Acetylcarnitine (C2) Propionylcarnitine (C3) | ↓ ↓ ↓ | Tears | Targeted/3/ 3 (C0 & 2As) | HPLC-MS | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoridis, K.; Gika, H.; Kotali, A. Acylcarnitines in Ophthalmology: Promising Emerging Biomarkers. Int. J. Mol. Sci. 2022, 23, 16183. https://doi.org/10.3390/ijms232416183
Theodoridis K, Gika H, Kotali A. Acylcarnitines in Ophthalmology: Promising Emerging Biomarkers. International Journal of Molecular Sciences. 2022; 23(24):16183. https://doi.org/10.3390/ijms232416183
Chicago/Turabian StyleTheodoridis, Konstantinos, Helen Gika, and Antigoni Kotali. 2022. "Acylcarnitines in Ophthalmology: Promising Emerging Biomarkers" International Journal of Molecular Sciences 23, no. 24: 16183. https://doi.org/10.3390/ijms232416183





