Extracellular Vesicles as Biomarkers in Liver Disease
Abstract
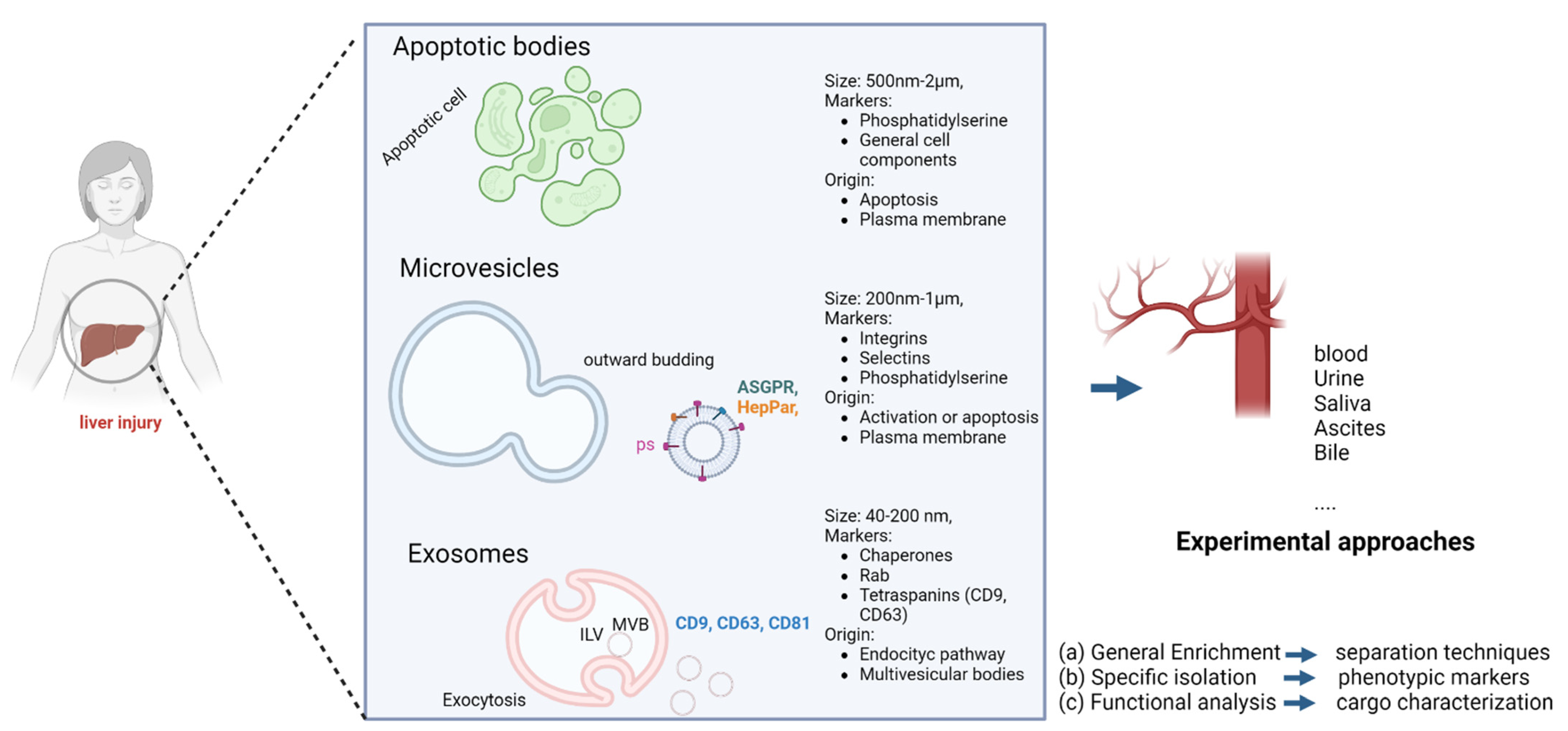
:1. Introduction
2. Biogenesis, Definition, and Classification of Extracellular Vesicles
3. Technology for Characterization and Isolation
4. Extracellular Vesicles as a Biomarker in Liver Disease
4.1. Non-Alcoholic Fatty Liver Disease (NAFLD)
4.2. Alcoholic Hepatitis
4.3. Viral Hepatitis
4.4. Fibrosis
4.5. Hepatobiliary Tumors: HCC and CCA
| Liver Disease | Surface Marker and/or Cargo | Sample Size | OUTCOMES | Methods | Ref. |
|---|---|---|---|---|---|
| NAFLD | CD14+ | NAFLD (n = 67); control (n = 44) | Patients with NAFL or NASH had significantly higher levels of CD14+ MVs (CD14+), which mediate the pathogenesis of NASH. | Flow cytometry | Kornek M. et al. Gastroenterology 2012. [6] |
| NAFLD | ASGR2 or CYP2E1 | NAFLD patients pre- and post-weight loss (n = 22); control (n = 6) | Plasma levels of EVs and hepatocyte-derived EVs are dynamic and decrease following NAFLD resolution due to weight loss surgery. | Nanoparticle tracking analysis | Nakao Y et al. Nanomedicine 2021 [8] |
| NASH with and without fibrosis | SLC27A5 ASGPR1 | Pre-cirrhotic NASH (n = 25); cirrhotic NASH (n = 25); control (n = 25) | Levels of ASGPR+ EVs were found to be increased 2-fold in pre-cirrhotic NASH and 3-fold in cirrhotic NASH compared to healthy controls. | Differential centrifugation, size exclusion; Chromatography and flow cytometry | Povero D et al. Hepatol Commun. 2022. [33] |
| Alcoholic hepatitis | miR-155 | Cirrhosis (n = 6); control (n = 5) | miR-155 as a mediator of alcohol-related regulation of autophagy and exosome production in hepatocytes and macrophages. | ExoQuick and nanoparticle tracking analysis | Babuta M at al. Hepatology 2019. [36] |
| Alcoholic hepatitis | miR-122 | ALD (n = 11) | Exosomes isolated from sera after alcohol consumption or from in vitro ethanol-treated hepatocytes contained miRNA-122. | Nanoparticle tracking analysis | Momen-Heravi F et al. Sci Rep. 2015. [38] |
| Alcoholic hepatitis | CYP2E1 | ALD (n = 14); control (n = 9) | Alcohol (ethanol) and/or its metabolites increased the amounts of EV proteins, including CYP2E1 and other P450 isoforms, that were secreted possibly from damaged hepatocytes. | Ultracentrifugation and ExoQuick | Cho YE et al. Hepatol Commun. 2017. [46] |
| Alcoholic hepatitis | CD3 CD4, CD68 CD11b, CD45 CD34, and ASGPR. | ALD (n = 101), 71 responders and 30 non-responders; control (n = 20) | Pre-therapy levels of CD34+ and ASGPR+ microvesicles are reliable non-invasive markers of steroid nonresponse and mortality in patients with severe alcoholic hepatitis. | Flow cytometry | Sukriti S et al. Aliment Pharmacol Ther. 2018. [49] |
| Alcoholic hepatitis | miR-155 | ALD (n = 8); control (n = 6) | The alcohol-related increase in number of circulating exosomes was observed in sera of human AH patients. | NanoSight and western blotting | Sehrawat TS, et al. Hepatology. 2021. [50] |
| Viral hepatitis | CD9, CD63, CD81/miR204-5p, miR181a-5p, miR143-3p, miR93-5p, miR122-5p | HCV (n = 16), before (T0) and after treatment (T6); control (n = 15) | Antifibrogenic miR204-5p, miR181a-5p, miR143-3p, miR93-5p, and miR122-5p were statistically underrepresented in T0 EVs compared to HD EVs, while miR204-5p and miR143-3p were statistically underrepresented in T6 EVs compared to control EVs. | Microbeads, proteomic, and western blot. | Montaldo C, et al. J Hepatol. 2021. [52] |
| Viral hepatitis | CD11a, CD14, CD147, and annexin V | Active hepatitis (n = 12); mild hepatitis (n = 10); and control (n = 8) | Patients with active hepatitis C had a significant increase in circulating MPs derived from CD4+ as well as CD8+ T cells compared to patients with mild hepatitis C and healthy controls, respectively. | Flow cytometry | Kornek et al. Hepatol. 2011. [53] |
| Viral hepatitis | CD31, CD41, and annexin V | HCV (n= 114) | Levels of both EMPs and PMPs decreased after sustained virological response and at follow-up. | Flow cytometry | Muñoz-Hernández R et al. Clin Transl Gastroenterol. 2020. [54] |
| Fibrosis | CD41a, CD42b, CD31, CD105, CD14, CD16, and CD284 | NAFLD with liver fibrosis (n = 26) | CD14+ and CD16+ EVs show potential capacity to predict liver fibrosis severity. | Flow cytometry | Welsh JA, et al. J Leukoc Biol 2018. [65] |
| Cirrhosis | CD31, CD41, CD235a+, and annexin V | Noninfected cirrhotic patients (n = 90); control (n = 10) | Microvesicle levels, mostly platelet-derived, were 2.5-fold higher in healthy volunteers compared with cirrhotic patients. Circulating small AV platelet-derived MV levels were lower in cirrhotic patients and inversely correlated with MELD score. | Flow cytometry | Weil D, et al. Clin Transl Gastroenterol. 2021 [66] |
| Cirrhosis | CD31, CD41, CD62, CD144, cytokeratin-18, and annexin V | Cirrhotic patients (n = 139) | Hepatocyte MV levels were 4.0-fold and 2.2-fold higher in patients with Child–Pugh C compared to those with Child–Pugh A or B liver disease, respectively.Hepatocyte MV levels correlated with HVPG but cannot identify patients with HVPG > 10 mmHG. Hepatocyte MV level > 65 U/L predicted 6-month mortality independently of Child–Pugh score and MELD score. | Flow cytometry and Elisa | Payancé A, et al. Hepatol. 2018. [67] |
| Hepatobiliary Tumors (HCC) | - | HCC patients (n = 55); cirrhosis (n = 40); and controls (n = 21) | MV levels were significantly reduced in the 1-month post-operative samples compared to those in the pre-operative samples. MV levels showed better performance than AFP for early detection of HCC. | Bicinchoninic acid assay | Wang W, et al. Cancer Biomark. 2013. [74] |
| Hepatobiliary Tumors (HCC and CCA) | EpCAM, CD147, ASGPR, CD133, and annexin V | Liver cancer (n = 172); cirrhosis (n = 54); and control (n = 202) | Annexin V+ EpCAM+ CD147+ taMPs were elevated in liver cancer (HCC and CCA). Annexin V+ EpCAM+ ASGPR1+ taMPs were increased in liver cancer compared to patients with cirrhosis. Annexin V+ EpCAM+ ASGPR1+ CD133+ taMPs allowed the distinction of liver malignancies. | Flow cytometry | Julich-Haertel H, et al. J Hepatol. 2017. [75] |
| Hepatobiliary Tumors (HCC) | EpCAM, CD63, CD147, GPC3, ASGPR 1 | Training cohort (n = 106) and validation cohort (n = 72) | EpCAM+ CD63+, CD147+ CD63+, and GPC3+ CD63+ were highly associated with early diagnosis of HCC (AUROC of 0.95 (95% CI = 0.90–0.99) with a sensitivity of 91% and a specificity of 90%). | Flow cytometry | Sun N, et al. Carcinoma. H Hepatol. 2022. [77] |
| Hepatobiliary Tumors (HCC) | PKH26 | HCC patients (n = 36); cirrhosis cohort (n = 26); NASH (n = 26); healthy donors (n = 38), (n = 23); HBV/HCV without liver cirrhosis (n = 25) | The HCC EV-derived molecular signatures exhibit great potential for noninvasive early detection of HCC from at-risk cirrhotic patients. | EV purification system (Click Chip), fluorescence microscopy, transmission electron microscopy and dynamic light scattering | Sun N, et al. Nature Comm. 2020. [78] |
| Hepatobiliary Tumors (HCC) | HepPar1+, CD144+, CD162+ | HCC patients (n = 15); liver cirrhosis (n = 5); and healthy controls (n = 5) | Levels of HepPar1+ MPs, measured before liver resection, were significantly higher in those who displayed early recurrence compared to those without recurrence. Endothelial-derived EVs (CD144+) or activated endothelial EVs (CD144+/CD62+) were not associated with HCC. | Flow cytometry | Abbate V, et al. Int J Mol Sci. 2017. [79] |
| Hepatobiliary Tumors (HCC) | miR-122, miR-148a, and miR-1246 | HCC patients (n = 5); liver cirrhosis (n = 5) | Serum exosomal level of miR-122, miR-148a, and miR-1246 was significantly higher in HCC than LC and normal control. | Transmission electron microscopy and western blot | Wang Y, et al. Med. 2018. [83] |
| Hepatobiliary Tumors (HCC) | miR-21 | HCC patients (n = 30); CHB patients (n = 30); healthy controls (n = 30) | miR-21 is enriched in serum exosomes which provides increased sensitivity for HCC detection than whole serum. | Transmission electron microscopy and western blot | Wang H, et al. Biomed Res Int. 2014. [79] |
| Hepatobiliary Tumors (HCC) | GRP78 and Asgr2 miR-10b-5Pp, miR-221-3p, miR-223-3p, miR-21-5p | HCV patients (n = 54); HBV patients (n = 40) HCC patients without HBV/HCV infection (n = 10) | Along with miR-21-5p, miR-10b-5p/miR-221-3p/miR-223-3p was found significantly upregulated in the exosome of HCC.Altered circulating hepatocyte-specific exosomal miRNAs were a risk factor for HCC development in both hepatitis B virus- and hepatitis C virus-infected patients. | NanoSight, transmission electron microscopy, and immune-blotting | Ghosh S, et al. Int J Cancer 2020. [86] |
| Hepatobiliary Tumors (HCC) | LINC00853 | HCC patients (n = 90); chronic hepatitis (n = 28); liver cirrhosis (n = 35); healthy controls (n = 29) | Levels of EV-LINC00853 were higher in HCC patients. EV-LINC00853 displayed excellent discriminatory ability in the diagnosis of all stages of HCC. | ExoQuick | Kim S et al. Mol Oncol. 2020. [87] |
| Hepatobiliary Tumors (CCA) | - | CCA patients (n = 5); pancreatic cancer (n = 20); nonmalignant (n = 15) | The median concentration of EVs was significantly higher in bile samples from patients with malignant common bile duct stenoses compare to controls or nonmalignant common bile duct stenoses. | NanoSight, transmission electron, and nanoparticle tracking analysis | Severino V et al. Gastroenterology 2017. [90] |
| Hepatobiliary Tumors (CCA) | CD9, CD63, CD81 | CCA patients (n = 43); HCC patients (n = 29); primary sclerosing cholangitis (PSC) (n = 30); and healthy control (n = 32). | Decrease in the EV size in CCA versus PSC patients.HCC patients showed a slight increase in serum EV concentration compared to the other three groups. The selection of biomarkers between CCA vs. control indicated that aminopeptidase N (AMPN), pantetheinase (VNN1), and polymeric immunoglobulin receptor (PIGR) show the best diagnostic capacity.Protein levels of VNN1, C-reactive protein (CRP), FIBG, IGHA1, A1AG1, and gamma-glutamyltransferase 1 are increased in serum EV of CCA patients compared to the other groups. | NanoSight, transmission electron, and nanoparticle tracking analysis | Arbelaiz A, et al. Hepatol. 2017 [91] |
5. EVs as Therapeutic Tool
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Zhu, H.; Wang, H. Extracellular Vesicles in Non-alcoholic Fatty Liver Disease and Alcoholic Liver Disease. Front. Physiol. 2021, 12, 707429. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazanave, S.C.; Mott, J.; Bronk, S.F.; Werneburg, N.W.; Fingas, C.D.; Meng, X.W.; Finnberg, N.; El-Deiry, W.; Kaufmann, S.; Gores, G.J. Death Receptor 5 Signaling Promotes Hepatocyte Lipoapoptosis. J. Biol. Chem. 2011, 286, 39336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornek, M.; Lynch, M.; Mehta, S.H.; Lai, M.; Exley, M.; Afdhal, N.H.; Schuppan, D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology 2012, 143, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimachi, K.; Matsumura, T.; Hirata, H.; Uchi, R.; Ueda, M.; Ueo, H.; Shinden, Y.; Iguchi, T.; Eguchi, H.; Shirabe, K.; et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br. J. Cancer 2015, 112, 532–538. [Google Scholar] [CrossRef]
- Nakao, Y.; Amrollahi, P.; Parthasarathy, G.; Mauer, A.S.; Sehrawat, T.S.; Vanderboom, P.; Nair, K.S.; Nakao, K.; Allen, A.M.; Hu, T.Y.; et al. Circulating extracellular vesicles are a biomarker for NAFLD resolution and response to weight loss surgery. Nanomedicine 2021, 36, 102430. [Google Scholar] [CrossRef]
- Sarin, S.K.; APASL ACLF Research Consortium (AARC) for APASL ACLF Working Party; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Hurley, J.H.; Boura, E.; Carlson, L.A.; Róycki, B. Membrane budding. Cell 2010, 143, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, M.; Raposo, G. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.M.; Salter, R.D. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J. Immunol. 2010, 185, 3740–3749. [Google Scholar] [CrossRef] [Green Version]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [Green Version]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Epple, L.M.; Griffiths, S.G.; Dechkovskaia, A.M.; Dusto, N.L.; White, J.; Ouellette, R.J.; Anchordoquy, T.J.; Bemis, L.; Graner, M.W. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS ONE 2012, 7, e42064. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, D.A.; Yankson, K.; Shukla, N.; Vilanilam, G.; Ticer, T.; Wolfram, J. Extracellular vesicle therapeutics for liver disease. J. Control. Release 2018, 273, 86–98. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Thery, C.; Clayton, A.; Amigorena, S.; Raposo, G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Chapter 3; Unit 3.22. [Google Scholar]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.T.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, R.; Ansa-Addo, E.; Stratton, D.; Antwi-Baffour, S.; Jorfi, S.; Kholia, S.; Krige, L.; Lange, S.; Inal, J. A filtration-based protocol to isolate human Plasma Membrane-derived Vesicles and exosomes from blood plasma. J. Immunol. Methods 2011, 371, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Inglis, H.; Norris, P.; Danesh, A. Techniques for the analysis of extracellular vesicles using flow cytometry. Cytometry A 2015, 87, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Soo, C.Y.; Song, Y.; Zheng, Y.; Campbell, E.C.; Riches, A.C.; Gunn-Moore, F.; Powis, S.J. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 2012, 136, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Kogej, K.; Božič, D.; Kobal, B.; Herzog, M.; Černe, K. Application of Dynamic and Static Light Scattering for Size and Shape Characterization of Small Extracellular Nanoparticles in Plasma and Ascites of Ovarian Cancer Patients. Int. J. Mol. Sci. 2021, 22, 12946. [Google Scholar] [CrossRef]
- Boyoglu, C.; He, Q.; Willing, G.; Boyoglu-Barnum, S.; Dennis, V.A.; Pillai, S.; Singh, S.R. Microscopic Studies of Various Sizes of Gold Nanoparticles and Their Cellular Localizations. ISRN Nanotechnol. 2013, 2013, 123838. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, D.; Nakao, Y.; Mauer, A.S.; Thompson, J.M.; Sehrawat, T.S.; Liao, C.Y.; Krishnan, A.; Lucien, F.; Guo, Q.; Liu, M.; et al. IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice with Steatohepatitis. Gastroenterology 2020, 159, 1487–1503.e17. [Google Scholar] [CrossRef]
- Povero, D.; Eguchi, A.; Niesman, I.R.; Andronikou, N.; de Mollerat du Jeu, X.; Mulya, A.; Berk, M.; Lazic, M.; Thapaliya, S.; Parola, M.; et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci. Signal. 2013, 6, ra88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Povero, D.; Yamashita, H.; Ren, W.; Subramanian, M.G.; Myers, R.P.; Eguchi, A.; Simonetto, D.A.; Goodman, Z.D.; Harrison, S.A.; Sanyal, A.J.; et al. Characterization and Proteome of Circulating Extracellular Vesicles as Potential Biomarkers for NASH. Hepatol. Commun. 2020, 4, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Mauer, A.S.; Lucien, F.; Raiter, A.; Bandla, H.; Mounajjed, T.; Yin, Z.; Glaser, K.J.; Yin, M.; et al. Characterization of Cellular Sources and Circulating Levels of Extracellular Vesicles in a Dietary Murine Model of Nonalcoholic Steatohepatitis. Hepatol. Commun. 2019, 3, 1235–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A.; Patters, B.J.; Kodidela, S.; Kumar, S. Extracellular Vesicles: Intercellular Mediators in Alcohol-Induced Pathologies. J. Neuroimmune Pharmacol. 2020, 15, 409–421. [Google Scholar] [CrossRef]
- Babuta, M.; Furi, I.; Bala, S.; Bukong, T.N.; Lowe, P.; Catalano, D.; Calenda, C.; Kodys, K.; Szabo, G. Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease. Hepatology 2019, 70, 2123–2141. [Google Scholar] [CrossRef]
- Bala, S.; Petrasek, J.; Mundkur, S.; Catalano, D.; Levin, I.; Ward, J.; Alao, H.; Kodys, K.; Szabo, G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012, 56, 1946–1957. [Google Scholar] [CrossRef] [Green Version]
- Momen-Heravi, F.; Bala, S.; Kodys, K.; Szabo, G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 2015, 5, 9991. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Shim, Y.R.; Seo, W.; Kim, M.H.; Choi, W.M.; Kim, H.H.; Kim, Y.E.; Yang, K.; Ryu, T.; Jeong, J.M.; et al. Mitochondrial Double-Stranded RNA in Exosome Promotes Interleukin-17 Production Through Toll-Like Receptor 3 in Alcohol-associated Liver Injury. Hepatology 2020, 72, 609–625. [Google Scholar] [CrossRef]
- Eguchi, A.; Yan, R.; Pan, S.Q.; Wu, R.; Kim, J.; Chen, Y.; Ansong, C.; Smith, R.D.; Tempaku, M.; Ohno-Machado, L.; et al. Comprehensive characterization of hepatocyte-derived extracellular vesicles identifies direct miRNA-based regulation of hepatic stellate cells and DAMP-based hepatic macrophage IL-1β and IL-17 upregulation in alcoholic hepatitis mice. J. Mol. Med. 2020, 98, 1021–1034. [Google Scholar] [CrossRef]
- Verma, V.K.; Li, H.; Wang, R.; Hirsova, P.; Mushref, M.; Liu, Y.; Cao, S.; Contreras, P.C.; Malhi, H.; Kamath, P.S.; et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J. Hepatol. 2016, 64, 651–660. [Google Scholar] [CrossRef]
- Saha, B.; Momen-Heravi, F.; Kodys, K.; Szabo, G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J. Biol. Chem. 2016, 291, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.; Momen-Heravi, F.; Furi, I.; Kodys, K.; Catalano, D.; Gangopadhyay, A.; Haraszti, R.; Satishchandran, A.; Iracheta-Vellve, A.; Adejumo, A.; et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology 2018, 67, 1986–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitkopf, K.; Haas, S.; Wiercinska, E.; Singer, M.V.; Dooley, S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol. Clin. Exp. Res. 2005, 29, 121S–131S. [Google Scholar] [CrossRef] [PubMed]
- Brandon-Warner, E.; Feilen, N.A.; Culberson, C.R.; Field, C.O.; Delemos, A.S.; Russo, M.W.; Schrum, L.W. Processing of miR17-92 Cluster in Hepatic Stellate Cells Promotes Hepatic Fibrogenesis During Alcohol-Induced Injury. Alcohol. Clin. Exp. Res. 2016, 40, 1430–1442. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.E.; Mezey, E.; Hardwick, J.P.; Salem, N.; Clemens, D.L.; Song, B.J. Increased ethanol-inducible cytochrome P450-2E1 and cytochrome P450 isoforms in exosomes of alcohol-exposed rodents and patients with alcoholism through oxidative and endoplasmic reticulum stress. Hepatol. Commun. 2017, 1, 675–690. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Morán, L.; Peng, J.; Salinas, B.; López-Alcántara, N.; Sydor, S.; Vilchez-Vargas, R.; Asensio, I.; Hao, F.; Zheng, K.; et al. Intestinal Epithelial Cell-Derived Extracellular Vesicles Modulate Hepatic Injury via the Gut-Liver Axis During Acute Alcohol Injury. Front. Pharmacol. 2020, 11, 603771. [Google Scholar] [CrossRef]
- Bissonnette, J.; Altamirano, J.; Devue, C.; Roux, O.; Payancé, A.; Lebrec, D.; Bedossa, P.; Valla, D.; Durand, F.; Ait-Oufella, H.; et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 2017, 66, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Sukriti, S.; Maras, J.S.; Bihari, C.; Das, S.; Vyas, A.K.; Sharma, S.; Hussain, S.; Shasthry, S.; Choudhary, A.; Premkumar, M.; et al. Microvesicles in hepatic and peripheral vein can predict nonresponse to corticosteroid therapy in severe alcoholic hepatitis. Aliment. Pharmacol. Ther. 2018, 47, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Sehrawat, T.S.; Arab, J.P.; Liu, M.; Amrollahi, P.; Wan, M.; Fan, J.; Nakao, Y.; Pose, E.; Navarro-Corcuera, A.; Dasgupta, D.; et al. Circulating Extracellular Vesicles Carrying Sphingolipid Cargo for the Diagnosis and Dynamic Risk Profiling of Alcoholic Hepatitis. Hepatology 2021, 73, 571–585. [Google Scholar] [CrossRef]
- Kakizaki, M.; Yamamoto, Y.; Yabuta, S.; Kurosaki, N.; Kagawa, T.; Kotani, A. The immunological function of extracellular vesicles in hepatitis B virus-infected hepatocytes. PLoS ONE 2018, 13, e0205886. [Google Scholar] [CrossRef]
- Montaldo, C.; Terri, M.; Riccioni, V.; Battistelli, C.; Bordoni, V.; D’Offizi, G.; Prado, M.G.; Trionfetti, F.; Vescovo, T.; Tartaglia, E.; et al. Fibrogenic signals persist in DAA-treated HCV patients after sustained virological response. J. Hepatol. 2021, 75, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Kornek, M.; Popov, Y.; Libermann, T.A.; Afdhal, N.H.; Schuppan, D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology 2011, 53, 230–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Hernández, R.; Ampuero, J.; Millán, R.; Gil-Gómez, A.; Rojas, Á.; Macher, H.C.; Gallego-Durán, R.; Gato, S.; Montero-Vallejo, R.; Rico, M.C.; et al. Hepatitis C Virus Clearance by Direct-Acting Antivirals Agents Improves Endothelial Dysfunction and Subclinical Atherosclerosis: HEPCAR Study. Clin. Transl. Gastroenterol. 2020, 11, e00203. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, K.; Ingram, A.; Austin, R.; Kapoor, A.; Tang, D.; Majeed, F.; Qureshi, T.; Al-Nedawi, K. Regulation of the tumor suppressor PTEN through exosomes: A diagnostic potential for prostate cancer. PLoS ONE 2013, 8, e70047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Targeted Drug Delivery to Hepatic Stellate Cells for the Treatment of Liver Fibrosis. J. Pharmacol. Exp. Ther. 2019, 370, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Seo, W.; Eun, H.S.; Kim, S.Y.; Yi, H.S.; Lee, Y.S.; Park, S.H.; Jang, M.J.; Jo, E.; Kim, S.C.; Han, Y.M.; et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology 2016, 64, 616–631. [Google Scholar] [CrossRef] [Green Version]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology 2016, 64, 2219–2233. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, E.; Perumal, E.; Subramaniyan, R.; Mustapha, N. Liver fibrosis: Extracellular vesicles mediated intercellular communication in perisinusoidal space. Hepatology 2022, 76, 275–285. [Google Scholar] [CrossRef]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology 2016, 150, 956–967. [Google Scholar] [CrossRef] [Green Version]
- Bruno, S.; Chiabotto, G.; Camussi, G. Extracellular Vesicles: A Therapeutic Option for Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 4255. [Google Scholar] [CrossRef]
- Huang, R.; Pan, Q.; Ma, X.; Wang, Y.; Liang, Y.; Dai, B.; Liao, X.; Li, M.; Miao, H. Hepatic Stellate Cell-Derived Microvesicles Prevent Hepatocytes from Injury Induced by APAP/H2O2. Stem Cells Int. 2016, 2016, 8357567. [Google Scholar] [CrossRef] [PubMed]
- Hirsova, P.; Ibrahim, S.H.; Verma, V.K.; Morton, L.A.; Shah, V.H.; LaRusso, N.F.; Gores, G.J.; Malhi, H. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: A novel paradigm for obesity-related liver disease. J. Surg. Res. 2014, 192, 268–275. [Google Scholar]
- Devhare, P.B.; Sasaki, R.; Shrivastava, S.; Di Bisceglie, A.M.; Ray, R.; Ray, R.B. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J. Virol. 2017, 91, e02225-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.A.; Scorletti, E.; Clough, G.F.; Englyst, N.A.; Byrne, C.D. Leukocyte extracellular vesicle concentration is inversely associated with liver fibrosis severity in NAFLD. J. Leukoc. Biol. 2018, 104, 631–639. [Google Scholar] [CrossRef]
- Weil, D.; Di Martino, V.; Mourey, G.; Biichle, S.; Renaudin, A.; Laheurte, C.; Cypriani, B.; Delabrousse, E.; Grandclément, E.; Thévenot, T.; et al. Small Annexin V-Positive Platelet-Derived Microvesicles Affect Prognosis in Cirrhosis: A Longitudinal Study. Clin. Transl. Gastroenterol. 2021, 12, e00333. [Google Scholar] [CrossRef]
- Payancé, A.; Silva-Junior, G.; Bissonnette, J.; Tanguy, M.; Pasquet, B.; Levi, C.; Roux, O.; Nekachtali, O.; Baiges, A.; Hernández-Gea, V.; et al. Hepatocyte microvesicle levels improve prediction of mortality in patients with cirrhosis. Hepatology 2018, 68, 1508–1518. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Xie, F.; Feng, S.; Yang, H.; Mao, Y. Extracellular vesicles in hepatocellular cancer and cholangiocarcinoma. Ann. Transl. Med. 2019, 7, 86. [Google Scholar] [CrossRef]
- Gandhi, C.R. Oxidative Stress and Hepatic Stellate Cells: A Paradoxical Relationship. Trends Cell Mol. Biol. 2012, 7, 1–10. [Google Scholar]
- Lee, Y.T.; Tran, B.V.; Wang, J.J.; Liang, I.Y.; You, S.; Zhu, Y.; Agopian, V.G.; Tseng, H.R.; Yang, J.D. The Role of Extracellular Vesicles in Disease Progression and Detection of Hepatocellular Carcinoma. Cancers 2021, 13, 3076. [Google Scholar] [CrossRef] [PubMed]
- Sberna, A.L.; Bouillet, B.; Rouland, A.; Brindisi, M.C.; Nguyen, A.; Mouillot, T.; Duvillard, L.; Denimal, D.; Loffroy, R.; Vergès, B.; et al. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver diseas. Diabet. Med. 2018, 35, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, H.; Zhou, Y.; Jie, S. Peripheral blood microvesicles are potential biomarkers for hepatocellular carcinoma. Cancer Biomark. 2013, 13, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Julich-Haertel, H.; Urban, S.K.; Krawczyk, M.; Willms, A.; Jankowski, K.; Patkowski, W.; Kruk, B.; Krasnodębski, M.; Ligocka, J.; Schwab, R.; et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2017, 67, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.A.; Bednarsch, J.; Joechle, K.; Amygdalos, I.; Czigany, Z.; Heij, L.; Ulmer, T.F.; Neumann, U.P. Prognostic biomarkers for cholangiocarcinoma (CCA): State of the art. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Zhang, C.; Lee, Y.; Tran, B.V.; Wang, J.; Kim, H.; Lee, J.; Zhang, R.Y.; Wang, J.J.; Hu, J.; et al. HCC EV ECG Score: An Extracellular Vesicle-based Protein Assay for Detection of Early-Stage Hepatocellular Carcinoma. Hepatology 2022. [Google Scholar] [CrossRef]
- Sun, N.; Lee, Y.-T.; Zhang, R.Y.; Kao, R.; Teng, P.-C.; Yang, Y.; Yang, P.; Wang, J.J.; Smalley, M.; Chen, P.-J.; et al. Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat. Commun. 2020, 11, 4489. [Google Scholar] [CrossRef]
- Abbate, V.; Marcantoni, M.; Giuliante, F.; Vecchio, F.M.; Gatto, I.; Mele, C.; Saviano, A.; Arciuolo, D.; Gaetani, E.; Ferrari, M.C.; et al. HepPar1-Positive Circulating Microparticles Are Increased in Subjects with Hepatocellular Carcinoma and Predict Early Recurrence after Liver Resection. Int. J. Mol. Sci. 2017, 18, 1043. [Google Scholar] [CrossRef] [Green Version]
- Lazar, S.; Goldfinger, L.E. Platelets and extracellular vesicles and their cross talk with cancer. Blood 2021, 137, 3192. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Bi, Y.; Kou, J.; Shi, J.; Piao, D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J. Exp. Clin. Cancer Res. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, C.; Zhang, P.; Guo, G.; Jiang, T.; Zhao, X.; Jiang, J.; Huang, X.; Tong, H.; Tian, Y. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018, 7, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hou, L.; Li, A.; Duan, Y.; Gao, H.; Song, X. Expression of Serum Exosomal MicroRNA-21 in Human Hepatocellular Carcinoma. Biomed. Res. Int. 2014, 2014, 864894. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.-P.; Wang, C.-Y.; Jin, X.-H.; Li, M.; Wang, F.-W.; Huang, W.-J.; Yun, J.-P.; Xu, R.-H.; Cai, Q.-Q.; Xie, D. Acidic microenvironment up-regulates exosomal mir-21 and mir-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics 2019, 9, 1965–1979. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Baek, G.O.; Ahn, H.R.; Sung, S.; Seo, C.W.; Cho, H.J.; Nam, S.W.; Cheong, J.Y.; Eun, J.W. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol. Oncol. 2020, 14, 2646–2659. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, L.; Wen, S.; Deng, D.; Wan, F.; He, X.; Tian, L.; Liang, L.; Wei, C.; Gao, K.; et al. RNA sequencing of plasma exosomes revealed novel functional long noncoding RNAs in hepatocellular carcinoma. Cancer Sci. 2020, 111, 3338–3349. [Google Scholar] [CrossRef]
- Yugawa, K.; Yoshizumi, T.; Mano, Y.; Itoh, S.; Harada, N.; Ikegami, T.; Kohashi, K.; Oda, Y.; Mori, M. Cancer-associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR-150-3p. Eur. J. Surg. Oncol. 2021, 47, 384–393. [Google Scholar] [CrossRef]
- Severino, V.; Dumonceau, J.-M.; Delhaye, M.; Moll, S.; Annessi-Ramseyer, I.; Robin, X.; Frossard, J.-L.; Farina, A. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology 2017, 153, 495–504.e8. [Google Scholar] [CrossRef] [Green Version]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Agüero, R.; Lacasta, A.; et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; Persano, S.; Shen, H.; Zhao, Y.; Blanco, E.; Ferrari, M.; Wolfram, J. Strategies for improving drug delivery: Nanocarriers and microenvironmental priming. Expert Opin. Drug Deliv. 2017, 14, 865–877. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Li, Y.; Hou, S.; Zhang, W.; Meng, Z.; Wang, S.; Jia, Q.; Tan, J.; Wang, R.; et al. Engineering a HEK-293T exosome-based delivery platform for efficient tumor-targeting chemotherapy/internal irradiation combination therapy. J. Nanobiotechnol. 2022, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.-X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Haga, H.; Yan, I.K.; Takahashi, K.; Matsuda, A.; Patel, T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl. Med. 2017, 6, 1262–1272. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef] [Green Version]
- Anger, F.; Camara, M.; Ellinger, E.; Germer, C.-T.; Schlegel, N.; Otto, C.; Klein, I. Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Improve Liver Regeneration After Ischemia Reperfusion Injury in Mice. Stem Cells Dev. 2019, 28, 1451–1462. [Google Scholar] [CrossRef]
- Povero, D.; Pinatel, E.M.; Leszczynska, A.; Goyal, N.P.; Nishio, T.; Kim, J.; Kneiber, D.; de Araujo Horcel, L.; Eguchi, A.; Ordonez, P.M.; et al. Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight 2019, 5, e125652. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Yao, X.; Jiang, T.; Wang, Y.; Xie, F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, R.; Kemper, S.; Brigstock, D.R. Extracellular Vesicles From Hepatocytes Are Therapeutic for Toxin-Mediated Fibrosis and Gene Expression in the Liver. Front. Cell Dev. Biol. 2020, 7, 368. [Google Scholar] [CrossRef]
- Fiore, E.; Domínguez, L.M.; Bayo, J.; Malvicini, M.; Atorrasagasti, C.; Rodriguez, M.; Cantero, M.J.; García, M.; Yannarelli, G.; Mazzolini, G. Human umbilical cord perivascular cells-derived extracellular vesicles mediate the transfer of IGF-I to the liver and ameliorate hepatic fibrogenesis in mice. Gene Ther. 2020, 27, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Panera, N.; Eguchi, A.; Johnson, C.D.; Papouchado, B.G.; de Araujo Horcel, L.; Pinatel, E.M.; Alisi, A.; Nobili, V.; Feldstein, A.E. Lipid-Induced Hepatocyte-Derived Extracellular Vesicles Regulate Hepatic Stellate Cells via MicroRNA Targeting Peroxisome Proliferator-Activated Receptor-γ. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, S.; Pasquino, C.; Sanchez, M.B.H.; Tapparo, M.; Figliolini, F.; Grange, C.; Chiabotto, G.; Cedrino, M.; Deregibus, M.C.; Tetta, C.; et al. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-alcoholic Steatohepatitis. Mol. Ther. 2020, 28, 479–489. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Hernández, R.; Rojas, Á.; Gato, S.; Gallego, J.; Gil-Gómez, A.; Castro, M.J.; Ampuero, J.; Romero-Gómez, M. Extracellular Vesicles as Biomarkers in Liver Disease. Int. J. Mol. Sci. 2022, 23, 16217. https://doi.org/10.3390/ijms232416217
Muñoz-Hernández R, Rojas Á, Gato S, Gallego J, Gil-Gómez A, Castro MJ, Ampuero J, Romero-Gómez M. Extracellular Vesicles as Biomarkers in Liver Disease. International Journal of Molecular Sciences. 2022; 23(24):16217. https://doi.org/10.3390/ijms232416217
Chicago/Turabian StyleMuñoz-Hernández, Rocío, Ángela Rojas, Sheila Gato, Javier Gallego, Antonio Gil-Gómez, María José Castro, Javier Ampuero, and Manuel Romero-Gómez. 2022. "Extracellular Vesicles as Biomarkers in Liver Disease" International Journal of Molecular Sciences 23, no. 24: 16217. https://doi.org/10.3390/ijms232416217





