Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice
Abstract
:1. Introduction
2. Results
2.1. Selection of Ethephon Concentration to Maximize Photosynthesis and Growth
2.2. Ethephon Reduces the Oxidative Stress Associated with High-Temperature Stress
2.3. Ethephon Accelerates Antioxidant Enzyme Activity under High-Temperature Stress
2.4. Effect of Ethephon on Proline, Nitrogen, and Sulfur Content, as Well as Nitrate Reductase Activity under High-Temperature Stress
2.5. Ethephon Promotes Photosynthesis, Growth, and Yield Attributes under High-Temperature Stress
2.6. Effect of Ethephon on Total Non-Structural Carbohydrate, Soluble Sugar, and Sucrose Contents When under High-Temperature Stress
2.7. Effect of Ethephon on the Calvin Cycle Enzymes under High-Temperature Stress
2.8. Effect of Ethephon on Sucrose Metabolism Enzymes under High-Temperature Stress
2.9. Effect of Ethephon on Starch Content and ADP-Glucose Pyrophosphorylase Activity
2.10. Effect of Ethephon on the Expression of Genes Encoding Core PSII Proteins
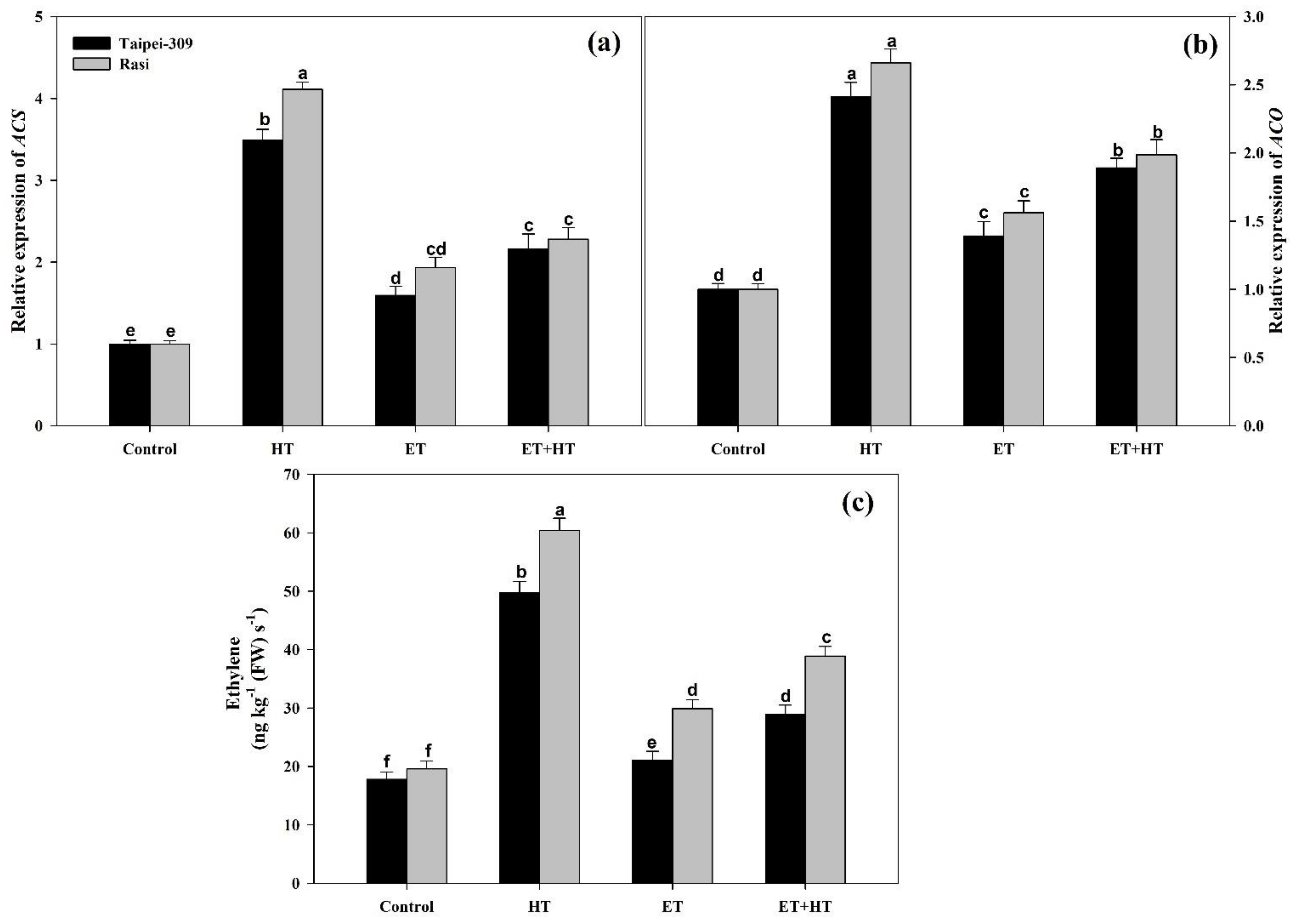
2.11. Effect of Ethephon on the Relative Expression of ACS and ACO, as Well as Ethylene Evolution, under High-Temperature Stress
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Material, Growth Conditions, and Experimental Design
4.3. Measurement of Photosynthetic Characteristics
4.4. Determination of Oxidative Stress Indicators
4.5. Estimation of Proline Content
4.6. Determination of Nitrogen and Sulfur Content
4.7. Determination of Nitrate Reductase Activity
4.8. Determination of Soluble Sugars, Sucrose, Starch and Total Non-Structural Carbohydrate Content
4.9. Determination of Growth and Yield Parameters
4.10. Determination of Antioxidant Enzyme Activities
4.11. Determination of Carbohydrate Metabolic Enzyme Activities
4.12. RNA Isolation and cDNA Synthesis
4.13. Quantitative Real-Time PCR Analysis
4.14. Estimation of Ethylene Evolution
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.R.; Singh, O. Study of impacts of global warming on climate change: Rise in sea level and disaster frequency. In Global Warming-Impacts and Future Perspective; Singh, B.R., Singh, O., Eds.; Intech Open: London, UK, 2012; p. 5816. [Google Scholar]
- Anderson, R.; E Bayer, P.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate change: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Masouleh, S.S.S.; Sassine, Y.N. Molecular and biochemical responses of horticultural plants and crops to heat stress. Ornam. Hortic. 2020, 26, 148–158. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Mohanty, S.; Tripathy, B.C. Role of Temperature Stress on Chloroplast Biogenesis and Protein Import in Pea. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Gautam, H.; Sehar, Z.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Khan, N.A. Nitric oxide enhances photosynthetic nitrogen and sulfur-use efficiency and activity of ascorbate-glutathione cycle to reduce high temperature stress-induced oxidative stress in rice (Oryza sativa L.) plants. Biomolecules 2021, 11, 305. [Google Scholar] [CrossRef]
- Prasad, P.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of Grain Sorghum to High Temperature Stress during Reproductive Development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Allakhverdiev, S.; Jajoo, A. Analysis of high temperature stress on the dynamics of antenna size and reducing side heterogeneity of Photosystem II in wheat leaves (Triticum aestivum). Biochim. Biophys. Acta 2011, 1807, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Haldimann, P.; Feller, U. Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2006, 120, 219–228. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Tewari, A.K.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme Temperature Responses, Oxidative Stress and Antioxidant Defense in Plants. In Abiotic Stress-Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; Intech Open: London, UK, 2013; pp. 169–205. [Google Scholar]
- Garris, A.J.; Tai, T.H.; Coburn, J.; Kresovich, S.; McCouch, S. Genetic Structure and Diversity in Oryza sativa L. Genetics 2005, 169, 1631–1638. [Google Scholar] [CrossRef] [Green Version]
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for Global Nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.; Datta, K.; Datta, S.K. Rice biofortification: High iron, zinc, and vitamin-A to fight against “hidden hunger”. Agronomy 2019, 9, 803. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V. High-Temperature Effects on Rice Growth, Yield, and Grain Quality. 2011, 111, 87–206. Adv. Agron. 2011, 111, 87–206. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Gourdji, S.M.; Sibley, A.M.; Lobell, D. Global crop exposure to critical high temperatures in the reproductive period: Historical trends and future projections. Environ. Res. Lett. 2013, 8, 024041. [Google Scholar] [CrossRef]
- Kondamudi, R.; Swamy, K.N.; Chakravarthy, D.V.N.; Vishnuprasanth, V.; Rao, Y.V.; Rao, P.R.; Voleti, S.R. Heat stress in rice-physiological mechanisms and adaptation strategies. In Crop Stress and its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 193–224. [Google Scholar]
- Suwa, R.; Hakata, H.; Hara, H.; El-Shemy, H.A.; Adu-Gyamfi, J.J.; Nguyen, N.T.; Fujita, K. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiol. Biochem. 2010, 48, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-X.; Feng, B.-H.; Chen, T.-T.; Fu, W.-M.; Li, H.-B.; Li, G.-Y.; Jin, Q.-Y.; Tao, L.-X.; Fu, G.-F. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ. Exp. Bot. 2018, 155, 718–733. [Google Scholar] [CrossRef]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, F.; Pantin, F.; Vile, D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant, Cell Environ. 2011, 34, 1563–1576. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Xia, S.; Wang, R.; Xiao, L. Phytohormonal quantification based on biological principles. 2017, 431–470. Horm. Metab. Signal. Plants 2017, 13, 431–470. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric oxide and hydrogen sulfide coordinately reduce glucose sensitivity and decrease oxidative stress via ascorbate-glutathione cycle in heat-stressed wheat (Triticum aestivum L.) plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; A Khan, N. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef] [Green Version]
- Haydari, M.; Maresca, V.; Rigano, D.; Taleei, A.; Shahnejat-Bushehri, A.A.; Hadian, J.; Sorbo, S.; Guida, M.; Manna, C.; Piscopo, M.; et al. Salicylic Acid and Melatonin Alleviate the Effects of Heat Stress on Essential Oil Composition and Antioxidant Enzyme Activity in Mentha × Piperita and Mentha Arvensis L. Antioxidants 2019, 8, 547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Prasad, P.V.; Siddique, K.H.; Nayyar, H. Plant growth-regulating molecules as thermoprotectants: Functional relevance and prospects for improving heat tolerance in food crops. J. Exp. Bot. 2019, 71, 569–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wen, C.-K. Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC, and ethephon. Plant Physiol. Biochem. 2010, 48, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Archambault, D.J.; Li, X.; Foster, K.R.; Jack, T.R. A Screening Test for the Determination of Ethylene Sensitivity. Environ. Monit. Assess. 2006, 115, 509–530. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, P.; Nagar, M.; Bharihoke, V.; Bhadoria, A.S. Ethephon, an organophosphorous, a fruit and vegetable ripener: Has potential hepatotoxic effects? J. Fam. Med. Prim. Care 2018, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I.R. Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front. Plant Sci. 2016, 7, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon mitigates nickel stress by modulat-ing antioxidant system, glyoxalase system and proline metabolism in Indian mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Per, T.S.; Khan, N.A. Ethephon increases photosynthetic-nitrogen use efficiency, proline and antioxidant metabolism to alleviate decrease in photosynthesis under salinity stress in mustard. Plant Signal. Behav. 2017, 12, e1297000. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N. Ethylene and Sulfur Coordinately Modulate the Antioxidant System and ABA Accumulation in Mustard Plants Under Salt Stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef]
- Ceusters, J.; de Poel, B.V. Ethylene exerts species-specific and age-dependent control of photosynthesis. Plant Physiol. 2018, 176, 2601–2612. [Google Scholar] [CrossRef] [Green Version]
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates’ stomatal aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef] [Green Version]
- Sehar, Z.; Iqbal, N.; Khan, M.I.R.; Masood, A.; Rehman, M.T.; Hussain, A.; AlAjmi, M.F.; Ahmad, A. Ethylene reduces glucose sensitivity and reverses photosynthetic repression through optimization of glutathione production in salt-stressed wheat (Triticum aestivum L.). Sci. Rep. 2021, 11, 12650. [Google Scholar] [CrossRef] [PubMed]
- Dusotoit-Coucaud, A.; Kongsawadworakul, P.; Maurousset, L.; Viboonjun, U.; Brunel, N.; Pujade-Renaud, V.; Chrestin, H.; Sakr, S. Ethylene stimulation of latex yield depends on the expression of a sucrose transporter (HbSUT1B) in rubber tree (Hevea brasiliensis). Tree Physiol. 2010, 30, 1586–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.H.; Fang, Y.J.; Fan, Y.J.; Wang, Y.; Qi, J.Y.; Tang, C.R. Expressional characterization of two class I trehalose-6-phosphate synthase genes in Hevea brasiliensis (para rubber tree) suggests a role in rubber production. New For. 2017, 48, 513–526. [Google Scholar] [CrossRef]
- Hemantaranjan, A.; Bhanu, A.N.; Singh, M.N.; Yadav, D.K.; Patel, P.K.; Singh, R.; Katiyar, D. Heat stress responses and thermotolerance. Adv. Plants Agric. Res. 2014, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xalxo, R.; Yadu, B.; Chandra, J.; Chandrakar, V.; Keshavkan, S. Alteration in carbohydrate metabolism modulates thermotolerance of plant under heat stress. In Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 77–115. [Google Scholar]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2011, 35, 259–270. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Mir, M.R.; Nazar, R.; Singh, S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol. 2008, 10, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Poór, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2021, 1–24. [Google Scholar] [CrossRef]
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.-S.P. Role of Ethylene and Its Cross Talk with Other Signaling Molecules in Plant Responses to Heavy Metal Stress. Plant Physiol. 2015, 169, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-S.; Yang, C.-Y. Ethylene-mediated signaling confers thermotolerance and regulates transcript levels of heat shock factors in rice seedlings under heat stress. Bot. Stud. 2019, 60, 1–12. [Google Scholar] [CrossRef]
- Jegadeesan, S.; Chaturvedi, P.; Ghatak, A.; Pressman, E.; Meir, S.; Faigenboim, A.; Rutley, N.; Beery, A.; Harel, A.; Weckwerth, W.; et al. Proteomics of Heat-Stress and Ethylene-Mediated Thermotolerance Mechanisms in Tomato Pollen Grains. Front. Plant Sci. 2018, 9, 1558. [Google Scholar] [CrossRef] [Green Version]
- Stearns, J.C.; Glick, B.R. Transgenic plants with altered ethylene biosynthesis or perception. Biotechnol. Adv. 2003, 21, 193–210. [Google Scholar] [CrossRef]
- Öztürk, L.; Demir, Y. Effects of putrescine and ethephon on some oxidative stress enzyme activities and proline content in salt stressed spinach leaves. Plant Growth Regul. 2003, 40, 89–95. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Jahan, B.; Alajmi, M.F.; Rehman, M.T.; Khan, N.A. Exogenously-sourced ethylene modulates defense mechanisms and promotes tolerance to zinc stress in mustard (Brassica juncea L.). Plants 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Maggio, A.; Miyazaki, S.; Veronese, P.; Fujita, T.; Ibeas, J.I.; Damsz, B.; Bressan, R.A. Does proline accumulation play an active role in stress-induced growth reduction? Plant J. 2002, 31, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Resurreccion, A.P.; Makino, A.; Bennett, J.; Mae, T. Effects of sulfur nutrition on the growth and photosynthesis of rice. Soil Sci. Plant Nutr. 2001, 47, 611–620. [Google Scholar] [CrossRef]
- Lunde, C.; Zygadlo, A.; Simonsen, H.T.; Nielsen, P.L.; Blennow, A.; Haldrup, A. Sulfur starvation in rice: The effect on photosynthesis, carbohydrate metabolism, and oxidative stress protective pathways. Physiol. Plantar. 2008, 134, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ashraf, M.; Hafeez, M. Thermotolerance of Pearl Millet and Maize at Early Growth Stages: Growth and Nutrient Relations. Biol. Plant. 2004, 48, 81–86. [Google Scholar] [CrossRef]
- Shah, N.; Paulsen, G. Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 2003, 257, 219–226. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, L.; Zhu, S.; Hou, J.; Chen, G.; Liu, F.; Liu, S.; Wang, C. Influence of heat stress on leaf morphology and nitrogen–carbohydrate metabolisms in two wucai (Brassica campestris L.) genotypes. Acta Soc. Bot. Pol. 2017, 86. [Google Scholar] [CrossRef]
- Cicchino, M.A.; Edreira, J.I.R.; Otegui, M.E. Maize Physiological Responses to Heat Stress and Hormonal Plant Growth Regulators Related to Ethylene Metabolism. Crop. Sci. 2013, 53, 2135–2146. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolás, E.; Torrecillas, A.; Sánchez-Blanco, M. High-Temperature Preconditioning and Thermal Shock Imposition Affects Water Relations, Gas Exchange and Root Hydraulic Conductivity in Tomato. Biol. Plant. 2003, 46, 203–208. [Google Scholar] [CrossRef]
- Pérez, P.; Alonso, A.; Zita, G.; Morcuende, R.; Martínez-Carrasco, R. Down-regulation of Rubisco activity under combined increases of CO2 and temperature minimized by changes in Rubisco kcat in wheat. Plant Growth Regul. 2011, 65, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Djanaguiraman, M.; Sheeba, J.A.; Devi, D.D.; Bangarusamy, U. Cotton Leaf Senescence can be Delayed by Nitrophenolate Spray Through Enhanced Antioxidant Defence System. J. Agron. Crop. Sci. 2009, 195, 213–224. [Google Scholar] [CrossRef]
- Goetz, M.; Guivarc’H, A.; Hirsche, J.; Bauerfeind, M.A.; González, M.-C.; Hyun, T.K.; Eom, S.H.; Chriqui, D.; Engelke, T.; Großkinsky, D.K.; et al. Metabolic Control of Tobacco Pollination by Sugars and Invertases. Plant Physiol. 2016, 173, 984–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-W.; Zhou, Z.-Q.; Yang, H.-X.; Wei, C.-X.; Wan, Y.-Y.; Wang, X.-J.; Bai, J.-G. Glucose application protects chloroplast ultrastructure in heat-stressed cucumber leaves through modifying antioxidant enzyme activity. Biol. Plant. 2015, 59, 131–138. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, P.; Fu, W.; Li, G.; Feng, B.; Chen, T.; Li, H.; Tao, L.; Fu, G. Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ. 2020, 43, 1273–1287. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Y.; Zhou, W.; Zhang, C. Effects of exogenously supplied sucrose on OsSUTs and OsSPSs transcript abundances and rice root ammonium assimilation. Acta Physiol. Plantar. 2016, 38, 1–15. [Google Scholar] [CrossRef]
- Lafta, A.M.; Lorenzen, J.H. Effect of High Temperature on Plant Growth and Carbohydrate Metabolism in Potato. Plant Physiol. 1995, 109, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of arsenic-induced oxidative stress and protein metabolism by diphenyleneiodonium, 24-epibrassinolide and proline in Glycine max L. Acta Bot. Croat. 2018, 77, 51–61. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Huang, B. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bentgrass cultivars. J. Am. Soc. Hort. Sci. 2000, 125, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.W.; Nie, Y.X.; Wan, Y.Y.; Chen, S.Y.; Sun, Y.; Wang, X.J.; Bai, J.G. Exogenous glucose regulates activities of antioxidant enzyme, soluble acid invertase and neutral invertase and alleviates dehydration stress of cucumber seedlings. Sci. Horticul. 2013, 162, 20–30. [Google Scholar] [CrossRef]
- Marias, D.E.; Meinzer, F.C.; Still, C. Impacts of leaf age and heat stress duration on photosynthetic gas exchange and foliar nonstructural carbohydrates in Coffea arabica. Ecol. Evol. 2017, 7, 1297–1310. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Prathap, V.; Tyagi, A. Correlation between expression and activity of ADP glucose pyrophosphorylase and starch synthase and their role in starch accumulation during grain filling under drought stress in rice. Plant Physiol. Biochem. 2020, 157, 239–243. [Google Scholar]
- Thitisaksakul, M.; Jiménez, R.C.; Arias, M.C.; Beckles, D.M. Effects of environmental factors on cereal starch biosynthesis and composition. J. Cereal Sci. 2012, 56, 67–80. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Activities of starch synthetic enzymes and contents of endogenous hormones in waxy maize grains subjected to post-silking water deficit. Sci. Rep. 2019, 9, 7059. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Fan, J.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Effects of ethylene on photosystem II and antioxidant enzyme activity in Bermuda grass under low temperature. Photosynth. Res. 2016, 128, 59–72. [Google Scholar] [CrossRef]
- Kim, J.; Chang, C.; Tucker, M.L. To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- Smet, D.; Depaepe, T.; Vandenbussche, F.; Callebert, P.; Nijs, I.; Ceulemans, R.; Van Der Straeten, D. The involvement of the phytohormone ethylene in the adaptation of Arabidopsis rosettes to enhanced atmospheric carbon dioxide concentrations. Environ. Exp. Bot. 2020, 177, 104128. [Google Scholar] [CrossRef]
- Yang, S.F.; E Hoffman, N. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Yang, S.F. Ethylene Evolution From 2-Chloroethylphosphonic Acid. Plant Physiol. 1969, 44, 1203–1204. [Google Scholar] [CrossRef]
- Biddle, E.; Kerfoot, D.G.S.; Kho, Y.H.; Russell, K.E. Kinetic Studies of the Thermal Decomposition of 2-Chloroethylphosphonic Acid in Aqueous Solution. Plant Physiol. 1976, 58, 700–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt Increase in the Level of Hydrogen Peroxide in Leaves of Winter Wheat Is Caused by Cold Treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lindner, R.C. Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol. 1944, 19, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnin, L.; Yien, C.H. Turbidimetric Determination of Available Sulfates. Soil Sci. Soc. Am. J. 1950, 15, 149–151. [Google Scholar] [CrossRef]
- Kuo, T.-M.; Warner, R.L.; Kleinhofs, A. In vitro stability of nitrate reductase from barley leaves. Phytochemistry 1982, 21, 531–533. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plantar. 2015, 37, 9. [Google Scholar] [CrossRef]
- Kuai, J.; Liu, Z.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Oosterhuis, D.M. Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relation ship with boll weight. Plant Sci. 2014, 223, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Usuda, H. The Activation State of Ribulose 1,5-bisphosphate Carboxylase in Maize Leaves in Dark and Light. Plant Cell Physiol. 1985, 26. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rao, I.M.; Terry, N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet: I. Changes in growth, gas exchange, and Calvin cycle enzymes. Plant Physiol. 1989, 90, 814–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalwade, S.B.; Devarumath, R.M. Functional Analysis of the Potential Enzymes Involved in Sugar Modulation in High and Low Sugarcane Cultivars. Appl. Biochem. Biotechnol. 2013, 172, 1982–1998. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowski, L.A.; Villand, P.; Luthi, E.; Olsen, O.A.; Preiss, J. Insensitivity of Barley Endosperm ADP-Glucose Pyrophosphorylase to 3-Phosphoglycerate and Orthophosphate Regulation. Plant Physiol. 1993, 101, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Turano, F.J.; Thakkar, S.S.; Fang, T.; Weisemann, J.M. Characterization and Expression of NAD(H)-Dependent Glutamate Dehydrogenase Genes in Arabidopsis. Plant Physiol. 1997, 113, 1329–1341. [Google Scholar] [CrossRef] [Green Version]






| Ethephon (mM) | Cultivars | Net Photosynthesis (µmol CO2 m−2 s−1) | Stomatal Conductance (mol m−2 s−1) | Intercellular CO2 Concentration (µmol CO2 mol−1) | SPAD Value | Maximal Quantum Yield of PSII Efficiency (Fv/Fm) |
|---|---|---|---|---|---|---|
| 0 | Taipei-309 | 16.8 ± 0.8 de | 0.33 ± 0.011 efg | 193.8 ± 11.5 bcd | 37.5 ± 1.24 cde | 0.804 ± 0.020 bcd |
| Rasi | 15.2 ± 0.7 ef | 0.27 ± 0.007 hi | 187.2 ± 10.0 cd | 34.7 ± 1.15 de | 0.791 ± 0.019 cde | |
| 0.4 | Taipei-309 | 19.9 ± 0.9 c | 0.37 ± 0.015 cde | 215.6 ± 14.2 abcd | 39.1 ± 1.28 cd | 0.830 ± 0.033 abc |
| Rasi | 18.7 ± 0.8 cd | 0.31 ± 0.011 fgh | 198.5 ± 11.9 bcd | 35.9 ± 1.20 de | 0.811 ± 0.032 bcd | |
| 0.8 | Taipei-309 | 21.2 ± 1.0 bc | 0.42 ± 0.019 b | 229.3 ± 15.7 ab | 40.9 ± 1.38 abc | 0.859 ± 0.021 abc |
| Rasi | 19.1 ± 0.9 cd | 0.35 ± 0.012 def | 219.7 ± 14.5 abc | 37.2 ± 1.22 cde | 0.826 ± 0.020 abc | |
| 1.2 | Taipei-309 | 23.6 ± 1.1 ab | 0.48 ± 0.021 a | 240.7 ± 16.4 a | 42.7 ± 1.40 ab | 0.871 ± 0.017 ab |
| Rasi | 20.4 ± 1.0 bc | 0.39 ± 0.016 bcd | 227.5 ±15.5 ab | 38.4 ± 1.26 cd | 0.841 ± 0.016 abc | |
| 1.6 | Taipei-309 | 25.3 ± 1.2 a | 0.50 ± 0.023 a | 251.6 ± 16.5 a | 44.2 ± 1.44 a | 0.892 ± 0.022 a |
| Rasi | 21.8 ± 1.0 bc | 0.41 ± 0.017 bc | 231.9 ± 16.4 ab | 39.6 ± 1.32 bc | 0.853 ± 0.021 abc | |
| 2.0 | Taipei-309 | 14.4 ± 0.7 ef | 0.29 ± 0.009 gh | 187.8 ± 10.0 cd | 33.8 ± 1.17 e | 0.776 ± 0.019 de |
| Rasi | 12.7 ± 0.6 f | 0.23 ±0.005 i | 176.4 ± 9.7 d | 28.5 ± 1.12 f | 0.759 ± 0.019 e |
| Ethephon (mM) | Cultivars | Plant Height (cm) | Shoot Fresh Weight (g Plant−1) | Root Fresh Weight (g Plant−1) | Shoot Dry Weight (g Plant−1) | Root Dry Weight (g Plant−1) |
|---|---|---|---|---|---|---|
| 0 | Taipei-309 | 38.2 ± 1.28 cd | 1.88 ± 0.05 abc | 0.65 ± 0.016 cde | 0.44 ± 0.011 cd | 0.068 ± 0.0017 cd |
| Rasi | 35.7 ± 1.10 d | 1.81 ± 0.03 cd | 0.57 ± 0.014 g | 0.37 ± 0.009 f | 0.057 ± 0.0014 fg | |
| 0.4 | Taipei-309 | 39.5 ± 1.33 cd | 1.90 ± 0.06 abc | 0.67 ± 0.026 bcd | 0.46 ± 0.018 bc | 0.070 ± 0.0028 c |
| Rasi | 36.8 ± 1.15 d | 1.84 ± 0.04 cd | 0.59 ± 0.023 fg | 0.39 ± 0.015 ef | 0.059 ± 0.0023 ef | |
| 0.8 | Taipei-309 | 41.7 ± 1.36 abc | 1.94 ± 0.06 abc | 0.68 ± 0.017 abc | 0.48 ± 0.012 ab | 0.073 ± 0.0018 bc |
| Rasi | 37.4 ± 1.22 d | 1.86 ± 0.04 bc | 0.60 ± 0.015 efg | 0.40 ± 0.010 ef | 0.061 ± 0.0015 ef | |
| 1.2 | Taipei-309 | 42.9 ± 1.39 ab | 1.99 ± 0.06 ab | 0.71 ± 0.014 ab | 0.49 ± 0.009 ab | 0.077 ± 0.0015 ab |
| Rasi | 38.7 ± 1.24 cd | 1.90 ± 0.06 abc | 0.62 ± 0.012 defg | 0.41 ± 0.008 de | 0.062 ± 0.0012 ef | |
| 1.6 | Taipei-309 | 43.6 ± 1.42 a | 2.03 ± 0.07 a | 0.73 ± 0.018 a | 0.51 ± 0.012 a | 0.079 ± 0.0019 a |
| Rasi | 39.3 ± 1.31 bcd | 1.92 ± 0.06 abc | 0.63 ± 0.015 cde | 0.42 ± 0.010 de | 0.064 ± 0.0016 de | |
| 2.0 | Taipei-309 | 31.4 ± 1.21 e | 1.63 ± 0.05 de | 0.51 ± 0.012 h | 0.33 ± 0.008 g | 0.052 ± 0.0013 g |
| Rasi | 27.5 ± 1.06 f | 1.51 ± 0.02 e | 0.42 ± 0.010 i | 0.26 ± 0.006 h | 0.041 ± 0.0010 h |
| Cultivars | Treatments | H2O2 Content | TBARS Content | SOD Activity | APX Activity | GR Activity |
|---|---|---|---|---|---|---|
| (nmol g−1 FW) | (U min−1 mg−1 Protein) | |||||
| Taipei-309 | Control | 31.8 ± 1.70 d | 12.4 ± 0.32 de | 7.89 ± 0.32 de | 1.35 ± 0.04 fg | 0.191 ± 0.005 ef |
| HT | 94.6 ± 4.30 b | 24.8 ± 1.17 b | 10.2 ± 0.49 c | 1.94 ± 0.09 e | 0.25 ± 0.005 d | |
| ET | 22.3 ± 1.32 e | 8.2 ± 0.23 f | 12.8 ± 0.52 b | 2.85 ± 0.12 c | 0.346 ± 0.011 b | |
| ET+HT | 44.7 ± 2.10 c | 14.1 ± 0.44 cd | 15.9 ± 0.56 a | 3.72 ± 0.13 a | 0.43 ± 0.01 a | |
| Rasi | Control | 35.1 ± 1.99 d | 13.1 ± 0.37 de | 6.92 ± 0.27 e | 1.18 ± 0.03 g | 0.172 ± 0.004 f |
| HT | 112.8 ± 5.02 a | 31.5 ± 1.22 a | 8.43 ± 0.34 d | 1.62 ± 0.07 f | 0.21 ± 0.005 e | |
| ET | 29.9 ± 1.59 de | 11.2 ± 0.28 e | 10.8 ± 0.41 c | 2.35 ± 0.10 d | 0.306 ± 0.009 c | |
| ET+HT | 52.4 ± 3.01 c | 15.8 ± 0.60 c | 13.7 ± 0.51 b | 3.14 ± 0.11 b | 0.368 ± 0.010 b | |
| Cultivars | Treatments | Nitrogen Content | Sulfur Content | Proline Content (mg g−1 FW) | Nitrate Reductase Activity (U min−1 mg−1 Protein) |
|---|---|---|---|---|---|
| (mg g−1 DW) | |||||
| Taipei-309 | Control | 33.8 ± 1.36 cd | 4.16 ± 0.22 bc | 13.6 ± 0.59 ef | 38.0 ± 1.45 c |
| HT | 25.3 ± 1.28 e | 3.25 ± 0.13 d | 18.1 ± 0.75 d | 29.9 ± 1.30 d | |
| ET | 45.4 ± 1.45 a | 4.83 ± 0.28 a | 24.5 ± 0.92 b | 52.7 ± 1.75 a | |
| ET+HT | 40.2 ± 1.40 b | 4.51 ± 0.24 ab | 28.7 ± 1.14 a | 46.5 ± 1.69 b | |
| Rasi | Control | 30.7 ± 1.34 d | 3.52 ± 0.17 cd | 11.8 ± 0.42 f | 33.0 ± 1.34 d |
| HT | 22.3 ± 1.24 e | 2.39 ± 0.10 e | 15.4 ± 0.66 e | 25.0 ± 1.25 e | |
| ET | 40.4 ± 1.42 b | 3.98 ± 0.20 bc | 20.7 ± 0.82 c | 44.2 ± 1.60 b | |
| ET+HT | 36.2 ± 1.37 bc | 3.71 ± 0.19 cd | 23.7 ± 0.89 b | 39.4 ± 1.49 c | |
| Cultivars | Treatments | No. of Tillers per Plant | No. of Panicle per Plant | Panicle Length (cm) | No. of Grains per Panicle | 1000 Grain Weight (g) |
|---|---|---|---|---|---|---|
| Taipei-309 | Control | 9.5 ± 0.45 bc | 7.7 ± 0.36 bc | 22.5 ± 0.91 ab | 117.6 ± 3.90 abc | 19.4 ± 0.97 bcd |
| HT | 7.7 ± 0.40 de | 6.1 ± 0.30 de | 20.4 ± 0.85 bc | 96.4 ± 2.34 de | 16.5 ± 0.65 de | |
| ET | 11.9 ± 0.61 a | 9.6 ± 0.47 a | 25.9 ± 1.08 a | 140.9 ± 4.67 a | 23.9 ± 1.33 a | |
| ET+HT | 11.1 ± 0.58 ab | 8.9 ± 0.41 ab | 24.9 ± 1.04 ab | 135.7 ± 4.31 ab | 22.4 ± 1.20 ab | |
| Rasi | Control | 8.7 ± 0.40 cd | 6.9 ± 0.25 cd | 20.7 ± 0.75 bc | 110.4 ± 2.91 bcd | 18.9 ± 0.86 cd |
| HT | 6.9 ± 0.35 e | 5.3 ± 0.20 e | 18.6 ± 0.65 c | 89.0 ± 1.94 e | 15.2 ± 0.51 e | |
| ET | 10.6 ± 0.50 ab | 8.4 ± 0.40 b | 23.3 ± 0.90 abc | 130.1 ± 3.92 ab | 22.9 ± 1.23 a | |
| ET+HT | 9.9 ± 0.47 bc | 7.8 ± 0.32 bc | 22.7 ± 0.87 abc | 124.6 ± 3.56 bc | 21.4 ± 1.15 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 1031. https://doi.org/10.3390/ijms23031031
Gautam H, Fatma M, Sehar Z, Iqbal N, Albaqami M, Khan NA. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. International Journal of Molecular Sciences. 2022; 23(3):1031. https://doi.org/10.3390/ijms23031031
Chicago/Turabian StyleGautam, Harsha, Mehar Fatma, Zebus Sehar, Noushina Iqbal, Mohammed Albaqami, and Nafees A. Khan. 2022. "Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice" International Journal of Molecular Sciences 23, no. 3: 1031. https://doi.org/10.3390/ijms23031031
APA StyleGautam, H., Fatma, M., Sehar, Z., Iqbal, N., Albaqami, M., & Khan, N. A. (2022). Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. International Journal of Molecular Sciences, 23(3), 1031. https://doi.org/10.3390/ijms23031031











