Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease
Abstract
1. Background
2. Inflammation and Immune Dysfunction in PD: A Cause or a Consequence?
2.1. Microglial Implication in PD
2.2. PD-Related Astrocytic Inflammation
2.3. Endothelial Inflammation Impact on PD
2.4. Peripheral Inflammation and PD
3. Nuclear Receptor Related-1 and Neuroinflammation Associated with Parkinson’s Disease
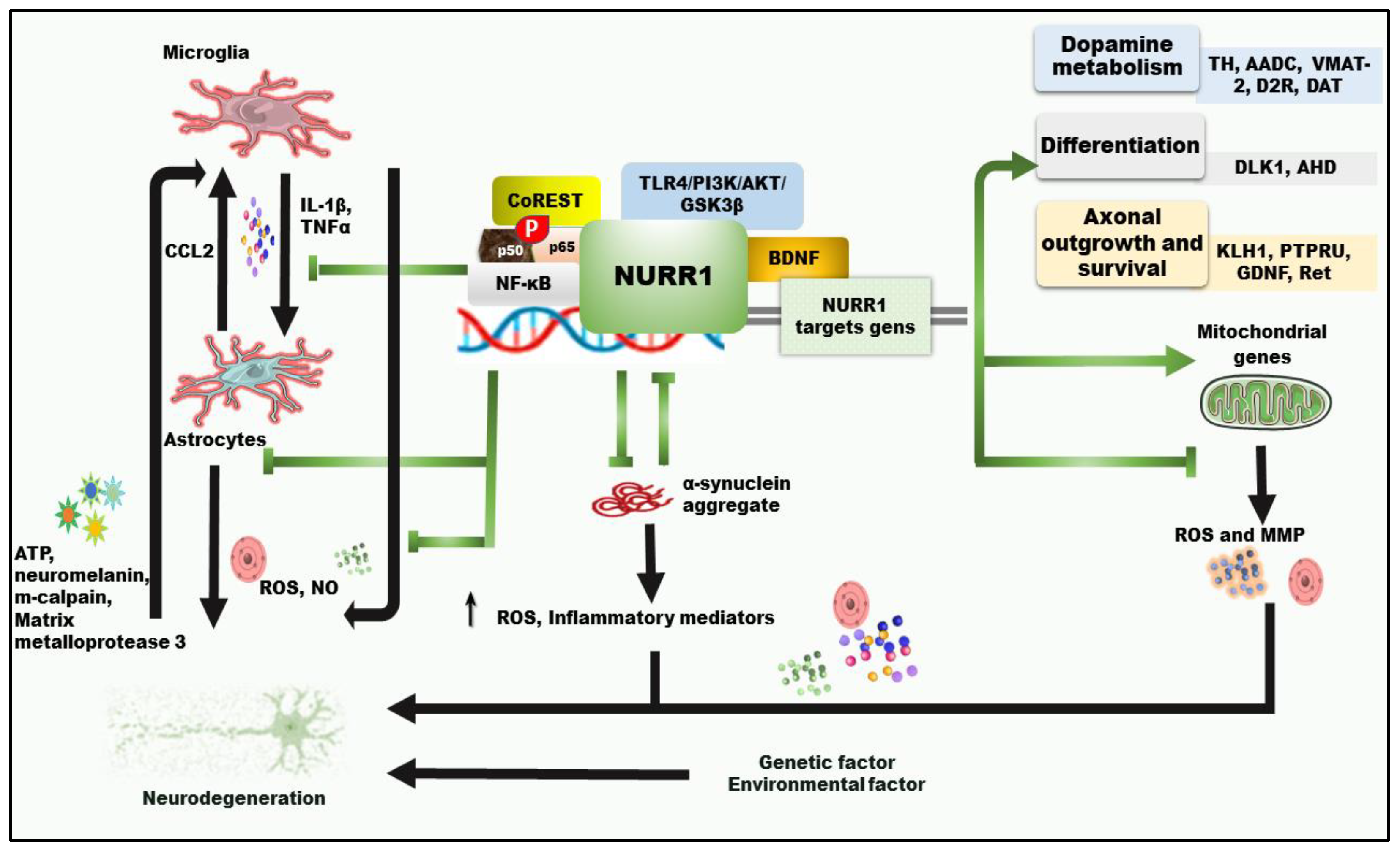
3.1. Functions of Nuclear Receptor Related-1 in Dopaminergic Neurons and PD
3.2. Involvement of Nuclear Receptor-Related Factor 1 in PD Neuroprotection and Neuroinflammation
3.2.1. The Role of NURR1 in α-Synuclein-Mediated Inflammation
3.2.2. The Impact of NURR1 in Neuroinflammation Caused by Mitochondrial Dysfunction and Oxidative Stress
3.2.3. Pyroptosis in PD and NURR1 Potential Impact
3.2.4. Alterations of NURR1 and Cytokines in PD
4. NURR1 as a Potential Neuroprotective and Anti-Inflammatory Target Therapy
| NURR1-Activating Compounds with Potential Neuroprotective and Anti-Inflammatory Target Therapy | ||||
|---|---|---|---|---|
| Compounds | Models | Methods | Main Outcomes | Ref. |
| AQ/CQ/HCQ | In vivo | AQ in 6-OHDA- lesioned rats | Interacts with LBD to modulate NURR1 transcriptional function and induces Nurr1 to suppress pro-inflammatory cytokine gene expression in microglia | [191] |
| In vitro | CQ in T cells | Promote Nurr1’s transcriptional activity by binding to LBD and up-regulating the expression of Nurr1, activating TREG cell differentiation. | [192] | |
| In vivo | HCQ in rat rotenone model | NURR1 expression is increased, NF-κB and pro-inflammatory cytokines (TNF-α, IL-1β) are inhibited, and GSK-3β activity is reduced. | [193] | |
| 1,1-bis(3′-indolyl)-1-(p-chlorophenyl) methane | In vitro | BV-2 reactive microglia (using LPS) | Enhance binding of NURR1 to the P65-binding site, reduce binding of P65 to inflammatory gene promoters, inhibit NF-κB-dependent gene expression | [194] |
| Daphnane-type and phorbol-type diterpenes | In vitro | BV-2 microglia cells | Activate Nurr1 and inhibit LPS-induced nitric oxide production | [195] |
| Isoxazolo-pyridinone analog | In vivo | Administered in C57BL/6 lactacystin-lesioned mice | Increase expression of Nurr1 and inhibit reactive microglia | [183,196] |
| SA00025 | In vivo | Administered in 6-OHDA rats primed with the toll-like receptor 3 double-stranded RNA inflammatory stimulant | Decrease the reactive microglia and IL-6. | [138] |
| Cilostazol | In vivo | Orally administered daily in rotenone rats | Hamper the NF-κB and TNFα, IL-1β, up-regulate Nurr1 and inhibit GSK-3β. | [197] |
| Nurr1 Modulators and Neuroprotective and Anti-Inflammatory Role | ||||
| Compounds | Models | Methods | Main Outcomes | Ref. |
| Memantine | In vitro | Administered in PC12 cells induced by 6-OHDA | Up-regulate NURR1 and eliminate the IL-6 and TNF-α. | [155] |
| Pramipexole | In vivo | Administered in 6-OHDA rats | Increases Nurr1 expression and impedes the elevated expression of NF-κB and α-synuclein. | [198] |
| NURR1/RXR agonist HX600 | In vitro | Reactive microglia, then exposed to LPS | Reduce the expression of nitric oxide synthase 2, macrophage receptor with collagenous structure, IL-1β, IL-6, and matrix metalloproteinase-9 and prevent inflammation-induced neuronal death | [160] |
| RXR agonist (IRX4204) | In vivo | Administered in 6-OHDA rats | Promote the survival of dopaminergic neurons in the SNc by activating cellular RXR-Nurr1 signaling | [199] |
| Retinoic acid-loaded polymeric nanoparticles | In vivo | Administered in MPTP mouse | Increase the expression levels of Nurr1 and Pitx3 | [200] |
| Bupleurum falcatum, Paeonia suffruticosa, and Angelica dahurica | In vivo and vitro | Administered in MPTP-induced mice and PC12 cells | Increase NURR1 expression in the SNc and protect the dopaminergic neurons. | [141] |
| Ginkgo biloba extract (EGb761) | In vivo | Administered in MPTP-induced mice | Regulate the expression of Nurr1 | [201] |
| Radix astragali ingredients (astragalus polysaccharide astraisoflavan) | In vitro | Administration in rat NSCs | Promote the expressions of sonic hedgehog, Nurr1, and Pitx3 mRNAs, and the proliferation of NSCs and induce NSCs differentiation toward dopaminergic neurons | [202] |
| Neuroprotective and anti-inflammatory role of NURR1 in genetic engineering and stem cell therapy form | ||||
| Genes | Models | Methods | Main Findings | Ref. |
| Nurr1 | In vitro | Overexpression in LPS-induced reactive microglia | Reduce the expression of pro-inflammatory cytokines (TNF-α, ILβ) | [189] |
| Overexpression in rat NSCs | Promote dopaminergic neuronal differentiation | [203] | ||
| Overexpression in mouse OBSCs | Generate mature-like mesencephalic dopaminergic neurons and a subpopulation of dopaminergic-gamma-aminobutyric acid neurons under long-term culture conditions. | [204] | ||
| Nurr1 + NRBE | In vitro | P19 embryonal carcinoma stem cells transfected by Nurr1, then exposed to NRBE | Induce P19 stem cell differentiation into dopaminergic neurons | [205] |
| Nurr1 + Pitx3 | In vitro | Overexpression in mouse iPSCs | Program iPSCs into functional dopaminergic-like neurons. | [206] |
| Combined transduction of Nurr1 and Pitx3 in murine and human embryonic stem cell cultures. | Synergistically promote terminal maturation to the midbrain dopaminergic neuron phenotype | [204,207] | ||
| NURR1 + BRN4 | In vivo | Co-transfect NSCs with Nurr1 and Brn4, then transplant into 6-OHDA rats | Increase the viability and maturity of dopaminergic neurons as well as the DA levels | [208] |
| Nurr1 + Ngn2 | In vitro | Co-transduced mouse embryonic OBSCs | Reduce the proportion of TH-positive neurons | [204] |
| Overexpression in the mouse midbrain progenitors | Increased production of dopaminergic neurons from midbrain progenitor cells | [209] | ||
| Co-expression in rat and mouse NSCs | Repress Nurr1-induced generation of TH+ cells in rat cultures, enhance Nurr1-induced dopaminergic cell yields in mouse NPCs | [210] | ||
| Nurr1 + Ascl1 | In vitro | Co-transduction and treatment by BDNF and neurotrophin-3 | Increased Nurr1-induced production of dopaminergic neurons | [203] |
| Nurr1 + Foxa2 | In vitro | Co-transduction in mouse NPCs | Induce the generation and differentiation of dopaminergic neurons, and their survival and resistance to toxic insult | [211] |
| In vivo | Co-transduction in 6-OHDA rats | |||
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Goldman, S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1996, 14, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.-B. Clinicopathological correlates of lewy body disease: Fundamental issues. J. Mov. Disord. 2010, 3, 11–14. [Google Scholar] [CrossRef]
- Cannon, J.R.; Greenamyre, J.T. Gene-environment interactions in Parkinson’s disease: Specific evidence in humans and mammalian models. Neurobiol. Dis. 2013, 57, 38–46. [Google Scholar] [CrossRef]
- Al-Nusaif, M.; Yang, Y.; Li, S.; Cheng, C.; Le, W. The role of NURR1 in metabolic abnormalities of Parkinson’s disease. Mol. Neurodegener. 2022, 17, 46. [Google Scholar] [CrossRef]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef]
- Thundyil, J.; Lim, K.L. DAMPs and neurodegeneration. Ageing Res. Rev. 2015, 24, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.I.; Robertson, S.A.; O’Keefe, L.V.; Fornarino, D.; Scott, A.; Lardelli, M.; Baune, B.T. The Enemy within: Innate Surveillance-Mediated Cell Death, the Common Mechanism of Neurodegenerative Disease. Front. Neurosci. 2016, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Zhao, M.; Zhang, Z.; Cui, L.-Y.; Zhou, X.; Zhang, W.; Chu, S.-F.; Zhang, D.-Y.; Chen, N.-H. Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization. Acta Pharmacol. Sin. 2020, 41, 523–534. [Google Scholar] [CrossRef]
- Orr, C.F.; Rowe, D.B.; Mizuno, Y.; Mori, H.; Halliday, G.M. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain 2005, 128, 2665–2674. [Google Scholar] [CrossRef]
- Hartmann, A. Postmortem studies in Parkinson’s disease. Dialogues Clin. Neurosci. 2004, 6, 281–293. [Google Scholar] [CrossRef]
- Tiwari, P.C.; Pal, R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin. Neurosci. 2017, 19, 71–80. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Carvey, P.M.; Hendey, B.; Monahan, A.J. The blood-brain barrier in neurodegenerative disease: A rhetorical perspective. J. Neurochem. 2009, 111, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Magistrelli, L.; Contaldi, E.; Vignaroli, F.; Gallo, S.; Colombatto, F.; Cantello, R.; Comi, C. Immune Response Modifications in the Genetic Forms of Parkinson’s Disease: What Do We Know? Int. J. Mol. Sci. 2022, 23, 3476. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Roth, T.L.; McGavern, D.B. Microglia development and function. Annu. Rev. Immunol. 2014, 32, 367–402. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Imamura, K.; Hishikawa, N.; Sawada, M.; Nagatsu, T.; Yoshida, M.; Hashizume, Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003, 106, 518–526. [Google Scholar] [CrossRef]
- He, M.; Dong, H.; Huang, Y.; Lu, S.; Zhang, S.; Qian, Y.; Jin, W. Astrocyte-Derived CCL2 is Associated with M1 Activation and Recruitment of Cultured Microglial Cells. Cell. Physiol. Biochem. 2016, 38, 859–870. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Salvi, V.; Sozio, F.; Sozzani, S.; Del Prete, A. Role of Atypical Chemokine Receptors in Microglial Activation and Polarization. Front. Aging Neurosci. 2017, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yao, H.-H.; Wu, J.-Y.; Ding, J.-H.; Sun, T.; Hu, G. Opening of microglial KATP channels inhibits rotenone-induced neuroinflammation. J. Cell. Mol. Med. 2008, 12, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-H.; Sun, H.-B.; Hu, Z.-L.; Lu, M.; Ding, J.-H.; Hu, G. Kir6.1/K-ATP channel modulates microglia phenotypes: Implication in Parkinson’s disease. Cell Death Dis. 2018, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Du, R.-H.; Lu, M.; Wang, C.; Ding, J.-H.; Wu, G.; Hu, G. The pore-forming subunit Kir6.1 of the K-ATP channel negatively regulates the NLRP3 inflammasome to control insulin resistance by interacting with NLRP3. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Scheiblich, H.; Dansokho, C.; Mercan, D.; Schmidt, S.V.; Bousset, L.; Wischhof, L.; Eikens, F.; Odainic, A.; Spitzer, J.; Griep, A.; et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 2021, 184, 5089–5106.e21. [Google Scholar] [CrossRef]
- Saijo, K.; Winner, B.; Carson, C.T.; Collier, J.G.; Boyer, L.; Rosenfeld, M.G.; Gage, F.H.; Glass, C.K. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 2009, 137, 47–59. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Leal, M.C.; Casabona, J.C.; Puntel, M.; Pitossi, F.J. Interleukin-1β and tumor necrosis factor-α: Reliable targets for protective therapies in Parkinson’s Disease? Front. Cell. Neurosci. 2013, 7, 53. [Google Scholar] [CrossRef]
- Lau, L.T.; Yu, A.C.-H. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma 2001, 18, 351–359. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.-I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.-S.; Kwon, S.-H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-L.; Long, C.-X.; Sun, L.; Xie, C.; Lin, X.; Cai, H. Astrocytic expression of Parkinson’s disease-related A53T α-synuclein causes neurodegeneration in mice. Mol. Brain 2010, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Morgado, J.; Gomes, F.C.A. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 2019, 11, 59. [Google Scholar] [CrossRef]
- Wu, F.; Liu, L.; Zhou, H. Endothelial cell activation in central nervous system inflammation. J. Leukoc. Biol. 2017, 101, 1119–1132. [Google Scholar] [CrossRef]
- Reyes, T.M.; Fabry, Z.; Coe, C.L. Brain endothelial cell production of a neuroprotective cytokine, interleukin-6, in response to noxious stimuli. Brain Res. 1999, 851, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Nagyőszi, P.; Wilhelm, I.; Farkas, A.E.; Fazakas, C.; Dung, N.T.-K.; Haskó, J.; Krizbai, I.A. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem. Int. 2010, 57, 556–564. [Google Scholar] [CrossRef]
- Guan, J.; Pavlovic, D.; Dalkie, N.; Waldvogel, H.J.; O’Carroll, S.J.; Green, C.R.; Nicholson, L.F. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013, 23, 154–164. [Google Scholar] [CrossRef]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood-Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef]
- Raasch, M.; Rennert, K.; Jahn, T.; Gärtner, C.; Schönfelder, G.; Huber, O.; Seiler, A.E.M.; Mosig, A.S. An integrative microfluidically supported in vitro model of an endothelial barrier combined with cortical spheroids simulates effects of neuroinflammation in neocortex development. Biomicrofluidics 2016, 10, 044102. [Google Scholar] [CrossRef]
- Perner, C.; Perner, F.; Gaur, N.; Zimmermann, S.; Witte, O.W.; Heidel, F.H.; Grosskreutz, J.; Prell, T. Plasma VCAM1 levels correlate with disease severity in Parkinson’s disease. J. Neuroinflammation 2019, 16, 94. [Google Scholar] [CrossRef]
- Contaldi, E.; Magistrelli, L.; Comi, C. T Lymphocytes in Parkinson’s Disease. J. Park. Dis. 2022, 12, S65–S74. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Benner, E.J.; Banerjee, R.; Reynolds, A.D.; Sherman, S.; Pisarev, V.M.; Tsiperson, V.; Nemachek, C.; Ciborowski, P.; Przedborski, S.; Mosley, R.L.; et al. Nitrated α-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE 2008, 3, e1376. [Google Scholar] [CrossRef]
- Cebrián, C.; Zucca, F.A.; Mauri, P.; Steinbeck, J.A.; Studer, L.; Scherzer, C.R.; Kanter, E.; Budhu, S.; Mandelbaum, J.; Vonsattel, J.P.; et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat. Commun. 2014, 5, 3633. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, M.S.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, M.A.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Bernardini, N.; Blandizzi, C.; Fornai, M. Enteric Glia at the Crossroads between Intestinal Immune System and Epithelial Barrier: Implications for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 9199. [Google Scholar] [CrossRef] [PubMed]
- Banati, R.B.; Daniel, S.E.; Blunt, S.B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov. Disord. 1998, 13, 221–227. [Google Scholar] [CrossRef]
- Nagatsu, T.; Mogi, M.; Ichinose, H.; Togari, A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural. Transm. Suppl. 2000, 277–290. [Google Scholar] [CrossRef]
- Knott, C.; Stern, G.; Wilkin, G.P. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 2000, 16, 724–739. [Google Scholar] [CrossRef]
- Shimoji, M.; Pagan, F.; Healton, E.B.; Mocchetti, I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotox. Res. 2009, 16, 318–328. [Google Scholar] [CrossRef]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E.C. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Doorn, K.J.; Moors, T.; Drukarch, B.; van de Berg, W.D.J.; Lucassen, P.J.; van Dam, A.-M. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol. Commun. 2014, 2, 90. [Google Scholar] [CrossRef] [PubMed]
- Brochard, V.; Combadière, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.-M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Earls, R.H.; Menees, K.B.; Chung, J.; Barber, J.; Gutekunst, C.-A.; Hazim, M.G.; Lee, J.-K. Intrastriatal injection of preformed alpha-synuclein fibrils alters central and peripheral immune cell profiles in non-transgenic mice. J. Neuroinflam. 2019, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gil, P.; Rodriguez-Perez, A.I.; Dominguez-Meijide, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Bidirectional Neural Interaction Between Central Dopaminergic and Gut Lesions in Parkinson’s Disease Models. Mol. Neurobiol. 2018, 55, 7297–7316. [Google Scholar] [CrossRef] [PubMed]
- Sznejder-Pacholek, A.; Joniec-Maciejak, I.; Wawer, A.; Ciesielska, A.; Mirowska-Guzel, D. The effect of α-synuclein on gliosis and IL-1α, TNFα, IFNγ, TGFβ expression in murine brain. Pharmacol. Rep. 2017, 69, 242–251. [Google Scholar] [CrossRef]
- Godoy, M.C.P.; Tarelli, R.; Ferrari, C.C.; Sarchi, M.I.; Pitossi, F.J. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain 2008, 131, 1880–1894. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Benner, E.J.; Mosley, R.L.; Destache, C.J.; Lewis, T.B.; Jackson-Lewis, V.; Gorantla, S.; Nemachek, C.; Green, S.R.; Przedborski, S.; Gendelman, H.E. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 9435–9440. [Google Scholar] [CrossRef]
- Reynolds, A.D.; Banerjee, R.; Liu, J.; Gendelman, H.E.; Mosley, R.L. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J. Leukoc. Biol. 2007, 82, 1083–1094. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, F.; Brownell, A.L.; Williams, K.; Chen, Y.I.; Livni, E.; Isacson, O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur. J. Neurosci. 2002, 15, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Na, S.J.; DiLella, A.G.; Lis, E.V.; Jones, K.; Levine, D.M.; Stone, D.J.; Hess, J.F. Molecular profiling of a 6-hydroxydopamine model of Parkinson’s disease. Neurochem. Res. 2010, 35, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Armentero, M.-T.; Levandis, G.; Bazzini, E.; Cerri, S.; Ghezzi, C.; Blandini, F. Adhesion molecules as potential targets for neuroprotection in a rodent model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 663–668. [Google Scholar] [CrossRef]
- McGeer, P.L.; Schwab, C.; Parent, A.; Doudet, D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol. 2003, 54, 599–604. [Google Scholar] [CrossRef]
- Barcia, C.; Bahillo, A.S.; Fernández-Villalba, E.; Bautista, V.; Poza, M.P.Y.; Fernández-Barreiro, A.; Hirsch, E.C.; Herrero, M.-T. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia 2004, 46, 402–409. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, W.; Wei, H.; Dean, M.N.; Standaert, D.G.; Cutter, G.R.; Benveniste, E.N.; Qin, H. Dysregulation of the Adaptive Immune System in Patients With Early-Stage Parkinson Disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1036. [Google Scholar] [CrossRef]
- Wang, P.; Yao, L.; Luo, M.; Zhou, W.; Jin, X.; Xu, Z.; Yan, S.; Li, Y.; Xu, C.; Cheng, R.; et al. Single-cell transcriptome and TCR profiling reveal activated and expanded T cell populations in Parkinson’s disease. Cell Discov. 2021, 7, 52. [Google Scholar] [CrossRef]
- Brodacki, B.; Staszewski, J.; Toczylowska, B.; Kozlowska, E.; Drela, N.; Chalimoniuk, M.; Stępien, A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFα, and INFγ concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci. Lett. 2008, 441, 158–162. [Google Scholar] [CrossRef]
- Baba, Y.; Kuroiwa, A.; Uitti, R.J.; Wszolek, Z.K.; Yamada, T. Alterations of T-lymphocyte populations in Parkinson disease. Park. Relat. Disord. 2005, 11, 493–498. [Google Scholar] [CrossRef]
- Li, T.; Yang, Z.; Li, S.; Cheng, C.; Shen, B.; Le, W. Alterations of NURR1 and Cytokines in the Peripheral Blood Mononuclear Cells: Combined Biomarkers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gao, H.; Luo, Q.; Wang, P.; Yang, X. The correlation of lymphocyte subsets, natural killer cell, and Parkinson’s disease: A meta-analysis. Neurol. Sci. 2017, 38, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.A.; Kannarkat, G.T.; Cintron, A.F.; Butkovich, L.M.; Fraser, K.B.; Chang, J.; Grigoryan, N.; Factor, S.A.; West, A.B.; Boss, J.M.; et al. LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Park. Dis. 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Spiegel, J.; Dillmann, U.; Grundmann, D.; Bürmann, J.; Faßbender, K.; Schäfer, K.-H.; Unger, M.M. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Park. Relat. Disord. 2018, 50, 104–107. [Google Scholar] [CrossRef]
- Reale, M.; Iarlori, C.; Thomas, A.; Gambi, D.; Perfetti, B.; Di Nicola, M.; Onofrj, M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav. Immun. 2009, 23, 55–63. [Google Scholar] [CrossRef]
- Pan-Montojo, F.; Anichtchik, O.; Dening, Y.; Knels, L.; Pursche, S.; Jung, R.; Jackson, S.; Gille, G.; Spillantini, M.G.; Reichmann, H.; et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 2010, 5, e8762. [Google Scholar] [CrossRef]
- Ahmed, S.; Kwatra, M.; Panda, S.R.; Murty, U.S.N.; Naidu, V.G.M. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav. Immun. 2021, 91, 142–158. [Google Scholar] [CrossRef]
- Han, X.; Sun, S.; Sun, Y.; Song, Q.; Zhu, J.; Song, N.; Chen, M.; Sun, T.; Xia, M.; Ding, J.; et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: Implications for Parkinson disease. Autophagy 2019, 15, 1860–1881. [Google Scholar] [CrossRef]
- Sherer, T.B.; Kim, J.-H.; Betarbet, R.; Greenamyre, J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Chao, Y.; Wong, S.C.; Tan, E.K. Evidence of inflammatory system involvement in Parkinson’s disease. BioMed Res. Int. 2014, 2014, 308654. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, X.; Wang, Y.; Cai, H.; Le, W. Nurr1Cd11bcre conditional knockout mice display inflammatory injury to nigrostriatal dopaminergic neurons. Glia 2020, 68, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999, 97, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaei, B.; Ito, T.; Joodmardi, E.; Mattsson, B.; Rouillard, C.; Carta, M.; Muramatsu, S.; Sumi-Ichinose, C.; Nomura, T.; Metzger, D.; et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J. Neurosci. 2009, 29, 15923–15932. [Google Scholar] [CrossRef]
- Alavian, K.N.; Jeddi, S.; Naghipour, S.I.; Nabili, P.; Licznerski, P.; Tierney, T.S. The lifelong maintenance of mesencephalic dopaminergic neurons by Nurr1 and engrailed. J. Biomed. Sci. 2014, 21, 27. [Google Scholar] [CrossRef]
- Jankovic, J.; Chen, S.; Le, W.D. The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog. Neurobiol. 2005, 77, 128–138. [Google Scholar] [CrossRef]
- Bäckman, C.; Perlmann, T.; Wallén, Å.; Hoffer, B.J.; Morales, M. A selective group of dopaminergic neurons express Nurr1 in the adult mouse brain. Brain Res. 1999, 851, 125–132. [Google Scholar] [CrossRef]
- Fan, X.; Luo, G.; Ming, M.; Pu, P.; Li, L.; Yang, D.; Le, W. Nurr1 expression and its modulation in microglia. Neuroimmunomodulation 2009, 16, 162–170. [Google Scholar] [CrossRef]
- Lallier, S.W.; Graf, A.E.; Waidyarante, G.R.; Rogers, L.K. Nurr1 expression is modified by inflammation in microglia. Neuroreport 2016, 27, 1120–1127. [Google Scholar] [CrossRef]
- Moon, M.; Jeong, I.; Kim, C.-H.; Kim, J.; Lee, P.K.J.; Mook-Jung, I.; Leblanc, P.; Kim, K.-S. Correlation between orphan nuclear receptor Nurr1 expression and amyloid deposition in 5XFAD mice, an animal model of Alzheimer’s disease. J. Neurochem. 2015, 132, 254–262. [Google Scholar] [CrossRef]
- Montarolo, F.; Raffaele, C.; Perga, S.; Martire, S.; Finardi, A.; Furlan, R.; Hintermann, S.; Bertolotto, A. Effects of isoxazolo-pyridinone 7e, a potent activator of the Nurr1 signaling pathway, on experimental autoimmune encephalomyelitis in mice. PLoS ONE 2014, 9, e108791. [Google Scholar] [CrossRef] [PubMed]
- Buervenich, S.; Carmine, A.; Arvidsson, M.; Xiang, F.; Zhang, Z.; Sydow, O.; Jönsson, E.G.; Sedvall, G.C.; Leonard, S.; Ross, R.G.; et al. NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am. J. Med. Genet. 2000, 96, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.R.; Harding, C.J.; Raines, S.; Tolley, K.; Parker, A.E.; Downey-Jones, M.; Needham, M.R. Nurr1 dependent regulation of pro-inflammatory mediators in immortalised synovial fibroblasts. J. Inflamm. 2005, 2, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perlmann, T.; Wallén-Mackenzie, A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004, 318, 45–52. [Google Scholar] [CrossRef]
- Le, W.-D.; Xu, P.; Jankovic, J.; Jiang, H.; Appel, S.H.; Smith, R.G.; Vassilatis, D.K. Mutations in NR4A2 associated with familial Parkinson disease. Nat. Genet. 2003, 33, 85–89. [Google Scholar] [CrossRef]
- Chu, Y.; Kompoliti, K.; Cochran, E.J.; Mufson, E.J.; Kordower, J.H. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J. Comp. Neurol. 2002, 450, 203–214. [Google Scholar] [CrossRef]
- Decressac, M.; Volakakis, N.; Björklund, A.; Perlmann, T. NURR1 in Parkinson disease—From pathogenesis to therapeutic potential. Nat. Rev. Neurol. 2013, 9, 629–636. [Google Scholar] [CrossRef]
- Liu, H.; Wei, L.; Tao, Q.; Deng, H.; Ming, M.; Xu, P.; Le, W. Decreased NURR1 and PITX3 gene expression in Chinese patients with Parkinson’s disease. Eur. J. Neurol. 2012, 19, 870–875. [Google Scholar] [CrossRef]
- Saucedo-Cardenas, O.; Quintana-Hau, J.D.; Le, W.-D.; Smidt, M.P.; Cox, J.J.; De Mayo, F.; Burbach, J.P.H.; Conneely, O.M. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 4013–4018. [Google Scholar] [CrossRef]
- Zetterström, R.H.; Solomin, L.; Jansson, L.; Hoffer, B.J.; Olson, L.; Perlmann, T. Dopamine neuron agenesis in Nurr1-deficient mice. Science 1997, 276, 248–250. [Google Scholar] [CrossRef]
- Le, W.; Conneely, O.M.; He, Y.; Jankovic, J.; Appel, S.H. Reduced Nurr1 expression increases the vulnerability of mesencephalic dopamine neurons to MPTP-induced injury. J. Neurochem. 1999, 73, 2218–2221. [Google Scholar] [PubMed]
- Kadkhodaei, B.; Alvarsson, A.; Schintu, N.; Ramsköld, D.; Volakakis, N.; Joodmardi, E.; Yoshitake, T.; Kehr, J.; Decressac, M.; Björklund, A.; et al. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, F.M.J.; van der Linden, A.J.A.; Wang, Y.; von Oerthel, L.; Sul, H.S.; Burbach, J.P.H.; Smidt, M.P. Identification of Dlk1, Ptpru and Klhl1 as novel Nurr1 target genes in meso-diencephalic dopamine neurons. Development 2009, 136, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; McKinney, C.; Lee, M.K.; Eells, J.B.; Phyillaier, M.A.; Nikodem, V.M. Regulation of GTP cyclohydrolase I expression by orphan receptor Nurr1 in cell culture and in vivo. J. Neurochem. 2007, 101, 142–150. [Google Scholar] [CrossRef]
- Luo, Y.; Henricksen, L.A.; Giuliano, R.E.; Prifti, L.; Callahan, L.M.; Federoff, H.J. VIP is a transcriptional target of Nurr1 in dopaminergic cells. Exp. Neurol. 2007, 203, 221–232. [Google Scholar] [CrossRef]
- Joksimovic, M.; Yun, B.A.; Kittappa, R.; Anderegg, A.M.; Chang, W.W.; Taketo, M.M.; McKay, R.D.G.; Awatramani, R.B. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat. Neurosci. 2009, 12, 125–131. [Google Scholar] [CrossRef]
- Barish, G.D.; Downes, M.; Alaynick, W.A.; Yu, R.T.; Ocampo, C.B.; Bookout, A.L.; Mangelsdorf, D.J.; Evans, R.M. A Nuclear Receptor Atlas: Macrophage activation. Mol. Endocrinol. 2005, 19, 2466–2477. [Google Scholar] [CrossRef]
- Pei, L.; Castrillo, A.; Chen, M.; Hoffmann, A.; Tontonoz, P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J. Biol. Chem. 2005, 280, 29256–29262. [Google Scholar] [CrossRef]
- Long-Smith, C.M.; Collins, L.; Toulouse, A.; Sullivan, A.M.; Nolan, Y.M. Interleukin-1β contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. J. Neuroimmunol. 2010, 226, 20–26. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Godoy, M.C.P.; Tarelli, R.; Chertoff, M.; Depino, A.M.; Pitossi, F.J. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1β in the substantia nigra. Neurobiol. Dis. 2006, 24, 183–193. [Google Scholar] [CrossRef]
- Yang, Y.X.; Latchman, D.S. Nurr1 transcriptionally regulates the expression of α-synuclein. Neuroreport 2008, 19, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Qi, H.; Cheng, C.; Wu, X.; Yang, Z.; Cai, H.; Chen, S.; Le, W. α-Synuclein Negatively Regulates Nurr1 Expression Through NF-κB-Related Mechanism. Front. Mol. Neurosci. 2020, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Parisiadou, L.; Sgobio, C.; Liu, G.; Yu, J.; Sun, L.; Shim, H.; Gu, X.-L.; Luo, J.; Long, C.-X.; et al. Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J. Neurosci. 2012, 32, 9248–9264. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Jang, Y.; Kim, C.-H.; Kim, W.; Toh, H.T.; Jeon, J.; Song, B.; Serra, A.; Lescar, J.; Yoo, J.Y.; et al. PGE1 and PGA1 bind to Nurr1 and activate its transcriptional function. Nat. Chem. Biol. 2020, 16, 876–886. [Google Scholar] [CrossRef]
- Rajan, S.; Toh, H.T.; Ye, H.; Wang, Z.; Basil, A.H.; Parnaik, T.; Yoo, J.Y.; Lim, K.-L.; Yoon, H.S. Prostaglandin A2 Interacts with Nurr1 and Ameliorates Behavioral Deficits in Parkinson’s Disease Fly Model. Neuromolecular Med. 2022, 24, 469–478. [Google Scholar] [CrossRef]
- Ji, R.; Sanchez, C.M.; Chou, C.L.; Chen, X.B.; Woodward, D.F.; Regan, J.W. Prostanoid EP1 receptors mediate up-regulation of the orphan nuclear receptor Nurr1 by cAMP-independent activation of protein kinase A, CREB and NF-κB. Br. J. Pharmacol. 2012, 166, 1033–1046. [Google Scholar] [CrossRef]
- Carrasco, E.; Casper, D.; Werner, P. PGE2 receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE2 neurotoxicity. J. Neurosci. Res. 2007, 85, 3109–3117. [Google Scholar] [CrossRef]
- Kawano, T.; Anrather, J.; Zhou, P.; Park, L.; Wang, G.; Frys, K.A.; Kunz, A.; Cho, S.; Orio, M.; Iadecola, C. Prostaglandin E2 EP1 receptors: Downstream effectors of COX-2 neurotoxicity. Nat. Med. 2006, 12, 225–229. [Google Scholar] [CrossRef]
- Lee, H.-J.; Suk, J.-E.; Patrick, C.; Bae, E.-J.; Cho, J.-H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.-J. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Shao, Q.-H.; Yan, W.-F.; Zhang, Z.; Ma, K.-L.; Peng, S.-Y.; Cao, Y.-L.; Yuan, Y.-H.; Chen, N.-H. Nurr1: A vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology 2019, 144, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Wallén, Å.; Castro, D.S.; Zetterström, R.H.; Karlén, M.; Olson, L.; Ericson, J.; Perlmann, T. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol. Cell. Neurosci. 2001, 18, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Decressac, M.; Kadkhodaei, B.; Mattsson, B.; Laguna, A.; Perlmann, T.; Björklund, A. α-Synuclein–Induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012, 4, 163ra156. [Google Scholar] [CrossRef] [PubMed]
- Volakakis, N.; Kadkhodaei, B.; Joodmardi, E.; Wallis, K.; Panman, L.; Silvaggi, J.; Spiegelman, B.M.; Perlmann, T. NR4A orphan nuclear receptors as mediators of CREB-dependent neuroprotection. Proc. Natl. Acad. Sci. USA 2010, 107, 12317–12322. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, F.; Caiazzo, M.; Greco, D.; Consales, C.; Leone, L.; Perrone-Capano, C.; D’Amato, L.C.; di Porzio, U. Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J. Neurochem. 2007, 102, 441–453. [Google Scholar] [CrossRef]
- Barneda-Zahonero, B.; Servitja, J.-M.; Badiola, N.; Miñano-Molina, A.J.; Fadó, R.; Saura, C.A.; Rodríguez-Alvarez, J. Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival*. J. Biol. Chem. 2012, 287, 11351–11362. [Google Scholar] [CrossRef]
- Baloh, R.H.; Enomoto, H.; Johnson, E.M., Jr.; Milbrandt, J. The GDNF family ligands and receptors—Implications for neural development. Curr. Opin. Neurobiol. 2000, 10, 103–110. [Google Scholar] [CrossRef]
- Smith, G.A.; Rocha, E.M.; Rooney, T.; Barnéoud, P.; McLean, J.R.; Beagan, J.; Osborn, T.; Coimbra, M.; Luo, Y.; Hallett, P.J.; et al. A Nurr1 agonist causes neuroprotection in a Parkinson’s disease lesion model primed with the toll-like receptor 3 dsRNA inflammatory stimulant poly(I:C). PLoS ONE 2015, 10, e0121072. [Google Scholar] [CrossRef]
- Zhang, T.; Jia, N.; Fei, E.; Wang, P.; Liao, Z.; Ding, L.; Yan, M.; Nukina, N.; Zhou, J.; Wang, G. Nurr1 is phosphorylated by ERK2 in vitro and its phosphorylation upregulates tyrosine hydroxylase expression in SH-SY5Y cells. Neurosci. Lett. 2007, 423, 118–122. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Liu, Y.; Zhao, H.; Zhao, S.; Yang, H. DJ-1 upregulates tyrosine hydroxylase gene expression by activating its transcriptional factor Nurr1 via the ERK1/2 pathway. Int. J. Biochem. Cell Biol. 2012, 44, 65–71. [Google Scholar] [CrossRef]
- Sim, Y.; Park, G.; Eo, H.; Huh, E.; Gu, P.S.; Hong, S.-P.; Pak, Y.K.; Oh, M.S. Protective effects of a herbal extract combination of Bupleurum falcatum, Paeonia suffruticosa, and Angelica dahurica against MPTP-induced neurotoxicity via regulation of nuclear receptor-related 1 protein. Neuroscience 2017, 340, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Kim, S.Y.; Gil, J.-E.; Byun, J.-S.; Cha, D.-W.; Ku, B.; Lee, W.; Kim, W.-K.; Oh, K.-J.; Lee, E.-W.; et al. Nurr1 performs its anti-inflammatory function by regulating RasGRP1 expression in neuro-inflammation. Sci. Rep. 2020, 10, 10755. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Kashiwagi, I.; Inoue, N.; Morita, R.; Hori, S.; Waldmann, H.; Rudensky, A.Y.; Ichinose, H.; Metzger, D.; Chambon, P.; et al. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat. Commun. 2011, 2, 269. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, J.P.; Murphy, E.P. Inflammation: A role for NR4A orphan nuclear receptors? Biochem. Soc. Trans. 2011, 39, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Kashiwagi, I.; Yoshida, R.; Fukaya, T.; Morita, R.; Kimura, A.; Ichinose, H.; Metzger, D.; Chambon, P.; Yoshimura, A. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013, 14, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Raveney, B.J.E.; Oki, S.; Yamamura, T. Nuclear receptor NR4A2 orchestrates Th17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS ONE 2013, 8, e56595. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, E.; Musich, P.R.; Lin, F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019, 25, 816–824. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Zhang, Q.; Itagaki, K.; Hauser, C.J. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 2010, 34, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Yang, F.; Zheng, Q.; Tang, W.; Li, J. The Potential Role of the NLRP3 Inflammasome Activation as a Link Between Mitochondria ROS Generation and Neuroinflammation in Postoperative Cognitive Dysfunction. Front. Cell. Neurosci. 2019, 13, 73. [Google Scholar] [CrossRef]
- van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef]
- Wei, X.; Gao, H.; Zou, J.; Liu, X.; Chen, D.; Liao, J.; Xu, Y.; Ma, L.; Tang, B.; Zhang, Z.; et al. Contra-directional Coupling of Nur77 and Nurr1 in Neurodegeneration: A Novel Mechanism for Memantine-Induced Anti-inflammation and Anti-mitochondrial Impairment. Mol. Neurobiol. 2016, 53, 5876–5892. [Google Scholar] [CrossRef]
- Carrieri, C.; Forrest, A.R.R.; Santoro, C.; Persichetti, F.; Carninci, P.; Zucchelli, S.; Gustincich, S. Expression analysis of the long non-coding RNA antisense to Uchl1 (AS Uchl1) during dopaminergic cells’ differentiation in vitro and in neurochemical models of Parkinson’s disease. Front. Cell. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Rasheed, M.; Deng, Y. The epigenetic mechanisms involved in mitochondrial dysfunction: Implication for Parkinson’s disease. Brain Pathol. 2022, 32, e13012. [Google Scholar] [CrossRef]
- Farshbaf, M.J.; Forouzanfar, M.; Ghaedi, K.; Kiani-Esfahani, A.; Peymani, M.; Nejati, A.S.; Izadi, T.; Karbalaie, K.; Noorbakhshnia, M.; Rahgozar, S.; et al. Nurr1 and PPARγ protect PC12 cells against MPP+ toxicity: Involvement of selective genes, anti-inflammatory, ROS generation, and antimitochondrial impairment. Mol. Cell. Biochem. 2016, 420, 29–42. [Google Scholar] [CrossRef]
- Volakakis, N.; Tiklova, K.; Decressac, M.; Papathanou, M.; Mattsson, B.; Gillberg, L.; Nobre, A.; Björklund, A.; Perlmann, T. Nurr1 and Retinoid X Receptor Ligands Stimulate Ret Signaling in Dopamine Neurons and Can Alleviate α-Synuclein Disrupted Gene Expression. J. Neurosci. 2015, 35, 14370–14385. [Google Scholar] [CrossRef]
- Loppi, S.; Kolosowska, N.; Kärkkäinen, O.; Korhonen, P.; Huuskonen, M.; Grubman, A.; Dhungana, H.; Wojciechowski, S.; Pomeshchik, Y.; Giordano, M.; et al. HX600, a synthetic agonist for RXR-Nurr1 heterodimer complex, prevents ischemia-induced neuronal damage. Brain Behav. Immun. 2018, 73, 670–681. [Google Scholar] [CrossRef]
- Spathis, A.D.; Asvos, X.; Ziavra, D.; Karampelas, T.; Topouzis, S.; Cournia, Z.; Qing, X.; Alexakos, P.; Smits, L.M.; Dalla, C.; et al. Nurr1:RXRα heterodimer activation as monotherapy for Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-Q.; Fang, Y.; Zheng, R.; Pu, J.-L.; Zhang, B.-R. NLRP3 Inflammasomes in Parkinson’s disease and their Regulation by Parkin. Neuroscience 2020, 446, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, Q.; Chen, G.; Li, P.A. Suppression of NLRP3 Inflammasome, Pyroptosis, and Cell Death by NIM811 in Rotenone-Exposed Cells as an in vitro Model of Parkinson’s Disease. Neurodegener. Dis. 2020, 20, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.A.; Rathinam, V.A.K.; Lee, S.-J.; Dolinay, T.; Lam, H.C.; Englert, J.A.; Rabinovitch, M.; Cernadas, M.; Kim, H.P.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Tu, Y.; Ding, D.; Yi, Q.; Sun, X.; Wang, Y.; Wang, K.; Zhu, M.; Mao, J. Dysfunctional Nurr1 promotes high glucose-induced Müller cell activation by up-regulating the NF-κB/NLRP3 inflammasome axis. Neuropeptides 2020, 82, 102057. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, R.; Liu, C.; Meng, W.; Pang, Q. Itaconate Attenuates Neuroinflammation and Exerts Dopamine Neuroprotection in Parkinson’s Disease through Inhibiting NLRP3 Inflammasome. Brain Sci. 2022, 12, 1255. [Google Scholar] [CrossRef]
- Dong, A.-Q.; Yang, Y.-P.; Jiang, S.-M.; Yao, X.-Y.; Qi, D.; Mao, C.-J.; Cheng, X.-Y.; Wang, F.; Hu, L.-F.; Liu, C.-F. Pramipexole inhibits astrocytic NLRP3 inflammasome activation via Drd3-dependent autophagy in a mouse model of Parkinson’s disease. Acta Pharmacol. Sin. 2022, 1–12. [Google Scholar] [CrossRef]
- Litteljohn, D.; Hayley, S. Cytokines as potential biomarkers for Parkinson’s disease: A multiplex approach. Methods Mol. Biol. 2012, 934, 121–144. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Narabayashi, H.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and transforming growth factor-α levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996, 211, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Sawada, M. Inflammatory process in Parkinson’s disease: Role for cytokines. Curr. Pharm. Des. 2005, 11, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.; Imamura, K.; Nagatsu, T. Role of cytokines in inflammatory process in Parkinson’s disease. J. Neural. Transm. Suppl. 2006, 70, 373–381. [Google Scholar] [CrossRef]
- Chen, X.-X.; Qian, Y.; Wang, X.-P.; Tang, Z.-W.; Xu, J.-T.; Lin, H.; Yang, Z.-Y.; Song, X.-B.; Lu, D.; Guo, J.-Z.; et al. Nurr1 promotes neurogenesis of dopaminergic neuron and represses inflammatory factors in the transwell coculture system of neural stem cells and microglia. CNS Neurosci. Ther. 2018, 24, 790–800. [Google Scholar] [CrossRef]
- Hofmann, K.W.; Schuh, A.F.S.; Saute, J.; Townsend, R.; Fricke, D.; Leke, R.; Souza, D.O.; Portela, L.V.; Chaves, M.L.F.; Rieder, C.R.M. Interleukin-6 serum levels in patients with Parkinson’s disease. Neurochem. Res. 2009, 34, 1401–1404. [Google Scholar] [CrossRef]
- Koziorowski, D.; Tomasiuk, R.; Szlufik, S.; Friedman, A. Inflammatory cytokines and NT-proCNP in Parkinson’s disease patients. Cytokine 2012, 60, 762–766. [Google Scholar] [CrossRef]
- Neumann, H.; Wekerle, H. Neuronal control of the immune response in the central nervous system: Linking brain immunity to neurodegeneration. J. Neuropathol. Exp. Neurol. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Le, W.; Pan, T.; Huang, M.; Xu, P.; Xie, W.; Zhu, W.; Zhang, X.; Deng, H.; Jankovic, J. Decreased NURR1 gene expression in patients with Parkinson’s disease. J. Neurol. Sci. 2008, 273, 29–33. [Google Scholar] [CrossRef]
- Lang, A.E.; Espay, A.J. Disease Modification in Parkinson’s Disease: Current Approaches, Challenges, and Future Considerations. Mov. Disord. 2018, 33, 660–677. [Google Scholar] [CrossRef]
- Li, S.; Jia, C.; Li, T.; Le, W. Hot Topics in Recent Parkinson’s Disease Research: Where We are and Where We Should Go. Neurosci. Bull. 2021, 37, 1735–1744. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef] [PubMed]
- Magistrelli, L.; Contaldi, E.; Comi, C. The Immune System as a Therapeutic Target for Old and New Drugs in Parkinson’s Disease. CNS Neurol. Disord.-Drug Targets 2022, 22, 66–70. [Google Scholar] [CrossRef]
- Dong, J.; Li, S.; Mo, J.-L.; Cai, H.-B.; Le, W.-D. Nurr1-Based Therapies for Parkinson’s Disease. CNS Neurosci. Ther. 2016, 22, 351–359. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Cho, D.-Y.; Azam, S.; Kim, I.-S.; Choi, D.-K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019, 56, 5799–5814. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Tao, S.; de la Vega, M.R.; Park, S.L.; Vonderfecht, A.A.; Jacobs, S.L.; Zhang, D.D.; Wondrak, G.T. The antimalarial amodiaquine causes autophagic-lysosomal and proliferative blockade sensitizing human melanoma cells to starvation- and chemotherapy-induced cell death. Autophagy 2013, 9, 2087–2102. [Google Scholar] [CrossRef]
- Anderson, D.J. Stem cells and pattern formation in the nervous system: The possible versus the actual. Neuron 2001, 30, 19–35. [Google Scholar] [CrossRef]
- Brundin, P.; Karlsson, J.; Emgård, M.; Schierle, G.S.K.; Hansson, O.; Petersén, Å.; Castilho, R.F. Improving the survival of grafted dopaminergic neurons: A review over current approaches. Cell Transplant. 2000, 9, 179–195. [Google Scholar] [CrossRef]
- Wolters, E.C.; Strekalova, T.; de Munter, J.P.J.M.; Kramer, B.W. Naive BM-derived stem cells (Neuro-Cells) may modify acute and chronic neurodegenerative disorders by modulating macrophage behaviors. Ageing Neurodegener. Dis. 2021, 1, 3. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, X.-X.; Wang, W.; Li, J.-J.; Wang, X.-P.; Tang, Z.-W.; Xu, J.-T.; Lin, H.; Yang, Z.-Y.; Li, L.-Y.; et al. Transplantation of Nurr1-overexpressing neural stem cells and microglia for treating parkinsonian rats. CNS Neurosci. Ther. 2020, 26, 55–65. [Google Scholar] [CrossRef]
- Tian, L.; Al-Nusaif, M.; Chen, X.; Li, S.; Le, W. Roles of Transcription Factors in the Development and Reprogramming of the Dopaminergic Neurons. Int. J. Mol. Sci. 2022, 23, 845. [Google Scholar] [CrossRef]
- Kim, C.-H.; Han, B.-S.; Moon, J.; Kim, D.-J.; Shin, J.; Rajan, S.; Nguyen, Q.-T.; Sohn, M.; Kim, W.-G.; Han, M.; et al. Nuclear receptor Nurr1 agonists enhance its dual functions and improve behavioral deficits in an animal model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 8756–8761. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-Y.; Jang, Y.; Kim, W.; Shin, J.; Toh, H.T.; Kim, C.-H.; Yoon, H.S.; Leblanc, P.; Kim, K.-S. Chloroquine modulates inflammatory autoimmune responses through Nurr1 in autoimmune diseases. Sci. Rep. 2019, 9, 15559. [Google Scholar] [CrossRef] [PubMed]
- Hedya, S.A.; Safar, M.M.; Bahgat, A.K. Hydroxychloroquine antiparkinsonian potential: Nurr1 modulation versus autophagy inhibition. Behav. Brain Res. 2019, 365, 82–88. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, B.R.; Popichak, K.A.; Hammond, S.L.; Jorgensen, B.A.; Phillips, A.T.; Safe, S.; Tjalkens, R.B. The Nurr1 Activator 1,1-Bis(3′-Indolyl)-1-(p-Chlorophenyl)Methane Blocks Inflammatory Gene Expression in BV-2 Microglial Cells by Inhibiting Nuclear Factor κB. Mol. Pharmacol. 2015, 87, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-S.; Van Minh, N.; Choi, H.-Y.; Byun, J.-S.; Kim, W.-G. Daphnane and Phorbol Diterpenes, Anti-neuroinflammatory Compounds with Nurr1 Activation from the Roots and Stems of Daphne genkwa. Biol. Pharm. Bull. 2017, 40, 2205–2211. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Xie, W.J.; Tuo, H.; Hintermann, S.; Jankovic, J.; Le, W. Anti-parkinsonian effects of Nurr1 activator in ubiquitin-proteasome system impairment induced animal model of Parkinson’s disease. CNS Neurol. Disord.-Drug Targets 2012, 11, 768–773. [Google Scholar] [CrossRef]
- Hedya, S.A.; Safar, M.M.; Bahgat, A.K. Cilostazol Mediated Nurr1 and Autophagy Enhancement: Neuroprotective Activity in Rat Rotenone PD Model. Mol. Neurobiol. 2018, 55, 7579–7587. [Google Scholar] [CrossRef]
- Gao, H.; Wang, D.; Wang, Y.-L.; Mao, J.-P.; Jiang, S.; Yang, X.-L. Pramipexole attenuates 6-OHDA-induced Parkinson’s disease by mediating the Nurr1/NF-κB pathway. Mol. Biol. Rep. 2021, 48, 3079–3087. [Google Scholar] [CrossRef]
- Wang, J.; Bi, W.; Zhao, W.; Varghese, M.; Koch, R.J.; Walker, R.H.; Chandraratna, R.A.; Sanders, M.E.; Janesick, A.; Blumberg, B.; et al. Selective brain penetrable Nurr1 transactivator for treating Parkinson’s disease. Oncotarget 2016, 7, 7469–7479. [Google Scholar] [CrossRef]
- Esteves, M.; Cristóvão, A.C.; Saraiva, T.; Rocha, S.M.; Baltazar, G.; Ferreira, L.; Bernardino, L. Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease. Front. Aging Neurosci. 2015, 7, 20. [Google Scholar] [CrossRef]
- Rojas, P.; Ruiz-Sánchez, E.; Rojas, C.; Ögren, S.O. Ginkgo biloba extract (EGb 761) modulates the expression of dopamine-related genes in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neuroscience 2012, 223, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Dou, L.; Shan, L.; Sun, Y.; Li, W. Proliferation and committed differentiation into dopamine neurons of neural stem cells induced by the active ingredients of radix astragali. Neuroreport 2018, 29, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-W.; Park, C.-H.; Bae, Y.-C.; Bae, J.-Y.; Chung, S.; Chang, M.-Y.; Koh, H.-C.; Lee, H.-S.; Hwang, S.-J.; Lee, K.-H.; et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells 2007, 25, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Traver, E.; Solís, O.; Díaz-Guerra, E.; Ortiz, Ó.; Vergaño-Vera, E.; Méndez-Gómez, H.R.; García-Sanz, P.; Moratalla, R.; Vicario-Abejón, C. Role of Nurr1 in the Generation and Differentiation of Dopaminergic Neurons from Stem Cells. Neurotox. Res. 2016, 30, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Beiki, R.; Khaghani, M.; Esmaeili, F.; Dehghanian, F. Synergistic Effects of Combined Nurr1 Overexpression and Natural Inducers on the More Efficient Production of Dopaminergic Neuron-Like Cells From Stem Cells. Front. Cell. Neurosci. 2021, 15, 803272. [Google Scholar] [CrossRef] [PubMed]
- Salemi, S.; Baktash, P.; Rajaei, B.; Noori, M.; Amini, H.; Shamsara, M.; Massumi, M. Efficient generation of dopaminergic-like neurons by overexpression of Nurr1 and Pitx3 in mouse induced Pluripotent Stem Cells. Neurosci. Lett. 2016, 626, 126–134. [Google Scholar] [CrossRef]
- Martinat, C.; Bacci, J.-J.; Leete, T.; Kim, J.; Vanti, W.B.; Newman, A.H.; Cha, J.H.; Gether, U.; Wang, H.; Abeliovich, A. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc. Natl. Acad. Sci. USA 2006, 103, 2874–2879. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, L.; Qin, J.; Tian, M.; Zhu, H.; Dong, C.; Zhao, H.; Jin, G. Transplantation of neural stem cells co-transfected with Nurr1 and Brn4 for treatment of Parkinsonian rats. Int. J. Dev. Neurosci. 2013, 31, 82–87. [Google Scholar] [CrossRef]
- Andersson, E.K.; Irvin, D.K.; Ahlsiö, J.; Parmar, M. Ngn2 and Nurr1 act in synergy to induce midbrain dopaminergic neurons from expanded neural stem and progenitor cells. Exp. Cell Res. 2007, 313, 1172–1180. [Google Scholar] [CrossRef]
- Park, C.-H.; Kang, J.S.; Yoon, E.-H.; Shim, J.-W.; Suh-Kim, H.; Lee, S.-H. Proneural bHLH neurogenin 2 differentially regulates Nurr1-induced dopamine neuron differentiation in rat and mouse neural precursor cells in vitro. FEBS Lett. 2008, 582, 537–542. [Google Scholar] [CrossRef][Green Version]
- Lee, H.-S.; Bae, E.-J.; Yi, S.-H.; Shim, J.-W.; Jo, A.-Y.; Kang, J.-S.; Yoon, E.-H.; Rhee, Y.-H.; Park, C.-H.; Koh, H.-C.; et al. Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells 2010, 28, 501–512. [Google Scholar] [CrossRef] [PubMed]
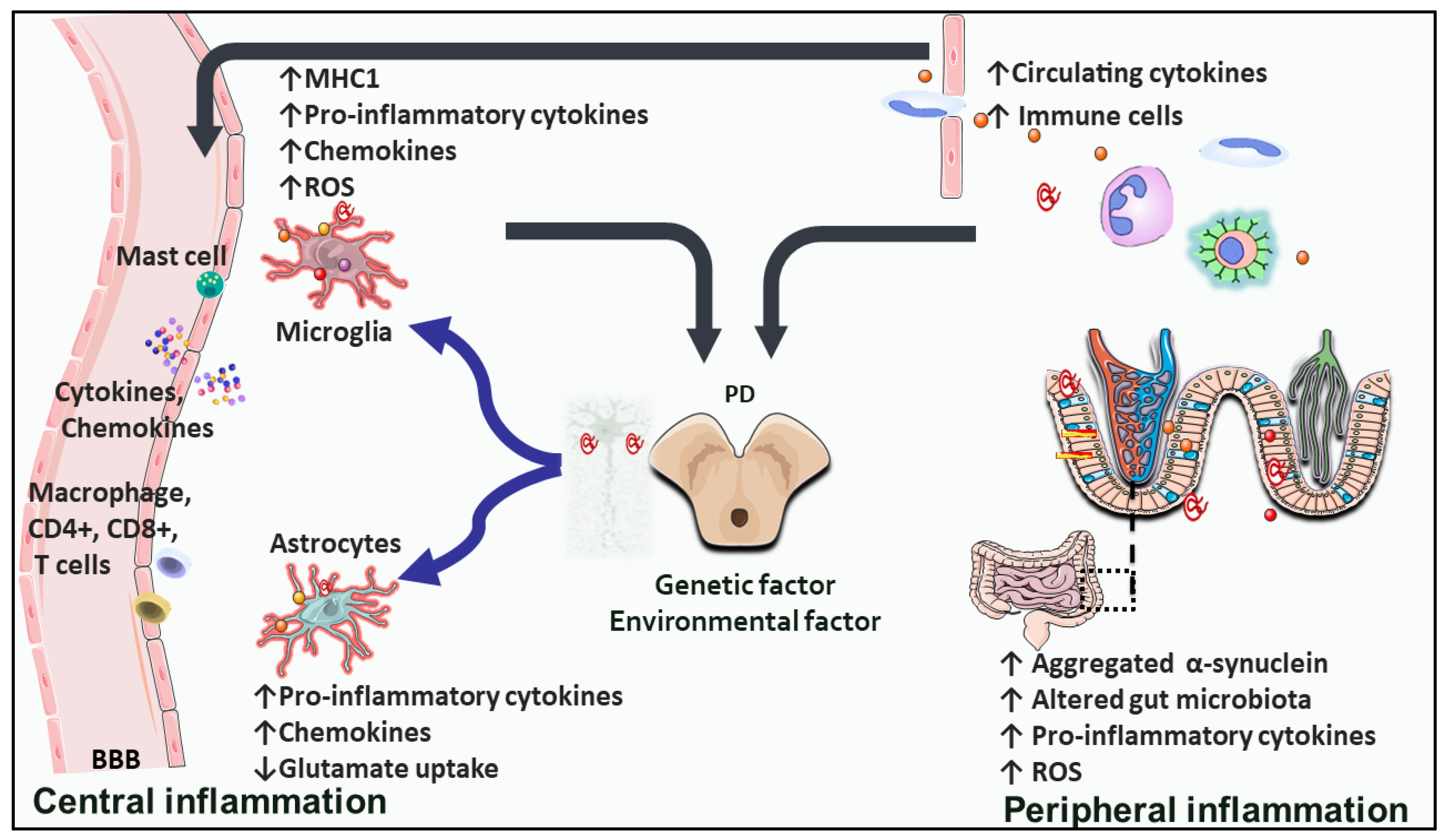
| PD Central Cellular and Molecular Inflammatory Changes | |||
|---|---|---|---|
| Models/Sample | Methods | Main Outcomes | Ref. |
| Human SNc | Compare PD to HC (Immunohistochemistry) |
| [26] |
| Human SNc | Compare PD to HC (Immunohistochemistry) |
| [57] |
| Human striatum and SNc | Compare PD to HC (Immunohistochemistry) |
| [27] |
| Human striatum | Compare PD to HC (ELISA) |
| [58] |
| Human striatum and SNc | Compare PD to HC (Immunohistochemistry) |
| [59] |
| Human SNc | Compare PD to HC (Immunohistochemistry and Western blot) |
| [60] |
| Human SNc | Compare PD to HC (Immunohistochemistry) |
| [61] |
| Human SNc | Compare PD to HC (Immunohistochemistry) |
| [62] |
| Human SNc | Compare PD to HC (Immunohistochemistry) |
| [63] |
| Mice striatum after α-synuclein seeds | Inject human PFF α-synuclein seeds or monomer |
| [64] |
| Mice nigrostriatal after oral DSS | Oral administration of DSS |
| [65] |
| Mice striatum after α-synuclein seeds injection | Inject α-synuclein into the striatum |
| [66] |
| Mice SNc after injecting LPS | Inject LPS into the 6-OHDA mice model |
| [67] |
| The mRNA profiles of microglia genes of mice | Compare microglia of specific pathogen-free and GF mice by flow cytometry and deep quantitative sequencing of the RNA transcripts |
| [68] |
| Mice SNc, midbrain after immune transfer cells | Adoptive transfer of copolymer-1 immune cells to MPTP mice model |
| [69] |
| Mice midbrain after transfer of Treg cells | Adoptive transfer of Treg cells to MPTP mice model |
| [70] |
| Recombinant α-synuclein to heterozygous Tlr-4+/− mice | Homeostatic astrocytes were incubated with recombinant α-synuclein |
| [71] |
| Rat striatum and SNc | Inject 6-OHDA in rat striatum |
| [72] |
| Rat striatum | Inject 6-OHDA in rat striatum |
| [73] |
| Rat striatum | Inject 6-OHDA in rat striatum |
| [74] |
| Monkey SNc | Intracarotid infusion of MPTP in rhesus monkeys |
| [75] |
| Monkey SNc | Inject MPTP in the vein of cynomolgus monkeys |
| [76] |
| PD Peripheral Cellular and Molecular Inflammatory Changes | |||
| Models/Sample | Methods | Main Outcomes | Ref. |
| Human PBMCs | Assess activation status by Flow cytometry in PD patients and HC |
| [77] |
| Human whole blood | Single-cell transcriptome and T cell receptor sequencing of PD patients and HC. |
| [78] |
| Human serum | Assess serum cytokine concentrations in PD patients and HC by flow cytometry |
| [79] |
| Human peripheral blood | Analysis of the peripheral T-lymphocyte populations in PD patients and HC |
| [80] |
| Human PBMCs | Assess the cytokines expression in PD patients, HC, and non-PD neurological disease controls |
| [81] |
| Human peripheral blood | Meta-analysis to assess the correlation between lymphocyte and natural killer cells in PD patients and HC. |
| [82] |
| Human PBMCs | Assess cytokines by flow cytometry in PD patients and HC. |
| [83] |
| Human fecal | Assess the intestinal inflammation fecal markers by ELISA |
| [84] |
| Human PBMCs | Investigate the levels of production and expression of cyto/chemokines by PBMCs in PD patients and HC through RT-PCR and ELISA |
| [85] |
| Leukocytes mice spleen after α-synuclein seeds in the striatum | Inject human PFF α-synuclein seeds or monomers into the striatum of mice |
| [64] |
| The colon of mice after injecting 6-OHDA | Inject 6-OHDA in the striatum and nigra of mice |
| [65] |
| Mice DMV after administration of rotenone | Enteral administration of rotenone (gastric tube) |
| [86] |
| Reactive microglia after being treated by MPP+ Mice SNc and serum after MPTP | MPP+ in reactive microglia LPS PD model MPTP was given subcutaneously in mice |
| [87] |
| Mice midbrain after injecting LPS | Inject LPS in SNc of A53Ttg/tg mice and MPTP/p-induced PD mice |
| [88] |
| Rat striatum and SNc after administration of rotenone | Rotenone chronically administers using subcutaneous osmotic minipumps |
| [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nusaif, M.; Lin, Y.; Li, T.; Cheng, C.; Le, W. Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 16184. https://doi.org/10.3390/ijms232416184
Al-Nusaif M, Lin Y, Li T, Cheng C, Le W. Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease. International Journal of Molecular Sciences. 2022; 23(24):16184. https://doi.org/10.3390/ijms232416184
Chicago/Turabian StyleAl-Nusaif, Murad, Yushan Lin, Tianbai Li, Cheng Cheng, and Weidong Le. 2022. "Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease" International Journal of Molecular Sciences 23, no. 24: 16184. https://doi.org/10.3390/ijms232416184
APA StyleAl-Nusaif, M., Lin, Y., Li, T., Cheng, C., & Le, W. (2022). Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease. International Journal of Molecular Sciences, 23(24), 16184. https://doi.org/10.3390/ijms232416184






