Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Results
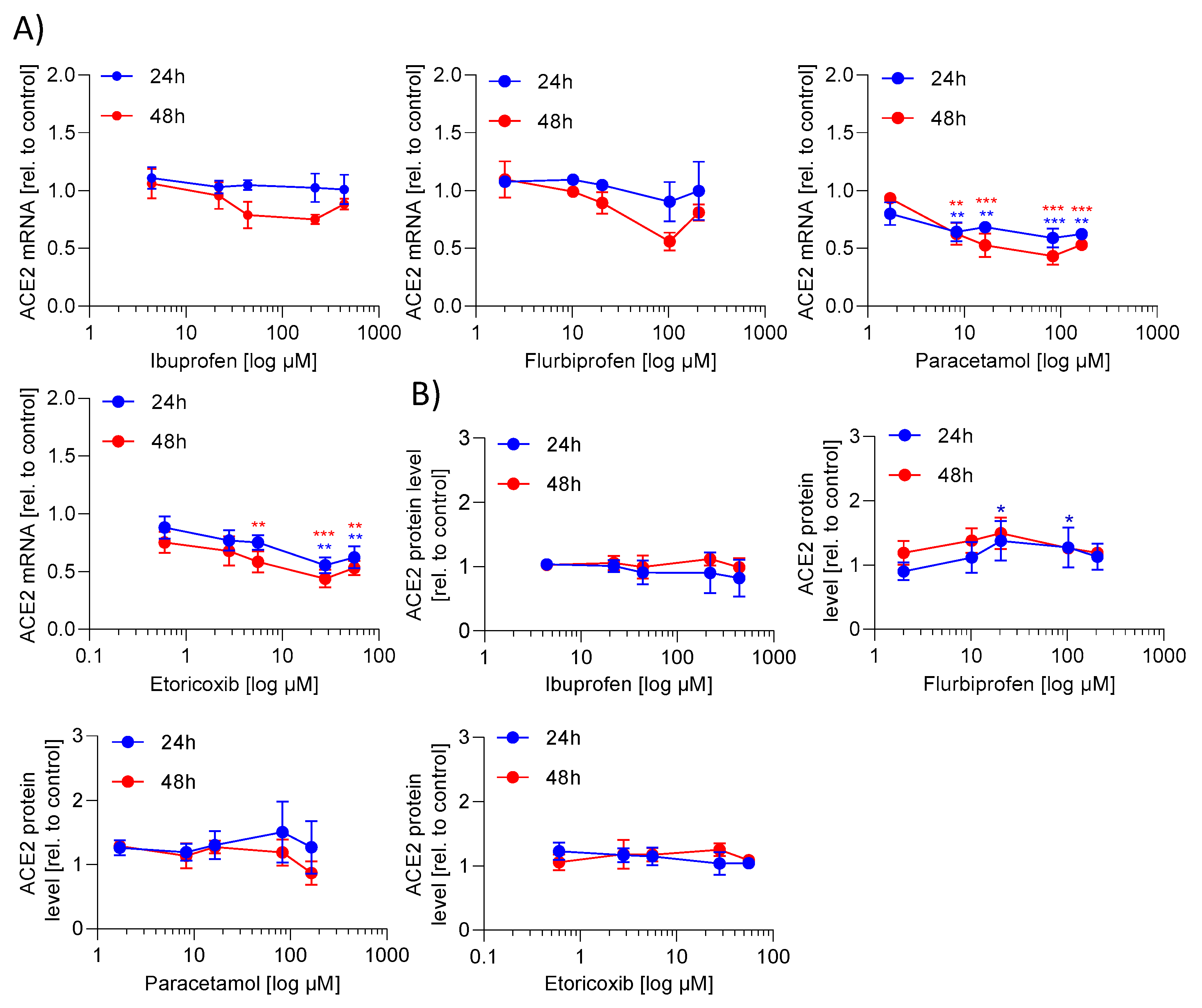
2.1. ACE2 mRNA and Protein Expression Is Not Regulated by NSAIDs and Paracetamol
2.2. ACE2 Activity Is Not Regulated by NSAIDs and Paracetamol
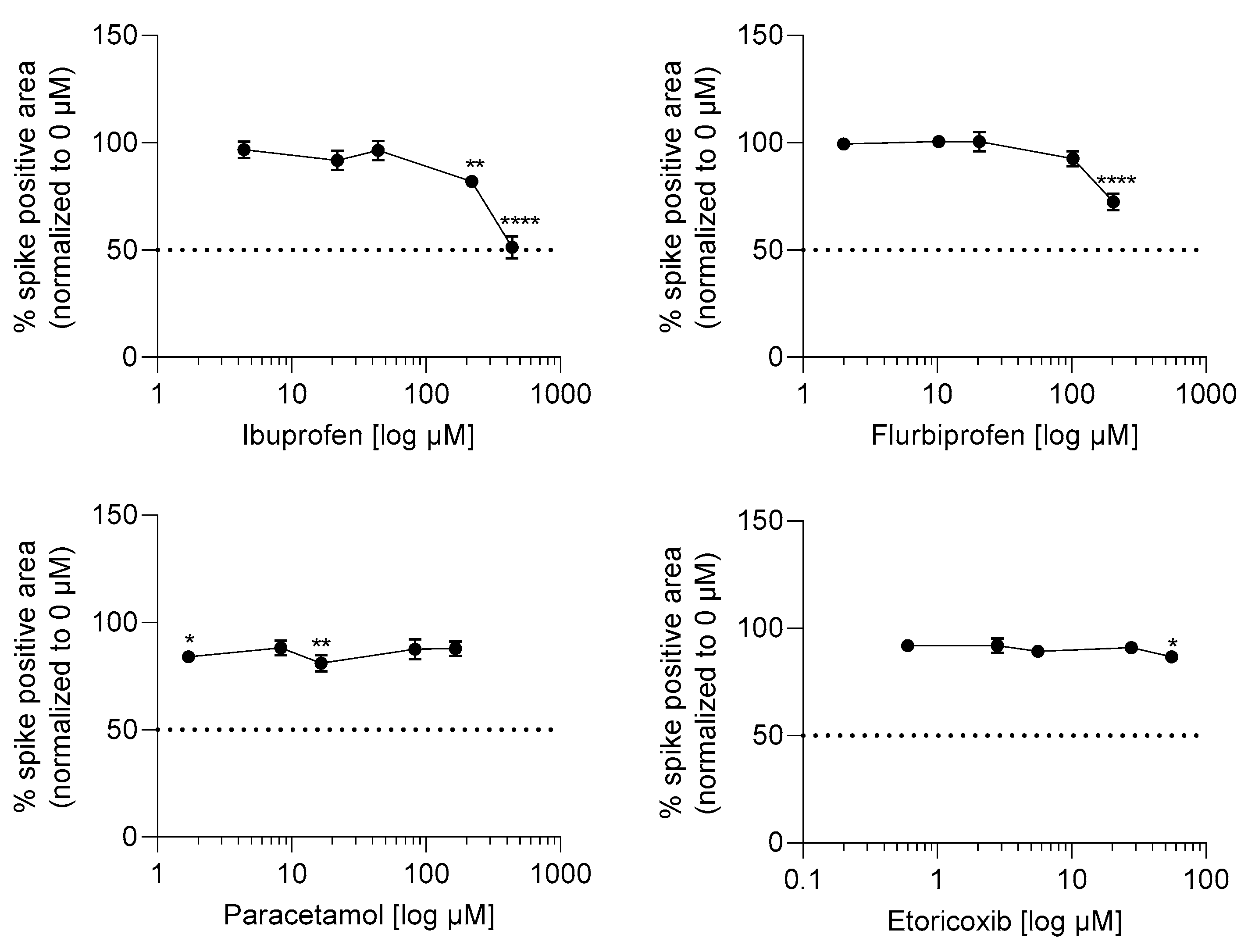
2.3. Ibuprofen and Flurbiprofen Reduced Infection Potential of SARS-CoV-2 at High Concentrations
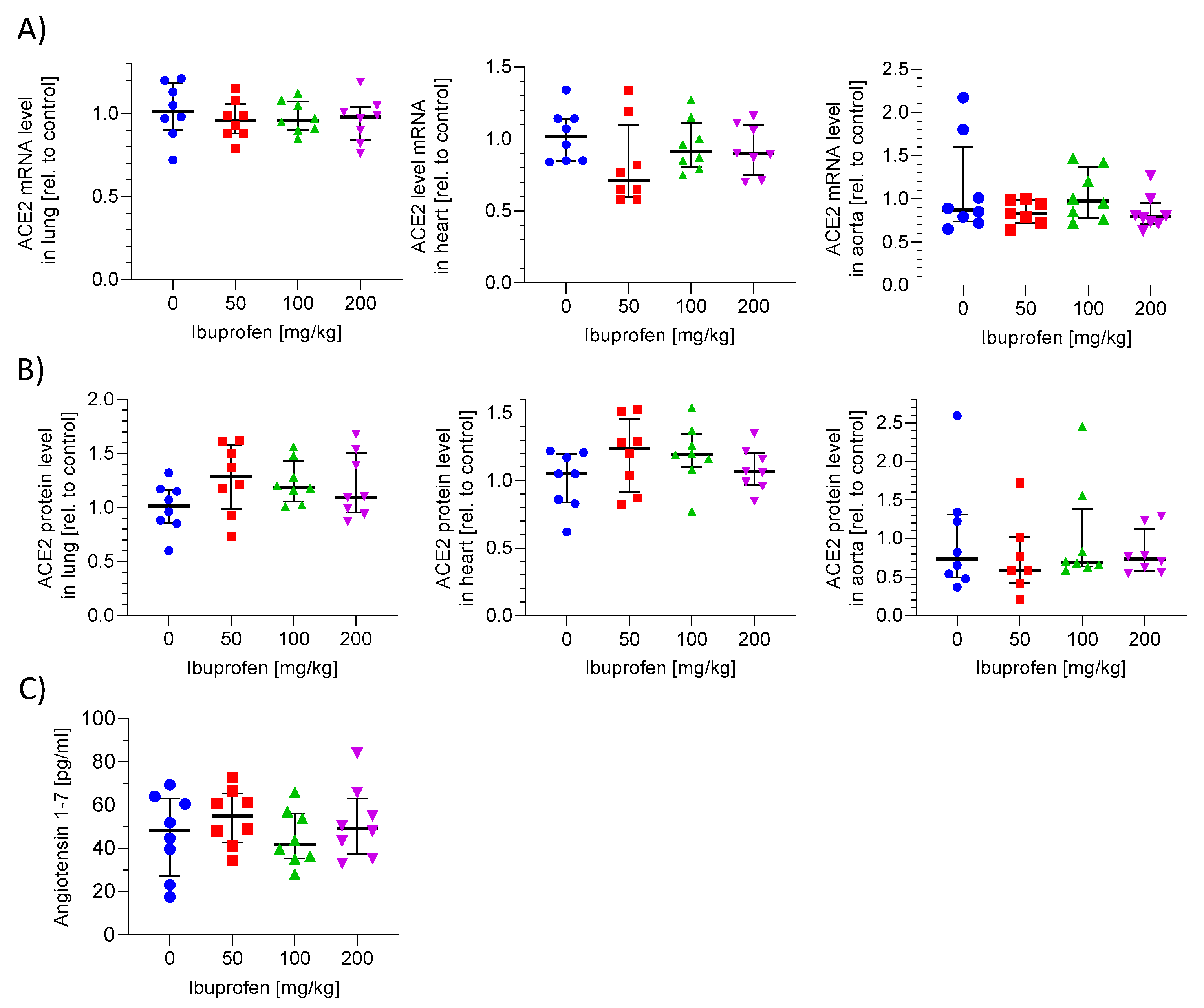
2.4. ACE2 mRNA and Protein Expression in Murine Lung, Heart and Aorta Is Not Influenced by Ibuprofen
2.5. ACE2 Activity in Plasma Is Not Altered by Ibuprofen Treatment
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Determination of mRNA Expression
4.3. Determination of Protein Expression
4.4. ACE2 Activity Assay of Soluble and Membrane Bound ACE2
4.5. Animal Study
4.5.1. Test Animals
4.5.2. Housing Conditions
4.5.3. Treatment Schedule
4.5.4. Sacrifice of the Animals and Blood Plasma and Organ Collection
4.6. Determination of Angiotensin 1–7 in Plasma
4.7. Infection Assay by Spike Protein Immunostaining
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Day, M. COVID-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ 2020, 368, m1086. [Google Scholar] [CrossRef] [Green Version]
- Moore, N.; Carleton, B.; Blin, P.; Bosco-Levy, P.; Droz, C. Does Ibuprofen Worsen COVID-19? Drug Saf. 2020, 43, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Clarke, N.; Wang, Z.; Fan, D.; Parajuli, N.; Basu, R.; Putko, B.; Kassiri, Z.; Turner, A.J.; Oudit, G.Y. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: A positive feedback mechanism in the RAS. J. Mol. Cell Cardiol. 2014, 66, 167–176. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.V.; Pfeffer, M.A.; Solomon, S.D. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Munch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Smart, L.; Fawkes, N.; Goggin, P.; Pennick, G.; Rainsford, K.D.; Charlesworth, B.; Shah, N. A narrative review of the potential pharmacological influence and safety of ibuprofen on coronavirus disease 19 (COVID-19), ACE2, and the immune system: A dichotomy of expectation and reality. Inflammopharmacology 2020, 28, 1141–1152. [Google Scholar] [CrossRef]
- Chen, J.S.; Alfajaro, M.M.; Wei, J.; Chow, R.D.; Filler, R.B.; Eisenbarth, S.C.; Wilen, C.B. Cyclooxgenase-2 is induced by SARS-CoV-2 infection but does not affect viral entry or replication. bioRxiv 2020. [Google Scholar] [CrossRef]
- Scarpignato, C. Piroxicam-beta-cyclodextrin: A GI safer piroxicam. Curr. Med. Chem. 2013, 20, 2415–2437. [Google Scholar] [CrossRef] [Green Version]
- Janssen, G.M.; Venema, J.F. Ibuprofen: Plasma concentrations in man. J. Int. Med. Res. 1985, 13, 68–73. [Google Scholar] [CrossRef]
- Szpunar, G.J.; Albert, K.S.; Bole, G.G.; Dreyfus, J.N.; Lockwood, G.F.; Wagner, J.G. Pharmacokinetics of flurbiprofen in man. I. Area/dose relationships. Biopharm. Drug Dispos. 1987, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pawasauskas, J.; Pergolizzi, J.V., Jr.; Lu, L.; Chen, Y.; Wu, S.; Jarrett, B.; Fain, R.; Hill, L.; Devarakonda, K. Pharmacokinetics of Oral and Intravenous Paracetamol (Acetaminophen) When Co-Administered with Intravenous Morphine in Healthy Adult Subjects. Clin. Drug Investig. 2018, 38, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, J.K.; Reynolds, J.K.; Remsberg, C.M.; Vega-Villa, K.R.; Davies, N.M. Clinical pharmacokinetic and pharmacodynamic profile of etoricoxib. Clin. Pharmacokinet. 2008, 47, 703–720. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Mishra, M.; Salemi, M.R.; Phinney, B.S.; Newens, J.L.; Gomes, A.V. Gender-specific changes in energy metabolism and protein degradation as major pathways affected in livers of mice treated with ibuprofen. Sci. Rep. 2020, 10, 3386. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Wang, C.; Chen, B.; Zhang, F.; Liu, Y.; Lu, Q.; Guo, H.; Yan, C.; Sun, H.; Hu, G.; et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology 2015, 131, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Pedrosa, M.A.; Garrido-Gil, P.; Labandeira, C.M.; Navarro, G.; Franco, R.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Interactions between ibuprofen, ACE2, renin-angiotensin system, and spike protein in the lung. Implications for COVID-19. Clin. Transl. Med. 2021, 11, e371. [Google Scholar] [CrossRef]
- Gallelli, L.; Galasso, O.; Urzino, A.; Sacca, S.; Falcone, D.; Palleria, C.; Longo, P.; Corigliano, A.; Terracciano, R.; Savino, R.; et al. Characteristics and clinical implications of the pharmacokinetic profile of ibuprofen in patients with knee osteoarthritis. Clin. Drug Investig. 2012, 32, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Kondo, E.; Kawaguchi, Y.; Kitamura, N.; Yasuda, K. Flurbiprofen concentration in soft tissues is higher after topical application than after oral administration. Bri. J. Clin. Pharmacol. 2013, 75, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Janssen, A.; Maier, T.J.; Schiffmann, S.; Coste, O.; Seegel, M.; Geisslinger, G.; Grosch, S. Evidence of COX-2 independent induction of apoptosis and cell cycle block in human colon carcinoma cells after S- or R-ibuprofen treatment. Eur. J. Pharmacol. 2006, 540, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, N.; Singh, R.; Rajput, S.P.; Saquib, Q. SARS-CoV-2: Understanding the Transcriptional Regulation of ACE2 and TMPRSS2 and the Role of Single Nucleotide Polymorphism (SNP) at Codon 72 of p53 in the Innate Immune Response against Virus Infection. Int. J. Mol. Sci. 2021, 22, 8660. [Google Scholar] [CrossRef]
- Lodhi, N.; Kossenkov, A.V.; Tulin, A.V. Bookmarking promoters in mitotic chromatin: Poly(ADP-ribose)polymerase-1 as an epigenetic mark. Nucleic Acids Res. 2014, 42, 7028–7038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, N.E.; Jaramillo, S.A.; Jones, A.N.; Vazquez, A.J.; Martz, M.; Versluis, L.M.; Raniere, M.O.; Nunnally, H.E.; Zarn, K.E.; Nottingham, R.; et al. Stenoparib, an Inhibitor of Cellular Poly(ADP-Ribose) Polymerase, Blocks Replication of the SARS-CoV-2 and HCoV-NL63 Human Coronaviruses In Vitro. mBio 2021, 12, e03495-20. [Google Scholar] [CrossRef]
- Nitulescu, G.M.; Paunescu, H.; Moschos, S.A.; Petrakis, D.; Nitulescu, G.; Ion, G.N.D.; Spandidos, D.A.; Nikolouzakis, T.K.; Drakoulis, N.; Tsatsakis, A. Comprehensive analysis of drugs to treat SARSCoV2 infection: Mechanistic insights into current COVID19 therapies (Review). Int. J. Mol. Med. 2020, 46, 467–488. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Lazureanu, V.; Coricovac, D.; Mioc, M.; Oancea, R.; Marcovici, I.; Pinzaru, I.; Soica, C.; Tsatsakis, A.M.; Cretu, O. SARS-CoV-2: Repurposed Drugs and Novel Therapeutic Approaches-Insights into Chemical Structure-Biological Activity and Toxicological Screening. J. Clin. Med. 2020, 9, 2084. [Google Scholar] [CrossRef]
- Terrier, O.; Dilly, S.; Pizzorno, A.; Chalupska, D.; Humpolickova, J.; Boura, E.; Berenbaum, F.; Quideau, S.; Lina, B.; Feve, B.; et al. Antiviral Properties of the NSAID Drug Naproxen Targeting the Nucleoprotein of SARS-CoV-2 Coronavirus. Molecules 2021, 26, 2593. [Google Scholar] [CrossRef]
- Drake, T.M.; Fairfield, C.J.; Pius, R.; Knight, S.R.; Norman, L.; Girvan, M.; Hardwick, H.E.; Docherty, A.B.; Thwaites, R.S.; Openshaw, P.J.M.; et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: A matched, prospective cohort study. Lancet Rheumatol. 2021, 3, e498–e506. [Google Scholar] [CrossRef]
- Moore, N.; Bosco-Levy, P.; Thurin, N.; Blin, P.; Droz-Perroteau, C. NSAIDs and COVID-19: A Systematic Review and Meta-analysis. Drug Saf. 2021, 44, 929–938. [Google Scholar] [CrossRef]
- Reus, P.; Schneider, A.K.; Ulshofer, T.; Henke, M.; Bojkova, D.; Cinatl, J.; Ciesek, S.; Geisslinger, G.; Laux, V.; Grattinger, M.; et al. Characterization of ACE Inhibitors and AT1R Antagonists with Regard to Their Effect on ACE2 Expression and Infection with SARS-CoV-2 Using a Caco-2 Cell Model. Life (Basel) 2021, 11, 810. [Google Scholar] [CrossRef]
- Xiao, F.; Burns, K.D. Measurement of Angiotensin Converting Enzyme 2 Activity in Biological Fluid (ACE2). Methods Mol. Biol. 2017, 1527, 101–115. [Google Scholar] [CrossRef]
- Mortensen, R.; Clemmensen, H.S.; Woodworth, J.S.; Therkelsen, M.L.; Mustafa, T.; Tonby, K.; Jenum, S.; Agger, E.M.; Dyrhol-Riise, A.M.; Andersen, P. Cyclooxygenase inhibitors impair CD4 T cell immunity and exacerbate Mycobacterium tuberculosis infection in aerosol-challenged mice. Commun. Biol. 2019, 2, 288. [Google Scholar] [CrossRef] [Green Version]
- Collier, A.F.; Gumerson, J.; Lehtimaki, K.; Puolivali, J.; Jones, J.W.; Kane, M.A.; Manne, S.; O’Neill, A.; Windish, H.P.; Ahtoniemi, T.; et al. Effect of Ibuprofen on Skeletal Muscle of Dysferlin-Null Mice. J. Pharmacol. Exp. Ther. 2018, 364, 409–419. [Google Scholar] [CrossRef]
- Bwire, G.M. Coronavirus: Why Men are More Vulnerable to COVID-19 Than Women? SN Compr. Clin. Med. 2020, 2, 874–876. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B.; Momen-Heravi, M. An Insight into the Sex Differences in COVID-19 Patients: What are the Possible Causes? Prehosp. Disaster Med. 2020, 35, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, A.; Bernard, R.; Gilis, A.; Gerlach, B.; Guillen, J.; Castagne, V.; Lefevre, I.A.; Ducrey, F.; Monk, L.; Bongiovanni, S.; et al. Introduction to the EQIPD quality system. Elife 2021, 10, e63294. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence |
|---|---|
| human ACE-for | 5′-AATGGGTCTTCAGTGCTCTC-3′ |
| human ACE-rev | 5′-GAGCCTCTCATTGTAGTC-3′ |
| human β-actin-for | 5′- CCAACCGCGAGAAGATGA-3′ |
| human β-actin-rev | 5′-CCAGAGGCGTACAGGGATAG-3′ |
| murine ACE2-for | 5′-TCCATTGGTCTTCTGCCATC-3′ |
| murine ACE2-rev | 5′-AACGATCTCCCGCTTCATCTC-3′ |
| murine β-actin-for | 5′-GGCTGTATTCCCCTCCATCG-3′ |
| murine β-actin-rev | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
| murine GAPD-for | 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| murine GAPDH-rev | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
| murine PPIA-for | 5′-GAGCTGTTTGCAGACAAAGTTC-3′ |
| murine PPIA-rev | 5′-CCCTGGCACATGAATCCTGG-3′ |
| Dose Ibuprofen | 0 mg/kg (n = 8) | 50 mg/kg (n = 8) | 100 mg/kg (n = 8) | 200 mg/kg (n = 8) |
|---|---|---|---|---|
| Body weight (g) (A) | ||||
| Mean | 27.9 | 27.3 | 27.4 | 26.8 |
| SEM | 0.14 | 0.23 | 0.15 | 0.33 |
| Amount (mL) water consumption per day (B) | ||||
| Mean | 5.6 | 5.8 | 5.7 | 5.9 |
| SEM | 0.10 | 0.13 | 0.12 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Bruin, N.; Schneider, A.-K.; Reus, P.; Talmon, S.; Ciesek, S.; Bojkova, D.; Cinatl, J.; Lodhi, I.; Charlesworth, B.; Sinclair, S.; et al. Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection. Int. J. Mol. Sci. 2022, 23, 1049. https://doi.org/10.3390/ijms23031049
de Bruin N, Schneider A-K, Reus P, Talmon S, Ciesek S, Bojkova D, Cinatl J, Lodhi I, Charlesworth B, Sinclair S, et al. Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection. International Journal of Molecular Sciences. 2022; 23(3):1049. https://doi.org/10.3390/ijms23031049
Chicago/Turabian Stylede Bruin, Natasja, Ann-Kathrin Schneider, Philipp Reus, Sonja Talmon, Sandra Ciesek, Denisa Bojkova, Jindrich Cinatl, Imran Lodhi, Bruce Charlesworth, Simon Sinclair, and et al. 2022. "Ibuprofen, Flurbiprofen, Etoricoxib or Paracetamol Do Not Influence ACE2 Expression and Activity In Vitro or in Mice and Do Not Exacerbate In-Vitro SARS-CoV-2 Infection" International Journal of Molecular Sciences 23, no. 3: 1049. https://doi.org/10.3390/ijms23031049






