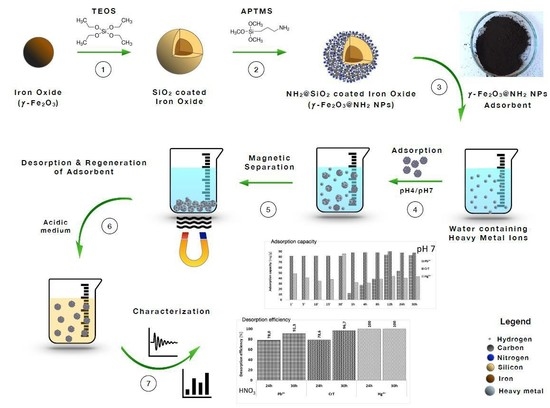
Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles
Abstract
1. Introduction
| Adsorbent | HM Ions | Tested pH | Adsorption Capacity | Desorption Efficiency | Reference |
|---|---|---|---|---|---|
| NH2-functionalized γ-Fe2O3 NPs (γ-Fe2O3@NH2 NPs) | Hg2+ | 4.0 | 16.2 mg/g | 100% | This work |
| CT-HPMNPs | 5.0 | 32.88 mg/g | ≅85% | [92] | |
| Rhodamine-hydrazide-modified Fe3O4 | 7.5 | 37.4 mg/g | - | [93] | |
| Nanocomposite based on Fe3O4 nanoparticles, chitosan nanoparticles, and polythiophene | 7.0 | 50 mg/g | - | [95] | |
| Fe3O4@SiO2-NH-HCG- (py) | 7.0 | 56 mg/g | 95% | [96] | |
| Fe3O4 nanoparticle coated with amino organic ligands and yam peel biomass | 7.0 | 60 mg/g | - | [94] | |
| Fe3O4@SiO2-NH-HCG- (pyd) | 7.0 | 77 mg/g | 95% | [96] | |
| NH2-functionalized γ-Fe2O3 NPs (γ-Fe2O3@NH2 NPs) | 7.0 | 85.6 mg/g | 100% | This work | |
| Armeniaca sibirica shell activated carbon (ASSAC) magnetized by nanoparticles (Fe3O4/ASSAC) | pH 2 | 97.1 mg/g | [97] | ||
| Activated carbon (XLAC) derived from Xanthoceras sorbifolia Bunge hull | pH 5.5 | 235.6 mg·g | [98] | ||
| Cadmium sulfide nanoparticles doped in a nanoadsorbent fabricated from polycaprolactam (nylon 6) nanofibers (CdS/N6) | pH 5 | 162 mg g | [99] |
2. Results and Discussion
2.1. Properties of the Prepared γ-Fe2O3@NH2 NPs
2.1.1. Crystallographic Properties
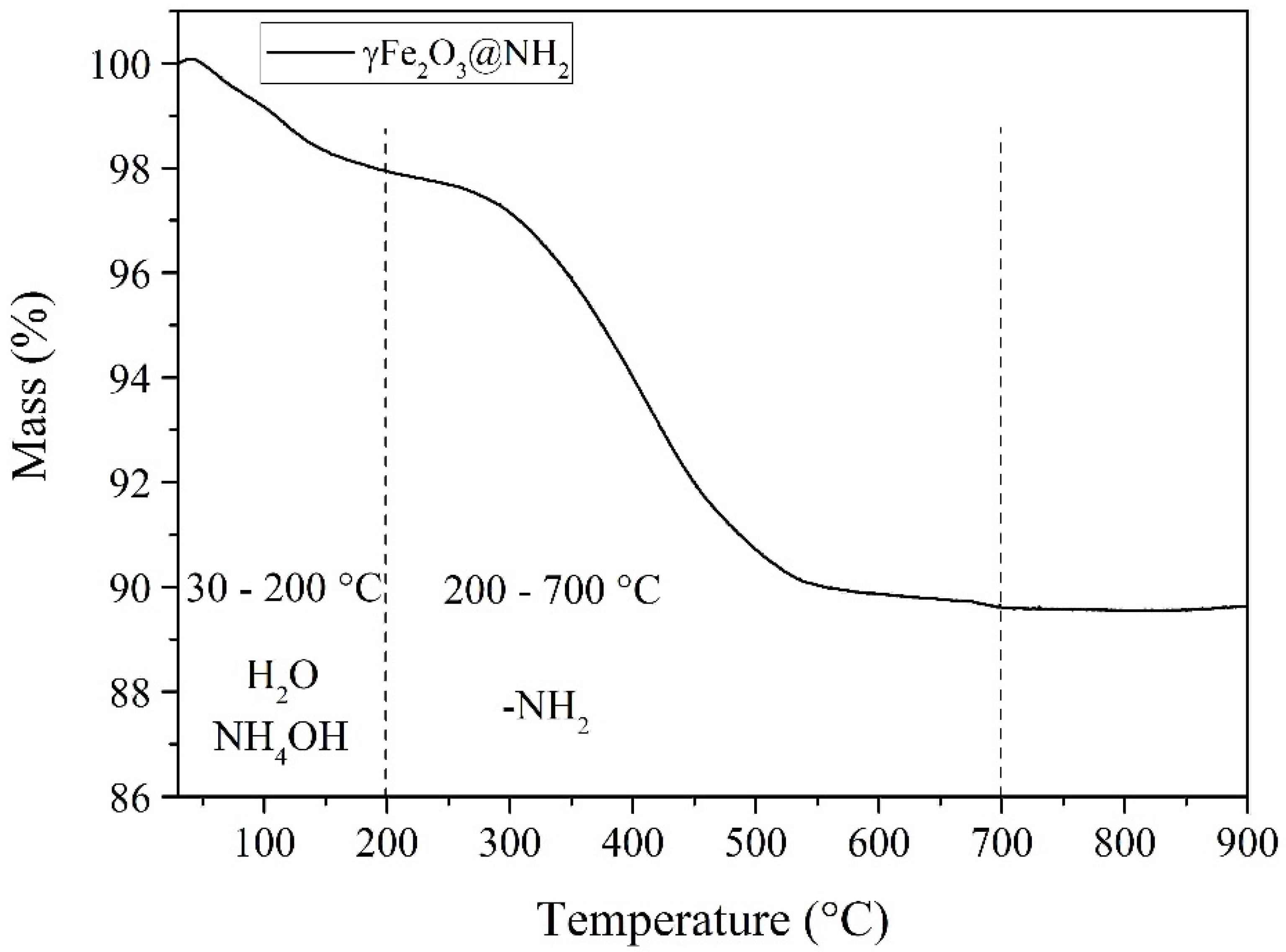
2.1.2. Thermogravimetric Properties
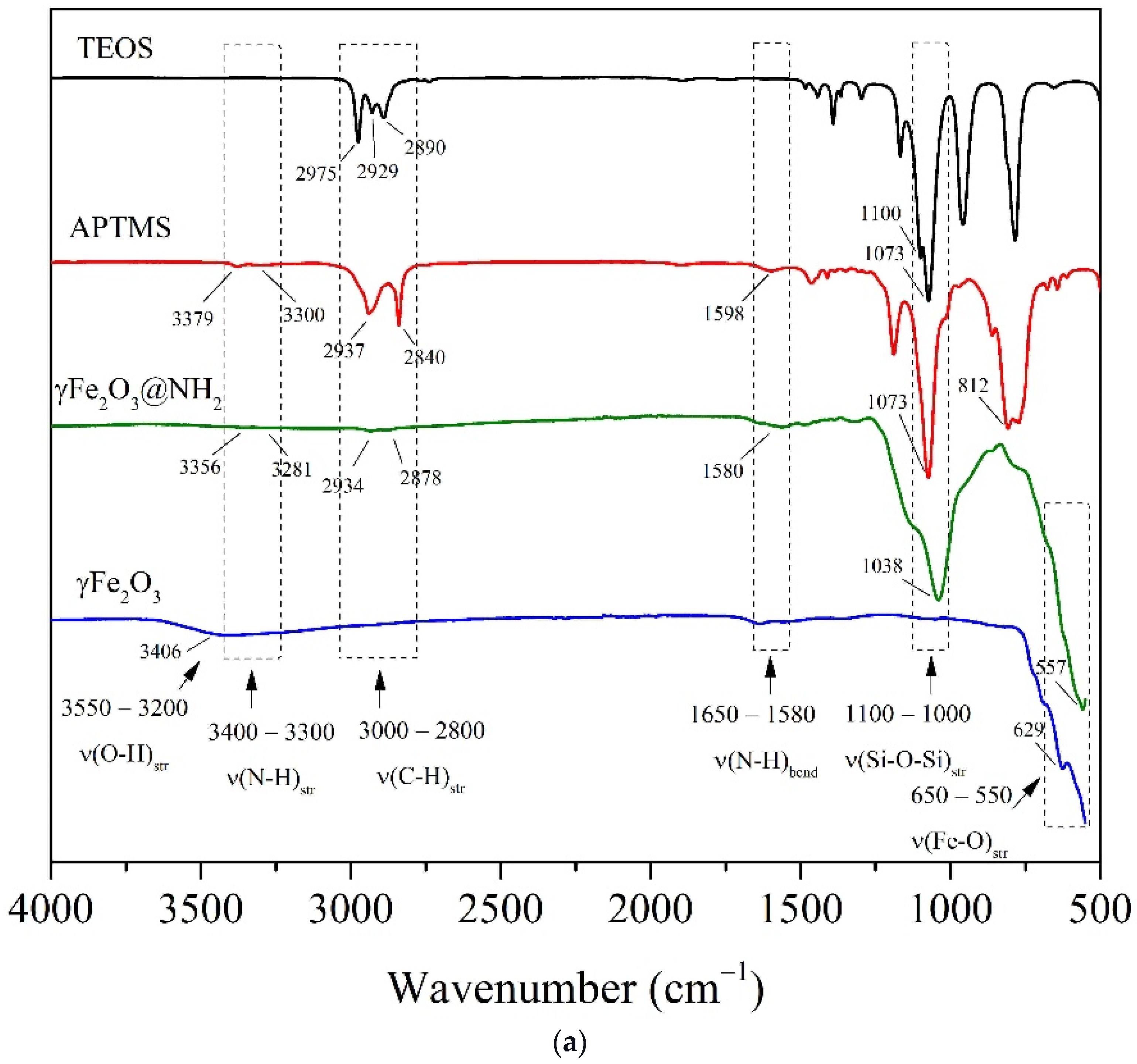
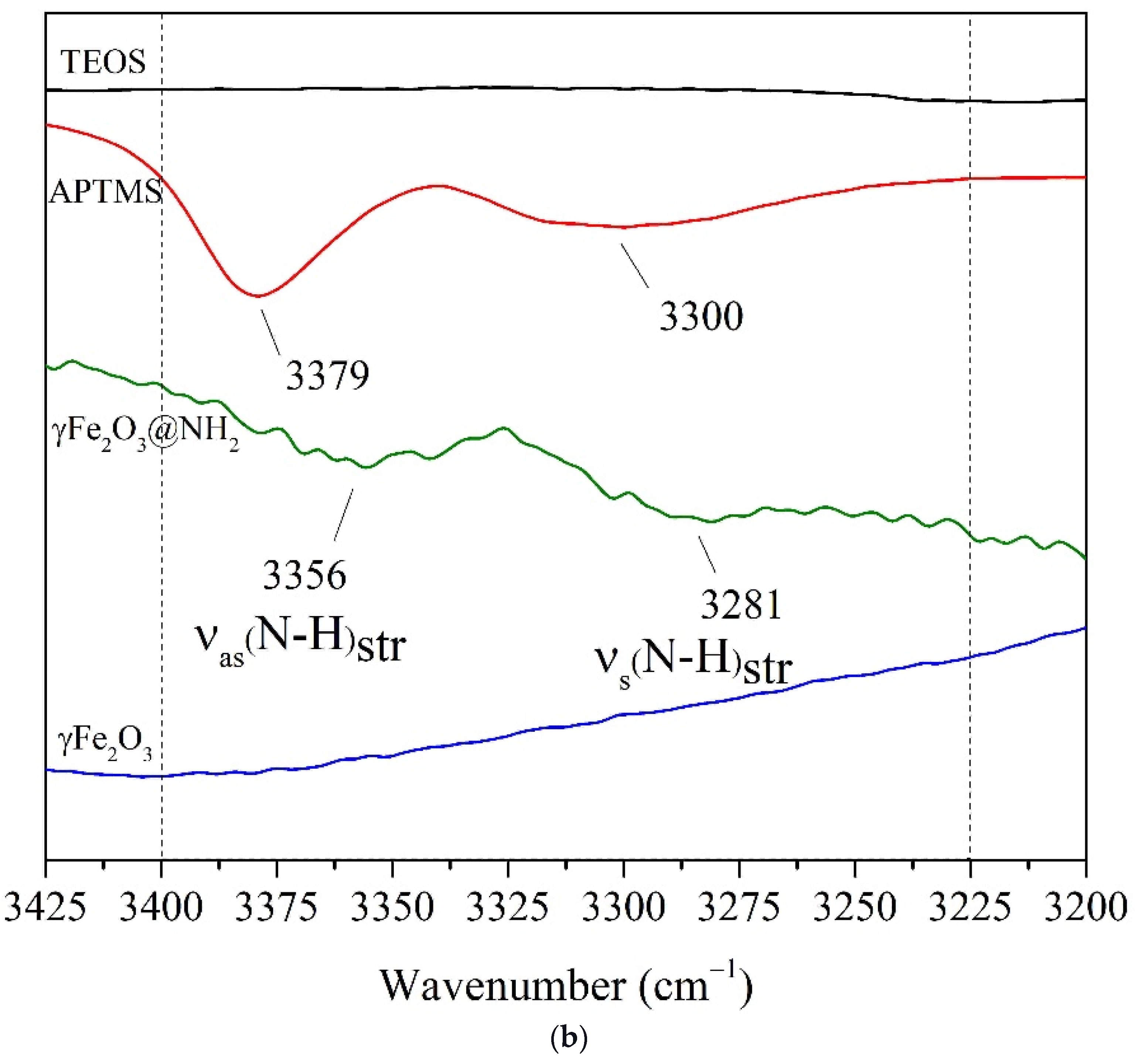
2.1.3. FTIR Spectroscopy
2.1.4. Specific Surface Area
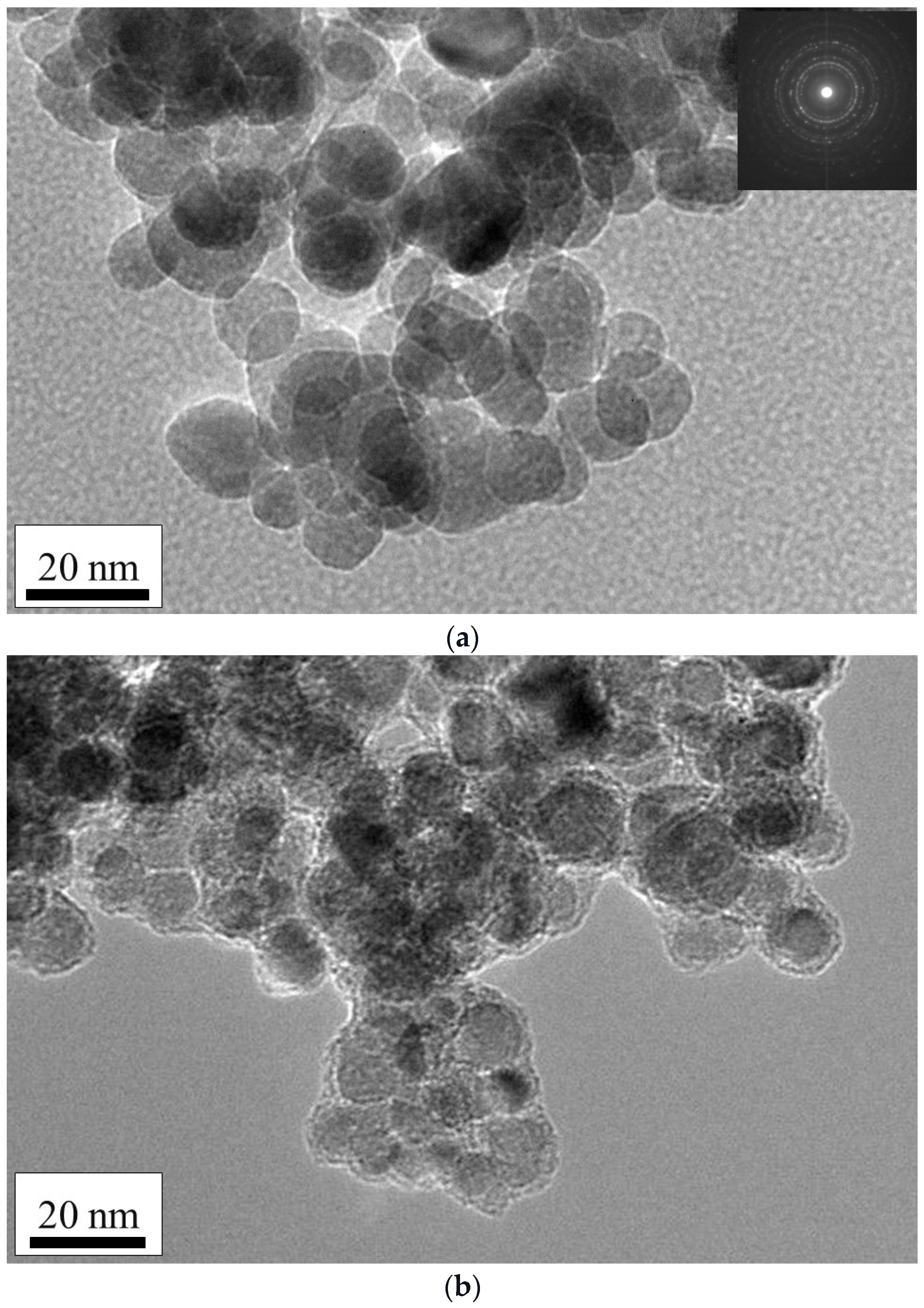
2.1.5. Morphological Properties
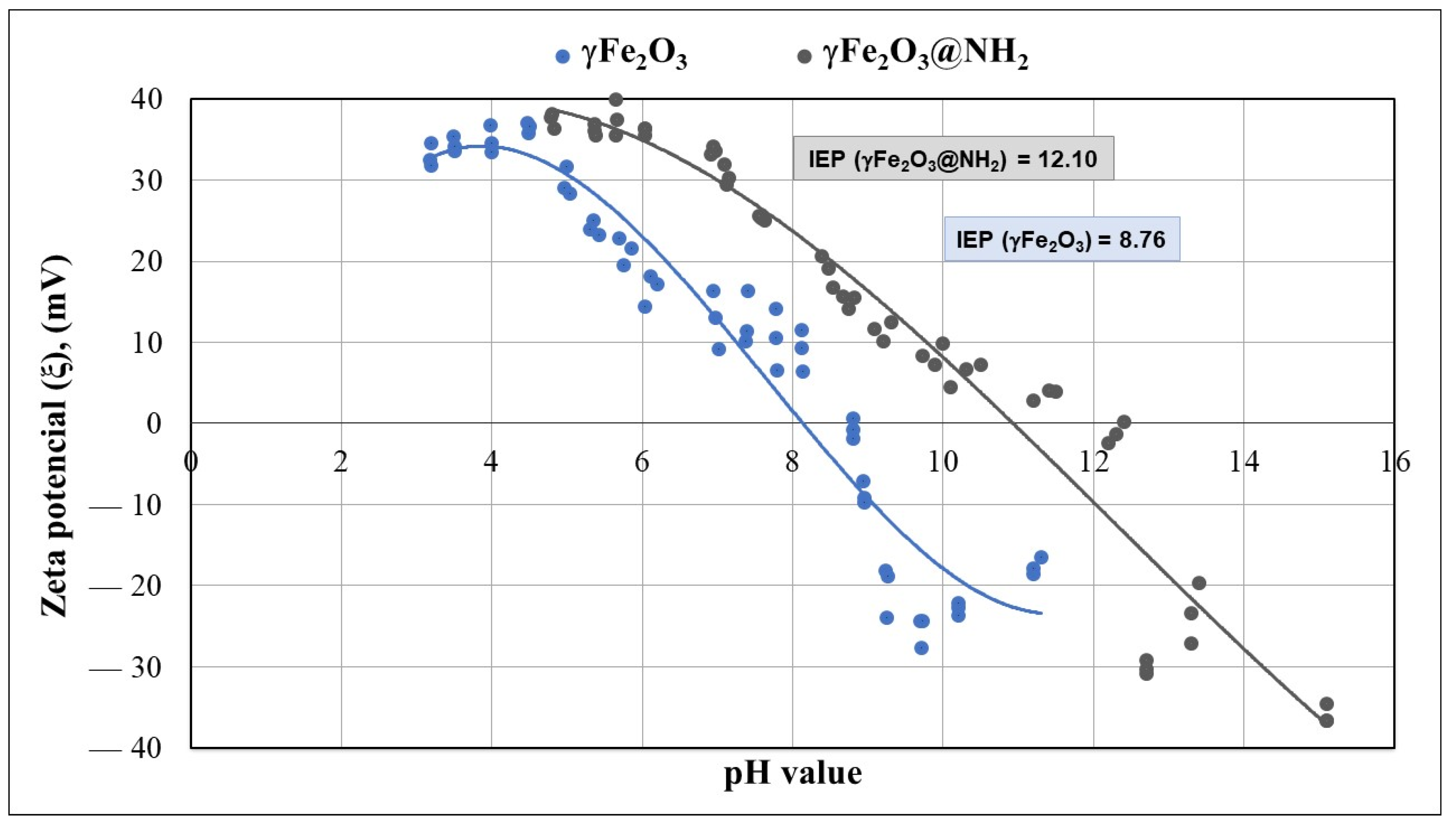
2.1.6. Zeta Potential
2.2. Adsorption Mechanisms
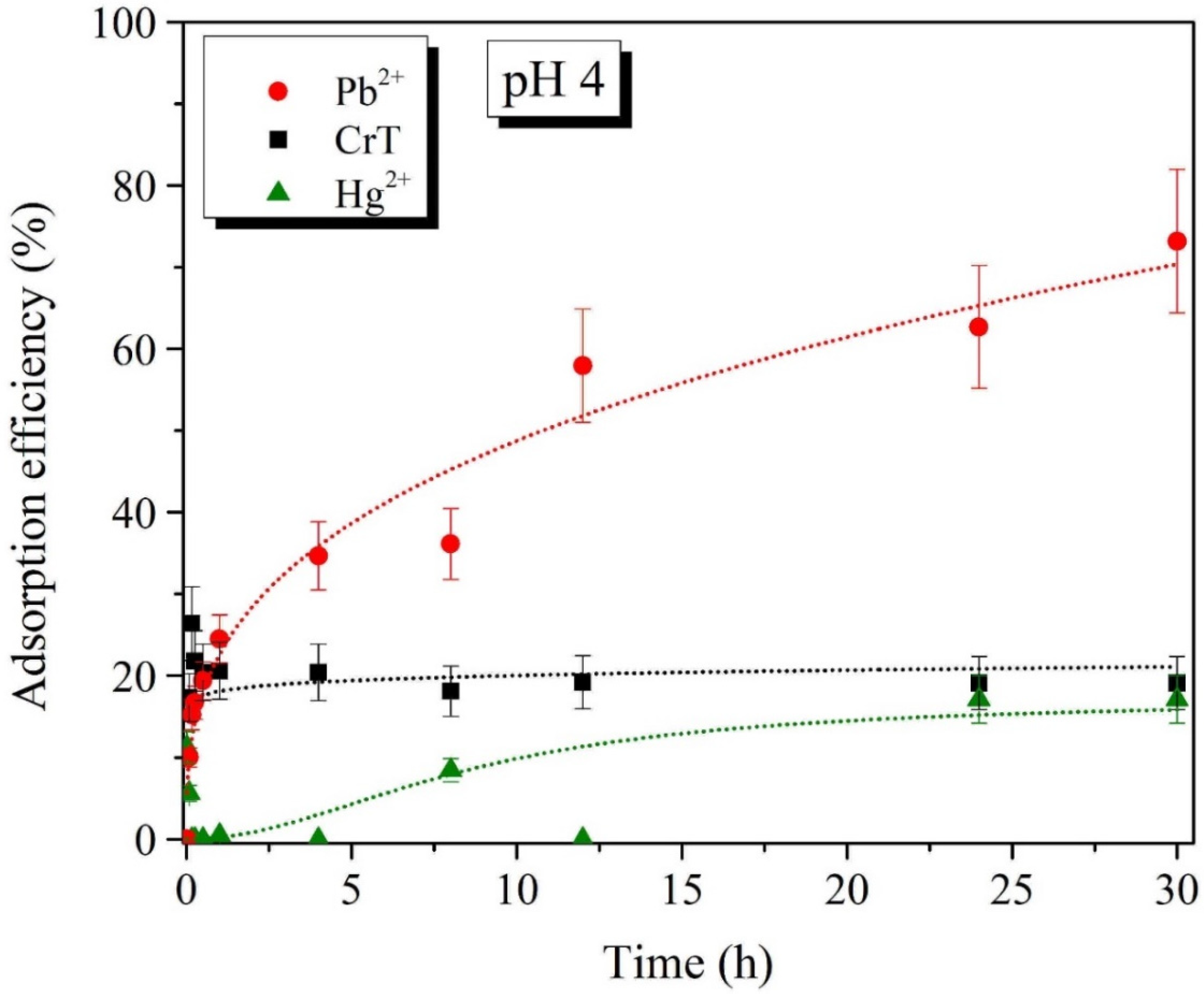
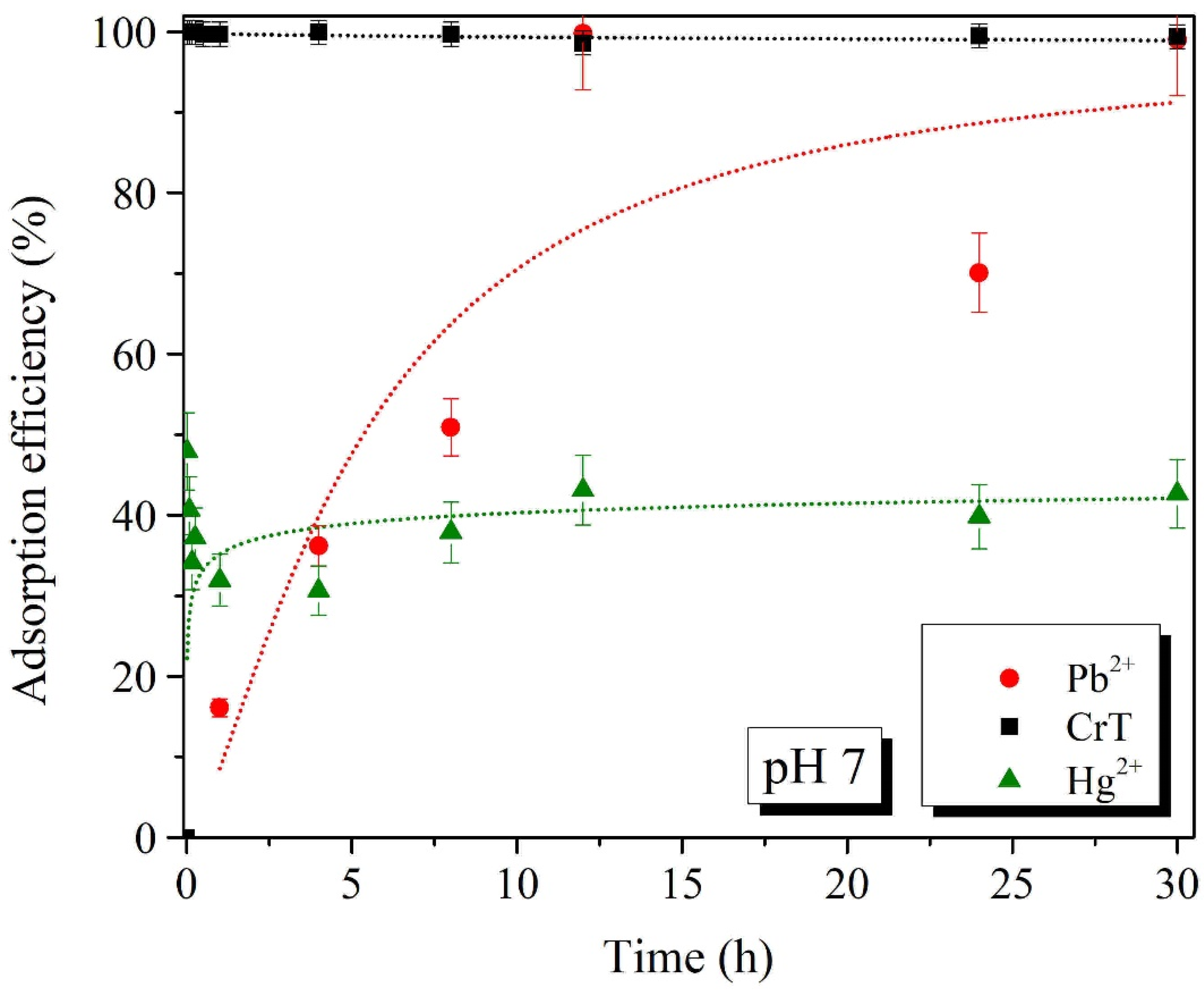
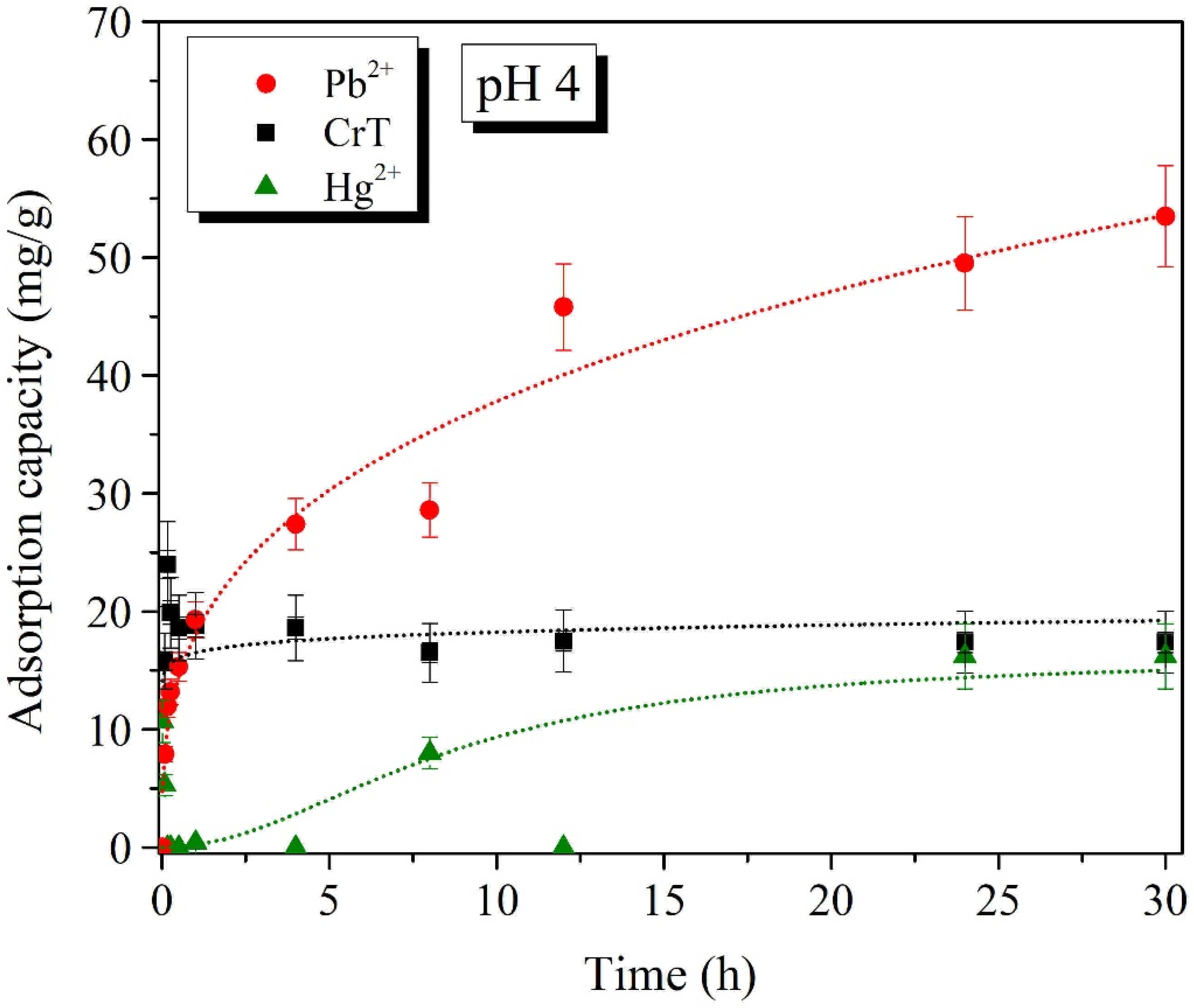
2.3. Effects of pH
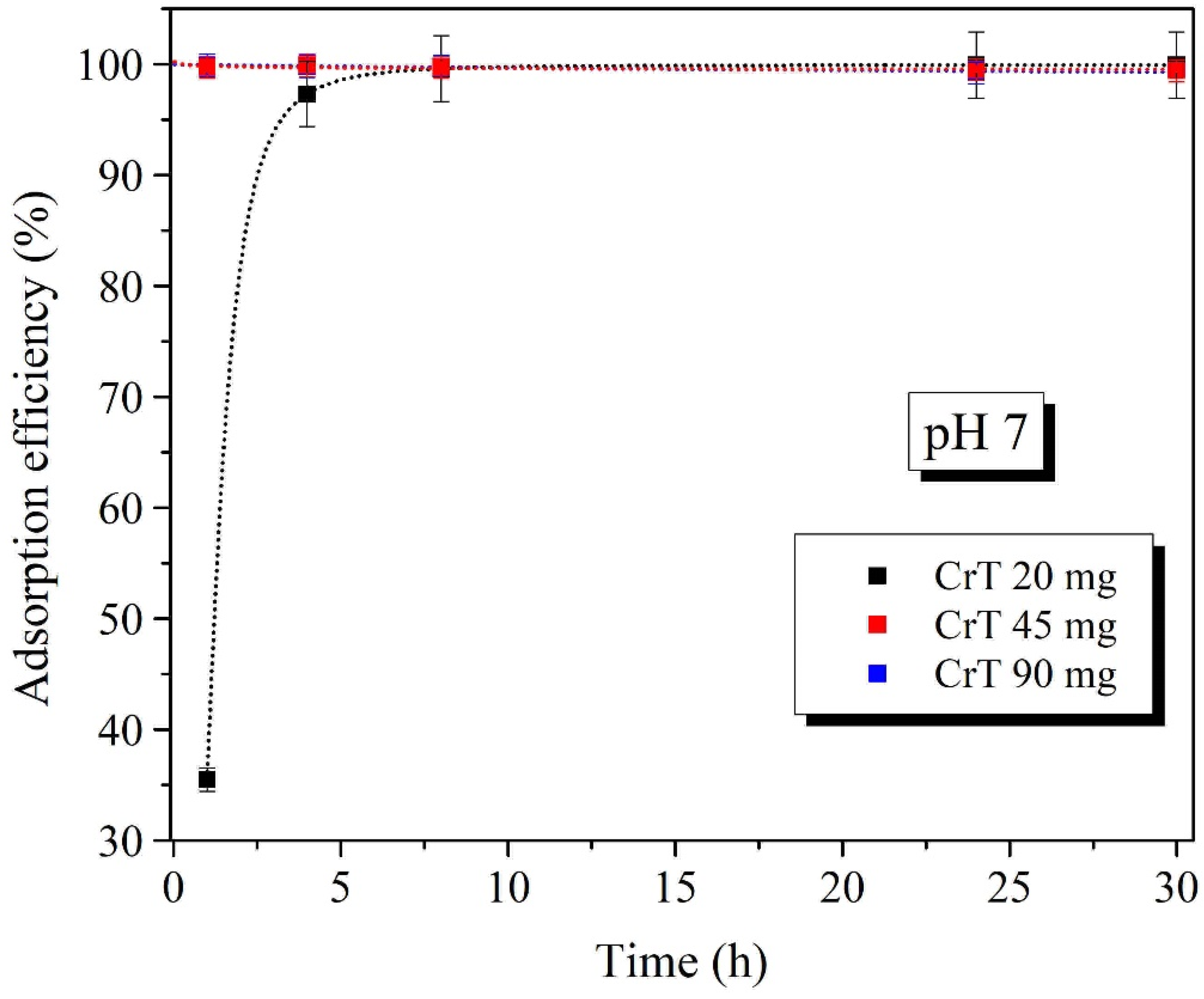
2.4. Effect of Adsorbent Mass
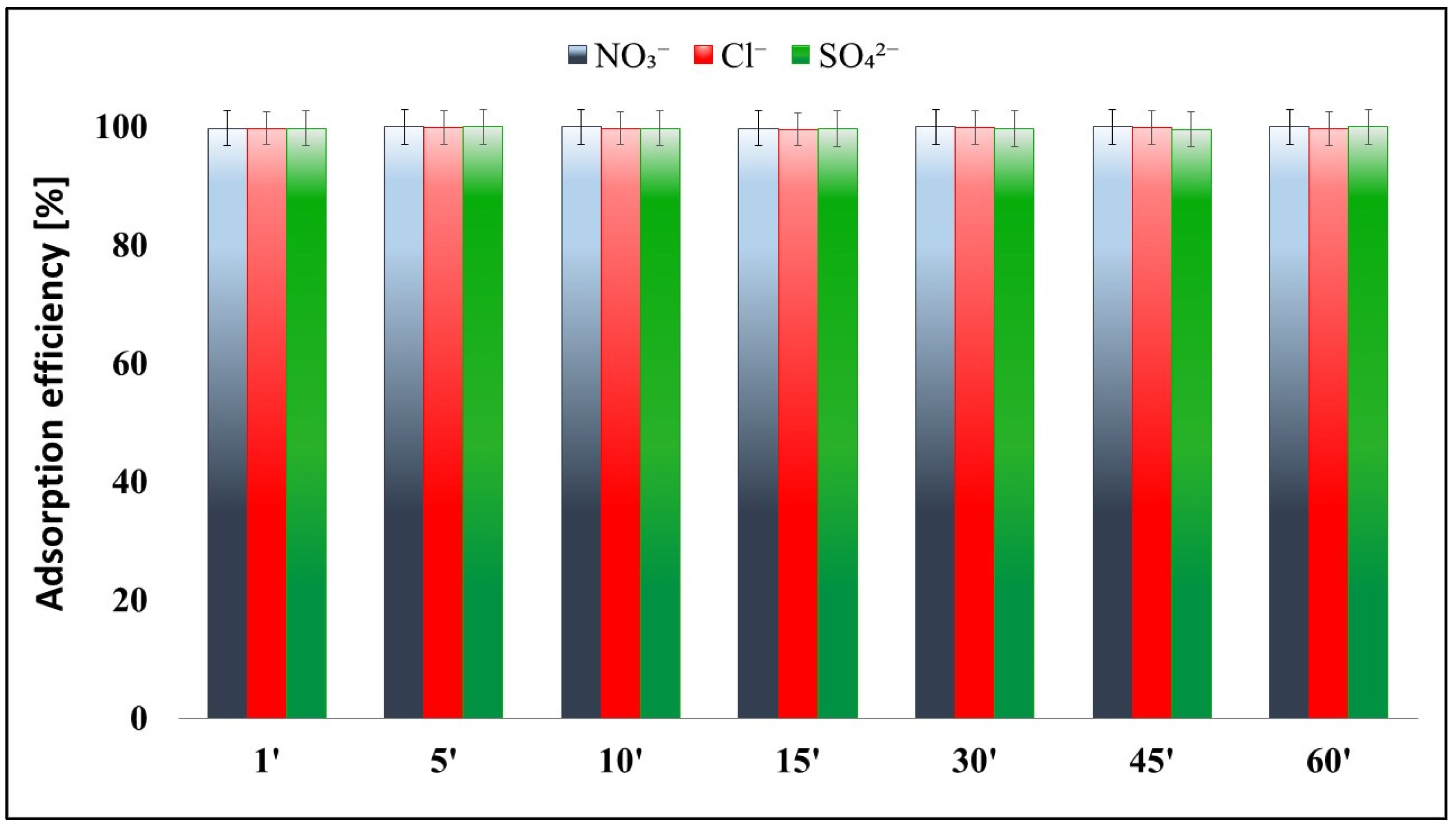
2.5. Effects of Anions (NO3−, Cl−, SO42−)
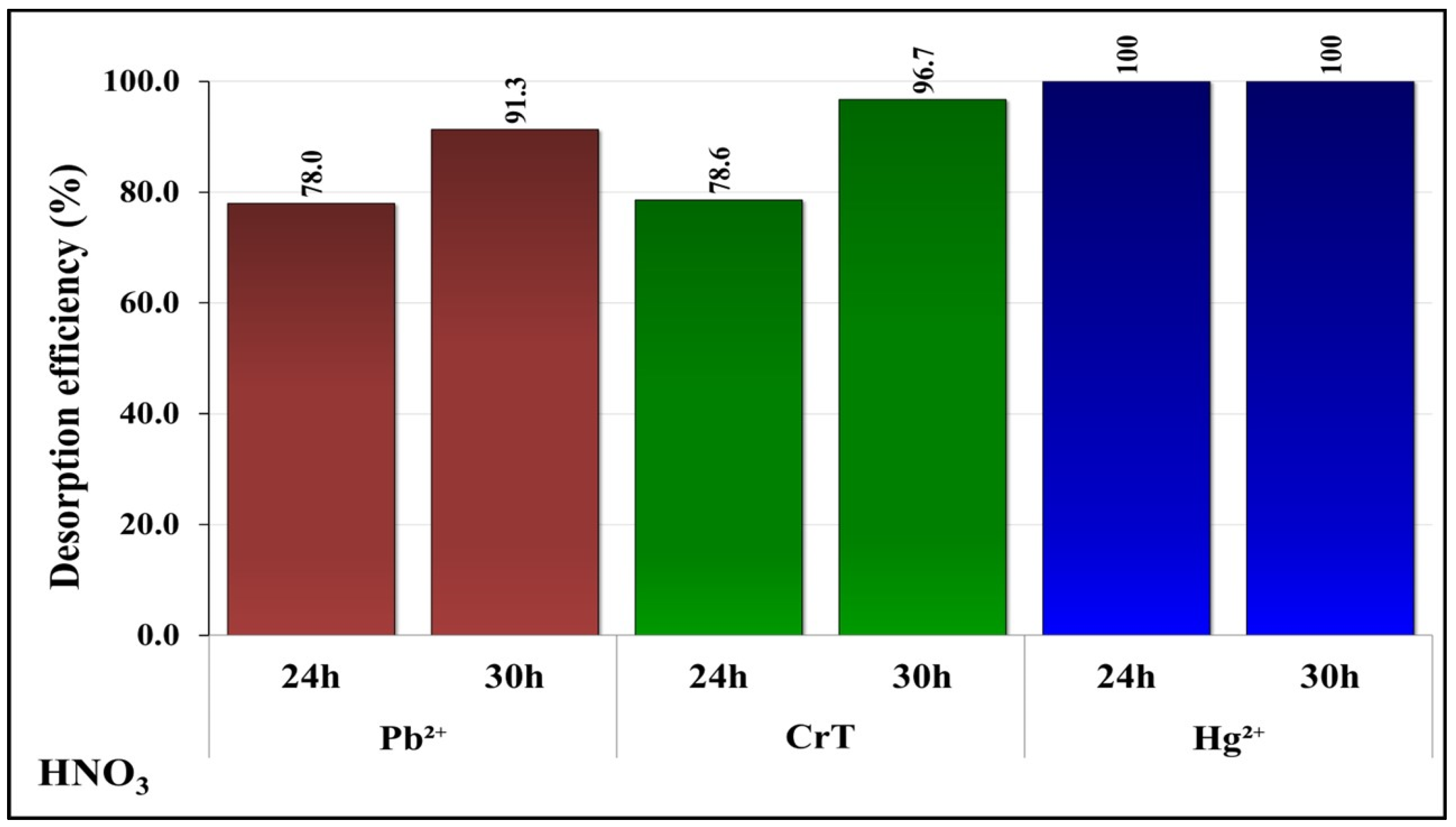
2.6. Desorption
3. Methods and Materials
3.1. Materials
3.2. Synthesis of MNPs
3.3. Stabilization of MNPs
3.4. Functionalization of MNPs
3.5. Characterization of Amino-Functionalized γ-Fe2O3 MNPs
3.6. Adsorption of Heavy Metal Ions in Aqueous Solutions
3.7. Desorption of HM Ions and Regeneration Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Auerbach, R.; Bokelmann, K.; Stauber, R.; Gutfleisch, O.; Schnell, S.; Ratering, S. Critical raw materials—Advanced recycling technologies and processes: Recycling of rare earth metals out of end-of-life magnets by bioleaching with various bacteria as an example of an intelligent recycling strategy. Miner. Eng. 2019, 134, 104–117. [Google Scholar] [CrossRef]
- European Commission; Blengini, G.; Latunussa, C.; Eynard, U.; Matos, C.; Georgitzikis, K.; Pavel, C.; Carrara, S.; Mancini, L.; Unguru, M.; et al. Study on the EU’s List of Critical Raw Materials (2020); Factsheets on Critical Raw Materials; Final Report; European Commission: Luxembourg, 2020. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for Strategic Technologies and Sectors in the EU, A Foresight Study; European Commission: Luxembourg, 2020; ET-04-20-034-EN-N; ISBN 978-92-76-15336-8. [Google Scholar] [CrossRef]
- Skirrow, R.G.; Huston, D.L.; Mernagh, T.P.; Thorne, J.P.; Dulfer, H.; Senior, A.B. Critical Commodities for a High-Tech World: Australia’s Potential to Supply Global Demand; Geoscience Australia: Canberra, Australia, 2013.
- Huang, H.; Wang, Y.; Zhang, Y.; Niu, Z.; Li, X. Amino-functionalized graphene oxide for Cr (VI), Cu (II), Pb (II) and Cd (II) removal from industrial wastewater. Open Chem. 2020, 18, 97–107. [Google Scholar] [CrossRef]
- Girtan, M.; Wittenberg, A.; Grilli, M.L.; de Oliveira, D.P.S.; Giosuè, C.; Ruello, M.L. The Critical Raw Materials Issue be-tween Scarcity, Supply Risk, and Unique Properties. Materials 2021, 14, 1826. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). The Priority List of Hazardous Substances. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 30 August 2022).
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification. Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. Resour. Conserv. Recycl. 2020, 156, 105688. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhang, H.; Li, Y.; Zhang, Z.; Zhang, W. Recycling spent lithium-ion battery as adsorbents to remove aqueous heavy metals: Adsorption kinetics, isotherms, and regeneration assessment. J. Environ. Chem. Eng. 2021, in press. [CrossRef]
- Dhiman, V.; Kondal, N. ZnO Nanoadsorbents: A potent material for removal of heavy metal ions from wastewater. Colloid Interface Sci. Commun. 2021, 41, 100380. [Google Scholar] [CrossRef]
- Fu, X.; Feng, X.; Sommar, J.; Wang, S. A review of studies on atmospheric mercury in China. Sci. Total Environ. 2012, 421–422, 73–81. [Google Scholar] [CrossRef]
- Mousavi, A.; Chávez, R.D.; Ali, A.-M.S.; Cabaniss, S.E. Mercury in natural waters: A mini-review. Environ. Forensics 2011, 12, 14–18. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221–222, 1–18. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. Mercury contamination in mushroom samples from Tokat, Turkey. Bull. Environ. Contam. Toxicol. 2005, 74, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lin, H.; Zheng, W.; Tomanicek, S.J.; Johs, A.; Feng, X.; Elias, D.A.; Liang, L.; Gu, B. Oxidation and methylation of dissolved elemental mercury by anaerobic bacteria. Nat. Geosci. 2013, 6, 751–754. [Google Scholar] [CrossRef]
- Unceta, N.; Seby, F.; Malherbe, J.; Donard, O.F.X. Chromium speciation in solid matrices and regulation: A review. Anal. Bioanal. Chem. 2010, 397, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Calik, S.; Sőzűdogru, O.; Massara, T.M.; Yilmaz, A.E.; Bakirdere, S.; Katsou, E.; Komesli, O.T. Removal of heavy metals by a membrane bioreactor combined with activated carbon. Anal. Lett. 2020, 54, 1616–1626. [Google Scholar] [CrossRef]
- Henze, M.; Comeau, Y. Wastewater characterization. In Biological Wastewater Treatment: Principles Modelling and Design; IWA Publishing: London, UK, 2008; pp. 33–52. [Google Scholar]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Lia, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Delil, A.D.; Köleli, N.; Dağhan, H.; Bahçeci, G. Recovery of heavy metals from canola (Brassica napus) and soybean (Glycine max) biomasses using electrochemical process. Environ. Technol. Innov. 2020, 17, 100559. [Google Scholar] [CrossRef]
- Dong, L.; Hou, L.; Wang, Z.; Gu, P.; Chen, G.; Jiang, R. A new function of spent activated carbon in BAC process: Removing heavy metals by ion exchange mechanism. J. Hazard. Mater. 2018, 359, 76–84. [Google Scholar] [CrossRef]
- Bauman, M.; Košak, A.; Lobnik, A.; Petrinić, I.; Luxbacher, T. Nanofiltration membranes modified with alkoxysilanes: Sur-face characterization using zeta-potential. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 110–117. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Nikouzad, S.K. A positively charged composite loose nanofiltration membrane for water purification from heavy metals. J. Membr. Sci. 2020, 611, 118205. [Google Scholar] [CrossRef]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Alibardi, L.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Impacts of coagula-tion-flocculation treatment on the size distribution and bioavailability of trace metals (Cu, Pb, Ni, Zn) in municipal wastewater. Water Res. 2018, 128, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, S.; Pan, S.-Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2020, 385, 123911. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Water purification using different waste fruit cortexes for the removal of heavy metals. J. Taibah Univ. Sci. 2016, 10, 700–708. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna, Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Gul, A.; Khaligh, N.G.; Julkapli, N.M. Surface modification of Carbon-Based Nanoadsorbents for the Advanced Wastewater Treatment. J. Mol. Struct. 2021, 1235, 130148. [Google Scholar] [CrossRef]
- Kegl, T.; Ban, I.; Lobnik, A.; Košak, A. Synthesis and characterization of novel γ-Fe2O3-NH4OH@SiO2(APTMS) nanoparticles for dysprosium adsorption. J. Hazard. Mater. 2019, 378, 120764. [Google Scholar] [CrossRef]
- Da’na, E.; Awad, A. Regeneration of spent activated carbon obtained from home filtration system and applying it for heavy metals adsorption. J. Environ. Chem. Eng. 2017, 5, 3091–3099. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, S.; Wang, J.; Yu, S.; Wang, Y. Amino-functionalized core-shell magnetic mesoporous composite microspheres for Pb (II) and Cd (II) removal. J. Environ. Sci. 2013, 25, 830–837. [Google Scholar] [CrossRef]
- Ahmadi, A.; Heidarzadeh, S.; Mokhtari, A.R.; Darezereshki, E.; Harouni, H.A. Optimization of heavy metal removal from aqueous solutions by maghemite (γ-Fe2O3) nanoparticles using response surface methodology. J. Geochem. Explor. 2014, 147, 151–158. [Google Scholar] [CrossRef]
- Qian, J.; Yang, T.; Zhang, W.; Lei, Y.; Zhang, C.; Ma, J.; Zhang, C. Preparation of NH2-Functionalized Fe2O3 and Its Chitosan Composites for the Removal of Heavy Metal Ions. Sustainability 2019, 11, 5186. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Kołodyńska, D.J.; Krukowska, P. Thomas. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363, ISSN 1385-8947. [Google Scholar] [CrossRef]
- Lo, S.-F.; Wang, S.-Y.; Tsai, M.-J.; Lin, L.-D. Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- Sujatha, S.; Sivarethinamohan, R. A critical review of Cr (VI) ion effect on mankind and its amputation through adsorption by activated carbon. Mater. Today Proc. 2021, 37, 1158–1162. [Google Scholar] [CrossRef]
- You, J.; Wang, L.; Zhao, Y.; Bao, W. A review of amino-functionalized magnetic nanoparticles for water treatment: Features and prospects. J. Clean. Prod. 2021, 281, 124668. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef]
- Zhao, Y.-G.; Shen, H.-Y.; Pan, S.-D.; Hu, M.-Q.; Xia, Q.-H. Preparation and characterization of ami-no-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium (VI) ions. J. Mater. Sci. 2010, 45, 5291–5301. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Colvin, V.L. Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Ritu, D. Ambashta, Mika Sillanpaa. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef]
- Mandel, K.; Hutter, F.; Gellermann, C.; Sextl, G. Modified Superparamagnetic Nanocomposite Microparticles for Highly Selective Hg(II) or Cu(II) Separation and Recovery from Aqueous Solutions. ACS Appl. Mater. Interfaces 2012, 4, 5633–5642. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, T.-J.; Yu, K.N.; Kim, B.G.; Park, S.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.-K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Jane Cypriyana, J.P.J.; Saigeetha, S.; Lavanya Agnes Angalene, J.; Samrot, A.V.; Suresh Kumar, S.; Ponniah, P.; Chakravarthi, S. Overview on toxicity of nanoparticles, its mechanism, models used in toxicity studies and disposal methods. Biocatal. Agric. Biotechnol. 2021, 36, 102117. [Google Scholar] [CrossRef]
- Douglas, L.A.; Baugher Brigitta, M.; Baugher Collen, R.; Schneider Duane, A.; Rahimian, K. Substituent effects on the sol-gel chemistry of organotrialkoxysilanes. Chem. Mater. Am. Chem. Soc. 2000, 12, 3624–3632. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, UK, 1990; ISBN 0-12-134970-5. [Google Scholar]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes: A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Colilla, M.; Vallet-Regí, M. Biocompatibility, Surface Engineering, and Delivery of Drugs, Genes and Other Molecules. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Zhang, J.; Zhai, S.; Li, S.; Xiao, Z.; Song, Y.; An, Q.; Tian, G. Pb (II) removal of Fe3O4@SiO2–NH2 core–shell nanomaterials prepared via a controllable sol–gel process. Chem. Eng. J. 2013, 215–216, 461–471. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Q.; Liu, B.; Hu, T.; Liu, W.; Yao, J. Green synthesis of tannin-hexamethylendiamine based adsorbents for efficient removal of Cr (VI). J. Hazard. Mater. 2018, 352, 27–35. [Google Scholar] [CrossRef]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K.; Rahman, S.M. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams, Intercomparison of Thermodynamic Databases, Geological Survey of Japan Open File Report No. 419, National Institute of Advanced Industrial Science and Technology, Research Center for Deep Geological Environments. May 2005. Available online: https://www.nrc.gov/docs/ML1808/ML18089A638.pdf (accessed on 30 August 2022).
- De Zoubov, N.; Pourbaix, M. Mercury. In Atlas of Electrochemical Equilibria in Aqueous Solutions; Pourbaix, M., Ed.; Section 15.3; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations, 2nd ed.; Kreiger: New York, NY, USA, 1986. [Google Scholar]
- Kipton J Powell; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H.; Hefter, G. Chemical Speciation of Environmentally Significant Heavy Metals with Inorganic Ligands, Part 1: The Hg2+-Cl−, OH−, CO32−, SO42−, and PO43− Aqueous Systems. Pure Appl. Chem. 2005, 77, 739–800. [Google Scholar] [CrossRef]
- Sun, M.; Li, P.; Jin, X.; Ju, X.; Yan, W.; Yuan, J.; Xing, C. Heavy metal adsorption onto graphene oxide, amino group on magnetic nanoadsorbents and application for detection of Pb (II) by strip sensor. Food Agric. Immunol. 2018, 29, 1053–1073. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Liu, Y.; Jiang, L.; Song, B.; Li, M.; Ding, Y. Adsorption of Cu(II), Pb(II), and Cd(II) ions from acidic aqueous solutions by diethylenetriaminepentaacetic acid-modified magnetic graphene oxide. J. Chem. Eng. Data 2017, 62, 407–416. [Google Scholar] [CrossRef]
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 mesoporous silica by Melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu(II), and Cd(II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Wang, X. Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans. 2011, 40, 10945–10952. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.N. Rapid removal and recovery of Pb(II) from wastewater by magnetic nano adsorbents. J. Hazard. Mater. 2010, 184, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Jawed, A.; Saxena, V.; Pandey, L.M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J. Water Process Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef]
- Yong-Mei, H.; Man, C.; Zhong-Bo, H. Effective removal of Cu (II) ions from aqueous solution by ami-no-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Morales, M.d.P.; Mazarío, E. Effect of the Surface Charge on the Adsorption Capacity of Chromium (VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis. Water 2019, 11, 2372. [Google Scholar] [CrossRef]
- Srikhaow, A.; Butburee, T.; Pon-On, W.; Srikhirin, T.; Uraisin, K.; Suttiponpanit, K.; Chaveanghong, S.; Smith, S.M. Efficient Mercury Removal at Ultralow Metal Concentrations by Cysteine Functionalized Carbon-Coated Magnetite. Appl. Sci. 2020, 10, 8262. [Google Scholar] [CrossRef]
- Mandela, K.; Huttera, F. The magnetic nanoparticle separation problem. Nano Today 2012, 7, 485–487. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nano-materials: A review. J. Hazard. Mater. 2019, 378, 120764. [Google Scholar] [CrossRef]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Jainae, K.; Sukpirom, N.; Fuangswasdi, S.; Unob, F. Adsorption of Hg (II) from aqueous solutions by thiol-functionalized polymer-coated magnetic particles. J. Ind. Eng. Chem. 2015, 23, 273–278. [Google Scholar] [CrossRef]
- Viltužnik, B.; Lobnik, A.; Košak, A. The removal of Hg (II) ions from aqueous solutions by using thi-ol-functionalized cobalt ferrite magnetic nanoparticles. J. Sol-Gel Sci. Technol. 2015, 74, 199–207. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, Z.; Zhang, Z.; Ren, L.; Tao, T.; Yang, R.; Guo, Z. Synthesis of Magnetic Fe3O4@C Nanoparticles Modified with–SO3H and –COOH Groups for Fast Removal of Pb2+, Hg2+, and Cd2+ Ions. Eur. J. Inorg. Chem. 2014, 2014, 3172–3177. [Google Scholar] [CrossRef]
- Marimón-Bolívar, W.; Tejeda-Benítez, L.; Herrera, A.P. Removal of mercury (II) from water using magnetic nanoparticles coated with amino organic ligands and yam peel biomass. Environ. Nanotechnol. Monit. Manag. 2018, 10, 486–493. [Google Scholar] [CrossRef]
- Maitlo, H.A.; Kim, K.-H.; Kumar, V.; Kim, S.; Park, J.-W. Nanomaterials-based treatment options for chromium in aqueous environments. Environ. Int. 2019, 130, 104748. [Google Scholar] [CrossRef] [PubMed]
- Nicola, R.; Costisor, O.; Ciopec, M.; Negrea, A.; Lazau, R.; Ianasi, C.; Piciorus, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Kakavandi, B.; Kalantary, R.R.; Jafari, A.J.; Nasseri, S.; Ameri, A.; Esrafili, A.; Azari, A. Pb (II) adsorption onto a magnetic composite of activated carbon and superparamagnetic Fe3O4 nanoparticles:Experimental and modeling study. CLEAN–Soil Air Water 2015, 43, 1157–1166. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, C.; Lü, T.; Zhao, H. Removal of Pb(II) Ions from Wastewater by Using Polyethyleneimine-Functionalized Fe3O4 Magnetic Nanoparticles. Appl. Sci. 2020, 10, 948. [Google Scholar] [CrossRef]
- Gutiérrez-López, D.N.; Flores-Alamo, M.C.; Carreño-de-León, M.C.; Solache-Rios, M.J. Removal of Pb (II) from aqueous solution by using microspheres of Zea mays rachis–sodium alginate by batch and column systems. Water Supply 2020, 20, 2133–2144. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, G.; Wang, L.; Wang, W.; Wang, Y.; Wang, H.; Xu, W. Efficient removal of lead ions from aqueous solution using C-TiO2 adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2021, 865, 012040. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr (VI) and Cu (II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Removal and recovery of Cr (VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- Baghani, A.N.; Mahvi, A.H.; Gholami, M.; Rastkari, N.; Delikhoon, M. One-Pot synthesis, characterization and adsorption studies of amine-functionalized magnetite nanoparticles for removal of Cr (VI) and Ni (II) ions from aqueous solution: Ki-netic, isotherm and thermodynamic studies. J. Environ. Health Sci. Eng. 2016, 14, 11. [Google Scholar] [CrossRef]
- Trang, V.T.; Tam, L.T.; van Quy, N.; Phan, V.N.; van Tuan, H.; Huy, T.Q.; Dinh, N.X.; Le, A.-T. Enhanced adsorption efficiency of inorganic chromium (VI) ions by using carbonencapsulated hematite nanocubes. J. Sci. Adv. Mater. Devices 2020, 5, 392–399. [Google Scholar] [CrossRef]
- Puszkarewicz, A.; Kaleta, J. Chromium (VI) Adsorption on Modified Activated Carbons. Appl. Sci. 2019, 9, 3549. [Google Scholar] [CrossRef]
- Bafrooee, A.A.T.; Panahi, H.A.; Moniri, E.; Miralinaghi, M.; Hasani, A.H. Removal of Hg2+ by carboxyl-terminated hyper-branched poly(amidoamine) dendrimers grafted superparamagnetic nanoparticles as an efficient adsorbent. Environ. Sci. Pollut. Res. 2020, 27, 9547–9567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, D.; Wu, G.; Yang, N.; Wu, A. Modifying Fe3O4 microspheres with rhodamine hydrazide for se-lective detection and removal of Hg2+ ion in water. J. Hazard. Mater. 2013, 244–245, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Shen, H.; Xu, Q.; Luo, J.; Hu, M. Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions. J. Colloid Interface Sci. 2012, 365, 204–212. [Google Scholar] [CrossRef]
- Morsi, R.E.; Al-Sabagh, A.M.; Moustafa, Y.M.; ElKholy, S.G.; Sayed, M.S. Polythiophene modified chitosan/magnetite nanocomposites for heavy metals and selective mercury removal. Egyption J. Pet. 2018, 27, 1077–1085. [Google Scholar] [CrossRef]
- Chen, D.; Awut, T.; Liu, B.; Ma, Y.; Wang, T.; Nurulla, I. Functionalized magnetic Fe3O4 nanoparticles for removal of heavy metal ions from aqueous solutions. E-Polym. 2016, 16, 313–322. [Google Scholar] [CrossRef]
- Hao, Y.; Pan, Y.; Du, Q.; Li, X.; Wang, X. Nanoparticle Fe3O4 magnetized activated carbon from Armeniaca sibirica shell for the adsorption of Hg(II) ions. BioResources 2021, 16, 6100–6120. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Y.; Wang, X.; Chen, Z.; Li, C. Competitive Adsorption of Cadmium(II) and Mercury(II) Ions from Aqueous Solutions by Activated Carbon from Xanthoceras sorbifolia Bunge Hull. J. Chem. 2016, 4326351. [Google Scholar] [CrossRef]
- Sereshti, H.; Gaikani, H.; Rashidi Nodeh, H. The effective removal of mercury ions (Hg2+) from water using cadmium sulfide nanoparticles doped in polycaprolactam nanofibers: Kinetic and equilibrium studies. J. Iran. Chem. Soc. 2018, 15, 743–751. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 2001; pp. 96–102. ISBN 0-201-61091-4. [Google Scholar]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. Mesoporous silica functionalized with 2-mercaptopyridine: Synthesis, characterization and employment for Hg (II) adsorption. Microporous Mesoporous Mater. 2006, 89, 58–68. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. 2-Mercaptothiazoline modified mesoporous silica for mercury removal from aqueous media. J. Hazard. Mater. 2006, 134, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Quintanilla, D.; del Hierro, I.; Fajardo, M.; Sierra, I. Preparation of 2-mercaptobenzothiazole-derivatized mesoporous silica and removal of Hg (II) from aqueous solution. J. Environ. Monit. 2006, 8, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zou, H. Removal of aqueous Hg (II) by thiol-functionalized nonporous silica microspheres prepared by one-step sol–gel method. RSC Adv. 2020, 10, 18534–18542. [Google Scholar] [CrossRef] [PubMed]
- Atul Kumar, M.; Sharma, A.; Maurya, I.K.; Thakur, A.; Kumar, S. Synthesis of ultra-small iron oxide and doped iron oxide nanostructures and their antimicrobial activities. J. Taibah Univ. Sci. 2019, 13, 280–285. [Google Scholar] [CrossRef]
- Smith, B. Infrared Spectral Interpretation, A Systematic Approach, CRC Press: Boca Raton, FL, USA, 1999; 288p.
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas-Solid Systems with Special Reference to the Determination of Surface-Area and Porosity. Pure Appl. Chem. 1985, 57, 603. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: San Diego, CA, USA, 1999; ISBN 0-12-598920-2. [Google Scholar]
- Zasonska, B.A.; Líškova, A.; Kuricova, M.; Tulinska, J.; Pop-Georgievski, O.; Čiampor, F.; Vavra, I.; Dušinska, M.; Ilavska, S.; Horvathova, M.; et al. Functionalized porous silica&maghemite core-shell nanoparticles for applications in medicine: Design, synthesis, and immunotoxicity. Croat. Med. J. 2016, 57, 165–178. [Google Scholar] [CrossRef]
- Sun, M.; Cheng, G.; Ge, X.; Chen, M.; Wang, C.; Lou, L.; Xu, X. Aqueous Hg (II) immobilization by chitosan stabilized magnetic iron sulfide nanoparticles. Sci. Total Environ. 2018, 621, 1074–1083. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Nanotechnology in Industrial Wastewater Treatment; IWA Publishing: Durham, NC, USA, 2014; Volume 13. [Google Scholar] [CrossRef]
- Dinker, M.K.; Kulkarni, P.S. Recent Advances in Silica-Based Materials for the Removal of Hexavalent Chromium: A Review. J. Chem. Eng. Data 2015, 60, 2521–2540. [Google Scholar] [CrossRef]
- Alqadami, A.; Naushad, M.; Abdalla, M.A.; Anhamad, T.; Alothman, Z.A.; Alshehri, S.M. Sythesis and characterization of Fe3O4@TSC nanocomposite: Highly efficient removal of toxic metal ions from aqueous medium. RSC Adv. 2016, 6, 22679–22689. [Google Scholar] [CrossRef]
- Gallios, G.P.; Vaclavikova, M. Removal of chromium (VI) from water streams: A thermodynamic study. Environ. Chem. Lett. 2008, 6, 235–240. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Seif, A.; Ahmadi, T.S.; Zargar, B.; Rashidi, F.; Rouzbahani, G.M. Thermodynamic and kinetic studies for the adsorption of Hg (II) by nano-TiO2 from aqueous solution. Adv. Powder Technol. 2012, 23, 148–156. [Google Scholar] [CrossRef]
- López-Muñoz, M.J.; Arencibia, A.; Cerro, L.; Pascual, R.; Melgar, Á. Adsorption of Hg (II) from aqueous solutions using TiO2 and titanate nanotube adsorbents. Appl. Surf. Sci. 2016, 367, 91–100. [Google Scholar] [CrossRef]




| Adsorbent | HM Ions | Tested pH | Adsorption Capacity | Desorption Efficiency | Reference |
|---|---|---|---|---|---|
| γ-Fe2O3 NPs | Pb2+ | 7.5 | 10.55 mg/g | - | [35] |
| Fe3O4@SiO2 NPs | 6.0 | 14.9 mg/g | 95.7% | [82] | |
| NH2-functionalized Fe2O3/chitosan NPs | 5.0 | 32.46 mg/g | - | [36] | |
| NH2-functionalized Fe2O3 NPs | 5.0 | 39.30 mg/g | - | [36] | |
| Magnetic composite of activated carbon and superparamagnetic Fe3O4 NPs (Fe3O4@C magnetic composite) | 6.0 | 41.7 mg/g | >77% | [83] | |
| NH2-functionalized γ-Fe2O3 NPs (γ-Fe2O3@NH2 NPs) | 4.0 | 53.5 mg/g | 90.7% | This work | |
| Amino-functionalized graphene oxide (GO-NH2) | 5.0 | 53.9 mg/g | - | [4] | |
| Fe3O4@SiO2–NH2 NPs | 6.2 | 0.37 mmol/g 76.66 mg/g * | - | [43] | |
| Amino-functionalized Fe3O4@mesoporous SiO2 core-shell composite microspheres | 5.5 | 82.29 mg/g | - | [35] | |
| NH2-functionalized γ-Fe2O3 NPs (γ-Fe2O3@NH2 NPs) | 7.0 | 83.6 mg/g | 91.3% | This work | |
| Polyethylenimine (PEI)-functionalized Fe3O4 magnetic nanoparticles (MNPs) | pH 5.0 | 60.98 mg/g | [84] | ||
| Composite beads of Zea mays rachis (ZMR) and sodium alginate (AL) | pH 5.0 | 60 mg/g | [85] | ||
| Carbon-doped TiO2 (C-TiO2) | pH 6.5 | 28.7 mg/g | [86] |
| Adsorbent | HM Ions | Tested pH | Adsorption Capacity | Desorption Efficiency | Reference |
|---|---|---|---|---|---|
| Magnetic magnetite NPs (Fe3O4) | CrT/Cr3+/Cr6+/Cr(VI) | 4.0 | 8.67 mg/g | >75% | [87] |
| Iron oxide magnetic NPs (MNPs) | 2.5 | 15.0 mg/g | ≅100% | [72] | |
| Maghemite NPs (γ-Fe2O3) | 2.5 | 19.2 mg/g | 87.7% | [88] | |
| NH2-functionalized γ-Fe2O3 NPs (γ-Fe2O3@NH2 NPs) | 4.0 | 24.0 mg/g | - | This work | |
| Amino-functionalized magnetite NPs (NH2-Fe3O4) | 3.0 | 24.25 mg/g | 98.02% | [89] | |
| APTES@TEOS@MNP | 2.5 | 35.0 mg/g | ≅100% | [72] | |
| NH2-functionalized nanomagnetic polymer adsorbents (EDA-NMPs) | 2.5 | 37.6 mg/g | - | [44] | |
| NH2-functionalized nanomagnetic polymer adsorbents (DETA-NMPs) | 2.5 | 37.9 mg/g | - | [44] | |
| NH2-functionalized nanomagnetic polymer adsorbents (TETA-NMPs) | 2.5 | 38.5 mg/g | - | [44] | |
| NH2-functionalized nanomagnetic polymer adsorbents (TEPA-NMPs) | 2.0 | 40.0 mg/g | - | [44] | |
| Amino-functionalized graphene oxide (GO-NH2) | 2.0 | 90.4 mg/g | - | [4] | |
| NH2-functionalized γ-Fe2O3 NPs (γ-Fe2O3@NH2 NPs) | 7.0 | 90.4 mg/g | 96.7% | This work | |
| Carbon-encapsulated hematite nanocubes (αFe2O3@C) | pH 3 | 76.92 mg/g | [90] | ||
| Activated carbons | pH 2 | 4.35 mg/g | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allwin Mabes Raj, A.F.P.; Bauman, M.; Lakić, M.; Dimitrušev, N.; Lobnik, A.; Košak, A. Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles. Int. J. Mol. Sci. 2022, 23, 16186. https://doi.org/10.3390/ijms232416186
Allwin Mabes Raj AFP, Bauman M, Lakić M, Dimitrušev N, Lobnik A, Košak A. Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles. International Journal of Molecular Sciences. 2022; 23(24):16186. https://doi.org/10.3390/ijms232416186
Chicago/Turabian StyleAllwin Mabes Raj, A. F. P., Maja Bauman, Marijana Lakić, Nena Dimitrušev, Aleksandra Lobnik, and Aljoša Košak. 2022. "Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles" International Journal of Molecular Sciences 23, no. 24: 16186. https://doi.org/10.3390/ijms232416186
APA StyleAllwin Mabes Raj, A. F. P., Bauman, M., Lakić, M., Dimitrušev, N., Lobnik, A., & Košak, A. (2022). Removal of Pb2+, CrT, and Hg2+ Ions from Aqueous Solutions Using Amino-Functionalized Magnetic Nanoparticles. International Journal of Molecular Sciences, 23(24), 16186. https://doi.org/10.3390/ijms232416186