Normal Retinotopy in Primary Visual Cortex in a Congenital Complete Unilateral Lesion of Lateral Geniculate Nucleus in Human: A Case Study
Abstract
:1. Introduction
2. Results
2.1. Behavioral Results
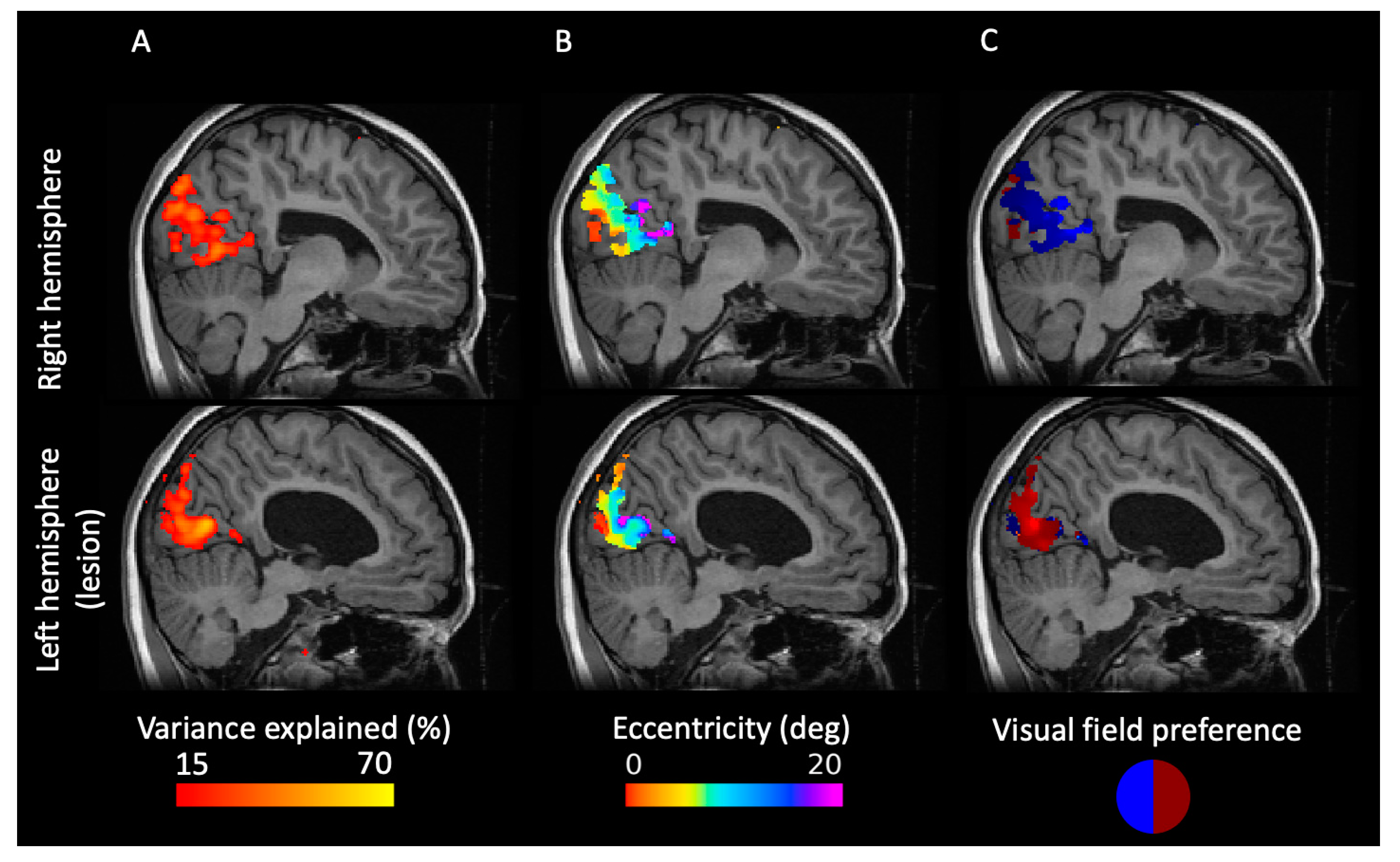
2.2. PRF Mapping Reveals a Retinotopic Map of the Visual Cortex
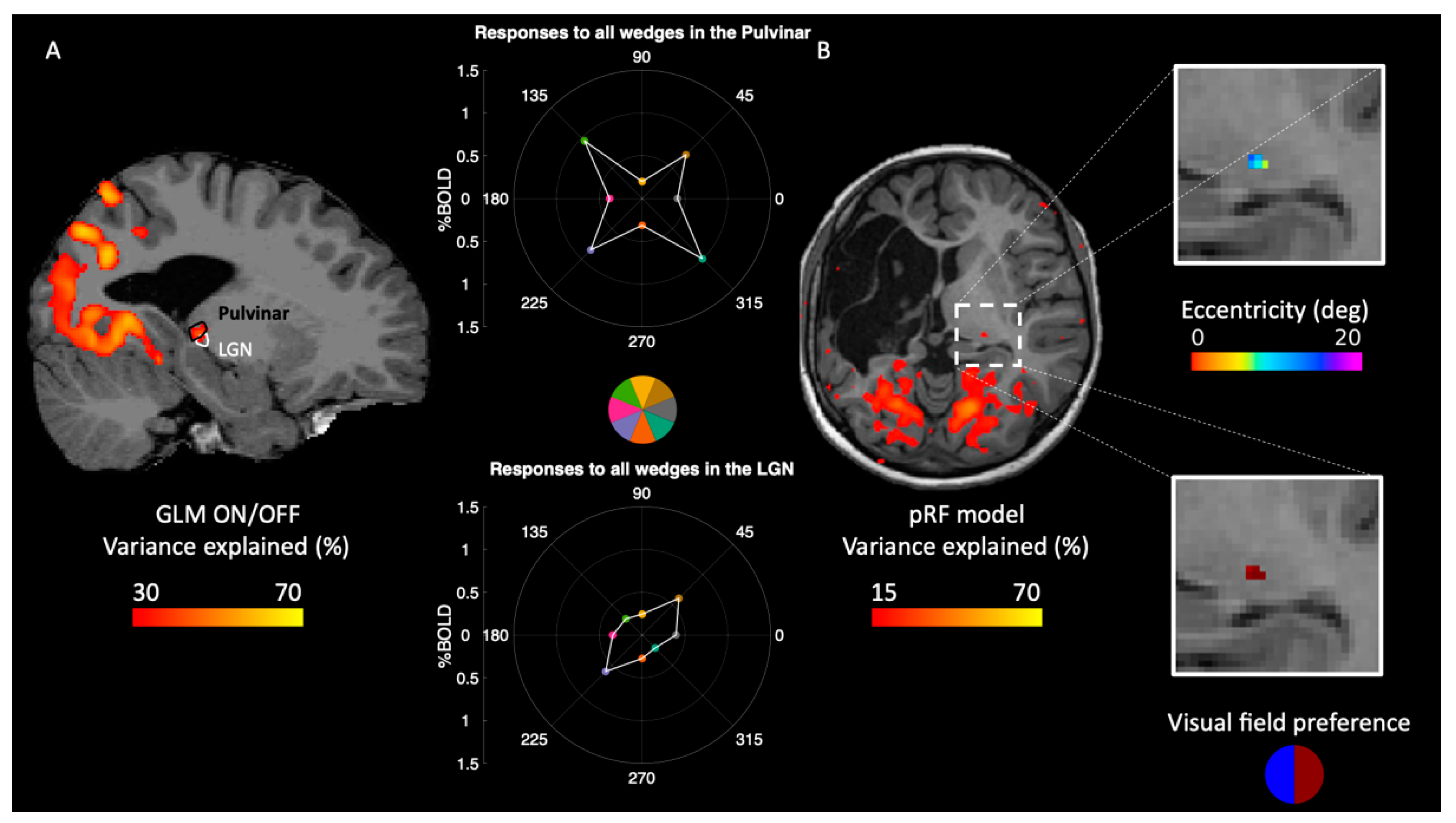
2.3. Reorganization of Responses in the Thalamus
2.4. Visual White Matter Bundles in Patient IB
3. Methods
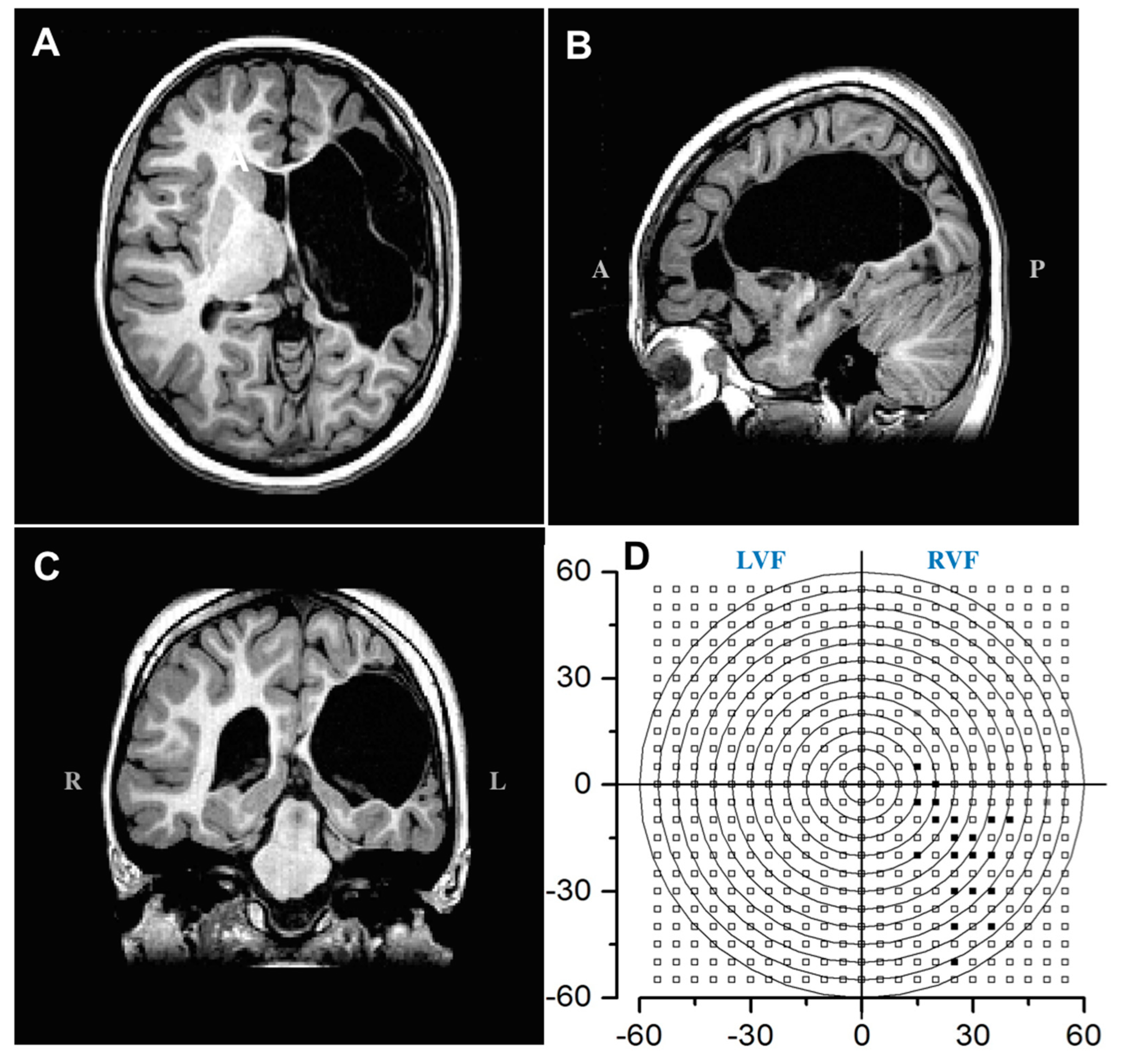
3.1. Clinical Description
3.2. Psychophysical Tests
3.3. Imaging Methods
3.3.1. Data Acquisition
3.3.2. Visual Stimulation for pRF Mapping
3.4. Functional Data Analysis
3.4.1. pRF Mapping
3.4.2. Analysis of Diffusion Data
3.4.3. Mapping the Pulvinar and LGN ROIs from an Atlas
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubel, D.H.; Wiesel, T.N. Receptive Fields, Binocular Interaction and Functional Architecture in the Cat’s Visual Cortex. J. Physiol. 1962, 160, 106–154. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Hubel, D.H. EXTENT OF RECOVERY FROM THE EFFECTS OF VISUAL DEPRIVATION IN KITTENS. J. Neurophysiol. 1965, 28, 1060–1072. [Google Scholar] [CrossRef]
- Petros, T.J.; Rebsam, A.; Mason, C.A. Retinal Axon Growth at the Optic Chiasm: To Cross or Not to Cross. Annu. Rev. Neurosci. 2008, 31, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Mcketton, L.; Kelly, K.R.; Schneider, K.A. Abnormal Lateral Geniculate Nucleus and Optic Chiasm in Human Albinism: Abnormal Visual System Morphology in Albinism. J. Comp. Neurol. 2014, 522, 2680–2687. [Google Scholar] [CrossRef]
- Puzniak, R.J.; Ahmadi, K.; Kaufmann, J.; Gouws, A.; Morland, A.B.; Pestilli, F.; Hoffmann, M.B. Quantifying Nerve Decussation Abnormalities in the Optic Chiasm. NeuroImage Clin. 2019, 24, 102055. [Google Scholar] [CrossRef]
- Ahmadi, K.; Fracasso, A.; van Dijk, J.A.; Kruijt, C.; van Genderen, M.; Dumoulin, S.O.; Hoffmann, M.B. Altered Organization of the Visual Cortex in FHONDA Syndrome. NeuroImage 2019, 190, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Tinelli, F.; Cicchini, G.M.; Arrighi, R.; Tosetti, M.; Cioni, G.; Morrone, M.C. Blindsight in Children with Congenital and Acquired Cerebral Lesions. Cortex 2013, 49, 1636–1647. [Google Scholar] [CrossRef]
- Ajina, S.; Pestilli, F.; Rokem, A.; Kennard, C.; Bridge, H. Human Blindsight Is Mediated by an Intact Geniculo-Extrastriate Pathway. eLife 2015, 4, e08935. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Keliris, G.A.; Papageorgiou, T.D.; Shao, Y.; Krapp, E.; Papageorgiou, E.; Stingl, K.; Bruckmann, A.; Schiefer, U.; Logothetis, N.K.; et al. Population Receptive Field Analysis of the Primary Visual Cortex Complements Perimetry in Patients with Homonymous Visual Field Defects. Proc. Natl. Acad. Sci. USA 2014, 111, E1656–E1665. [Google Scholar] [CrossRef] [Green Version]
- Wandell, B.A.; Smirnakis, S.M. Plasticity and Stability of Visual Field Maps in Adult Primary Visual Cortex. Nat. Rev. Neurosci. 2009, 10, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Teuber, H.-L. Recovery of Function After Brain Injury in Man. In Novartis Foundation Symposia; Porter, R., Fitzsimons, D.W., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 159–190. ISBN 978-0-470-72016-5. [Google Scholar]
- Perenin, M.T. Visual Function within the Hemianopic Field Following Early Cerebral Hemidecortication in Man—II. Pattern Discrimination. Neuropsychologia 1978, 16, 697–708. [Google Scholar] [CrossRef]
- Knyazeva, M.G.; Maeder, P.; Kiper, D.C.; Deonna, T.; Innocenti, G.M. Vision After Early-Onset Lesions of the Occipital Cortex: II. Physiological Studies. Neural Plast. 2002, 9, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Werth, R. Visual Functions without the Occipital Lobe or after Cerebral Hemispherectomy in Infancy. Eur. J. Neurosci. 2006, 24, 2932–2944. [Google Scholar] [CrossRef]
- Braddick, O.; Atkinson, J.; Hood, B.; Harkness, W.; Jackson, G.; Vargha-Khademt, F. Possible Blindsight in Infants Lacking One Cerebral Hemisphere. Nature 1992, 360, 461–463. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Villablanca, J.R.; Sonnier, B.J.; Levine, M.S. Reorganization of Pericruciate Cortical Projections to the Spinal Cord and Dorsal Column Nuclei after Neonatal or Adult Cerebral Hemispherectomy in Cats. Brain Res. 1986, 385, 343–355. [Google Scholar] [CrossRef]
- Carr, L.J.; Harrison, L.M.; Evans, A.L.; Stephens, J.A. Patterns of Central Motor Reorganization in Hemiplegic Cerebral Palsy. Brain 1993, 116, 1223–1247. [Google Scholar] [CrossRef]
- Muckli, L.; Naumer, M.J.; Singer, W. Bilateral Visual Field Maps in a Patient with Only One Hemisphere. Proc. Natl. Acad. Sci. USA 2009, 106, 13034–13039. [Google Scholar] [CrossRef] [Green Version]
- Chambers, T.M.; Agopian, A.J.; Lewis, R.A.; Langlois, P.H.; Danysh, H.E.; Weber, K.A.; Shaw, G.M.; Mitchell, L.E.; Lupo, P.J. Epidemiology of Anophthalmia and Microphthalmia: Prevalence and Patterns in Texas, 1999–2009. Am. J. Med. Genet. A. 2018, 176, 1810–1818. [Google Scholar] [CrossRef]
- Hoffmann, M.B.; Dumoulin, S.O. Congenital Visual Pathway Abnormalities: A Window onto Cortical Stability and Plasticity. Trends Neurosci. 2015, 38, 55–65. [Google Scholar] [CrossRef]
- Neveu, M.M.; Holder, G.E.; Ragge, N.K.; Sloper, J.J.; Collin, J.R.O.; Jeffery, G. Early Midline Interactions Are Important in Mouse Optic Chiasm Formation but Are Not Critical in Man: A Significant Distinction between Man and Mouse. Eur. J. Neurosci. 2006, 23, 3034–3042. [Google Scholar] [CrossRef]
- Mikellidou, K.; Kurzawski, J.W.; Frijia, F.; Montanaro, D.; Greco, V.; Burr, D.C.; Morrone, M.C. Area Prostriata in the Human Brain. Curr. Biol. 2017, 27, 3056–3060.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, S. Retinotopic Organization in Human Visual Cortex and the Spatial Precision of Functional MRI. Cereb. Cortex 1997, 7, 181–192. [Google Scholar] [CrossRef]
- Allen, E.J.; St-Yves, G.; Wu, Y.; Breedlove, J.L.; Prince, J.S.; Dowdle, L.T.; Nau, M.; Caron, B.; Pestilli, F.; Charest, I.; et al. A Massive 7T FMRI Dataset to Bridge Cognitive Neuroscience and Artificial Intelligence. Nat. Neurosci. 2021. [Google Scholar] [CrossRef]
- Arcaro, M.J.; Pinsk, M.A.; Kastner, S. The Anatomical and Functional Organization of the Human Visual Pulvinar. J. Neurosci. 2015, 35, 9848–9871. [Google Scholar] [CrossRef] [Green Version]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Saad, Z.S.; Glen, D.R.; Chen, G.; Beauchamp, M.S.; Desai, R.; Cox, R.W. A New Method for Improving Functional-to-Structural MRI Alignment Using Local Pearson Correlation. NeuroImage 2009, 44, 839–848. [Google Scholar] [CrossRef] [Green Version]
- Kay, K.N.; Rokem, A.; Winawer, J.; Dougherty, R.F.; Wandell, B.A. GLMdenoise: A Fast, Automated Technique for Denoising Task-Based FMRI Data. Front. Neurosci. 2013, 7, 1–15. [Google Scholar] [CrossRef]
- Dumoulin, S.O.; Wandell, B.A. Population Receptive Field Estimates in Human Visual Cortex. NeuroImage 2008, 39, 647–660. [Google Scholar] [CrossRef] [Green Version]
- Dumoulin, S.O.; Harvey, B.M. Reconstructing Human Population Receptive Field Properties. J. Vis. 2012, 12, 41–45. [Google Scholar] [CrossRef]
- Friston, K.J.; Fletcher, P.; Josephs, O.; Holmes, A.; Rugg, M.D.; Turner, R. Event-Related FMRI: Characterizing Differential Responses. NeuroImage 1998, 7, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himmelberg, M.M.; Kurzawski, J.W.; Benson, N.C.; Pelli, D.G.; Carrasco, M.; Winawer, J. Cross-Dataset Reproducibility of Human Retinotopic Maps. NeuroImage 2021, 244, 118609. [Google Scholar] [CrossRef]
- Wandell, B.A.; Dumoulin, S.O.; Brewer, A.A. Visual Field Maps in Human Cortex. Neuron 2007, 56, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Worsley, K.J.; Liao, C.H.; Aston, J.; Petre, V.; Duncan, G.H.; Morales, F.; Evans, A.C. A General Statistical Analysis for FMRI Data. NeuroImage 2002, 15, 1–15. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Calamante, F.; Gadian, D.G.; Connelly, A. Direct Estimation of the Fiber Orientation Density Function from Diffusion-Weighted MRI Data Using Spherical Deconvolution. NeuroImage 2004, 23, 1176–1185. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Calamante, F.; Connelly, A. MRtrix: Diffusion Tractography in Crossing Fiber Regions. Int. J. Imaging Syst. Technol. 2012, 22, 53–66. [Google Scholar] [CrossRef]
- Takemura, H.; Caiafa, C.F.; Wandell, B.A.; Pestilli, F. Ensemble Tractography. PLOS Comput. Biol. 2016, 12, e1004692. [Google Scholar] [CrossRef]
- Pestilli, F.; Yeatman, J.D.; Rokem, A.; Kay, K.N.; Wandell, B.A. Evaluation and Statistical Inference for Human Connectomes. Nat. Methods 2014, 11, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Caiafa, C.F.; Pestilli, F. Multidimensional Encoding of Brain Connectomes. Sci. Rep. 2017, 7, 11491. [Google Scholar] [CrossRef] [Green Version]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. NeuroImage 2011, 54, 2033–2044. [Google Scholar] [CrossRef] [Green Version]
- Collins, D.L.; Holmes, C.J.; Peters, T.M.; Evans, A.C. Automatic 3-D Model-based Neuroanatomical Segmentation. Hum. Brain Mapp. 1995, 3, 190–208. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A Probabilistic Atlas and Reference System for the Human Brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, P.; Bour, L.J.; Barth, P.G.; Wenniger-prick, L.; Verbeeten, B. Non-Decussating Retinal-Fugal Fibre Syndrome: An Inborn Achiasmatic Malformation Associated with Visuotopic Misrouting, Visual Evoked Potential Ipsilateral Asymmetry and Nystagmus. Brain 1995, 118, 1195–1216. [Google Scholar] [CrossRef] [PubMed]
- Bridge, H.; Thomas, O.; Jbabdi, S.; Cowey, A. Changes in Connectivity after Visual Cortical Brain Damage Underlie Altered Visual Function. Brain 2008, 131, 1433–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyon, D.C.; Nassi, J.J.; Callaway, E.M. A Disynaptic Relay from Superior Colliculus to Dorsal Stream Visual Cortex in Macaque Monkey. Neuron 2010, 65, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Kurzawski, J.W.; Mikellidou, K.; Morrone, M.C.; Pestilli, F. The Visual White Matter Connecting Human Area Prostriata and the Thalamus Is Retinotopically Organized. Brain Struct. Funct. 2020, 225, 1839–1853. [Google Scholar] [CrossRef]
- Bourne, J.A.; Morrone, M.C. Plasticity of Visual Pathways and Function in the Developing Brain: Is the Pulvinar a Crucial Player? Front. Syst. Neurosci. 2017, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Tanaka, S. Retinal Projections to the Pulvinar Nucleus of the Macaque Monkey: A Re-Investigation Using Autoradiography. Exp. Brain Res. 1984, 57, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Oehler, R.; Cowey, A. Retinal Ganglion Cells That Project to the Dorsal Lateral Geniculate Nucleus in the Macaque Monkey. Neuroscience 1984, 12, 1101–1123. [Google Scholar] [CrossRef]
- Warner, C.E.; Kwan, W.C.; Bourne, J.A. The Early Maturation of Visual Cortical Area MT Is Dependent on Input from the Retinorecipient Medial Portion of the Inferior Pulvinar. J. Neurosci. 2012, 32, 17073–17085. [Google Scholar] [CrossRef]
- Warner, C.E.; Kwan, W.C.; Wright, D.; Johnston, L.A.; Egan, G.F.; Bourne, J.A. Preservation of Vision by the Pulvinar Following Early-Life Primary Visual Cortex Lesions. Curr. Biol. 2015, 25, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Biagi, L.; Crespi, S.A.; Tosetti, M.; Morrone, M.C. BOLD Response Selective to Flow-Motion in Very Young Infants. PLOS Biol. 2015, 13, e1002260. [Google Scholar] [CrossRef] [Green Version]
- Tamietto, M.; Morrone, M.C. Visual Plasticity: Blindsight Bridges Anatomy and Function in the Visual System. Curr. Biol. 2016, 26, R70–R73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antón-Bolaños, N.; Sempere-Ferràndez, A.; Guillamón-Vivancos, T.; Martini, F.J.; Pérez-Saiz, L.; Gezelius, H.; Filipchuk, A.; Valdeolmillos, M.; López-Bendito, G. Prenatal Activity from Thalamic Neurons Governs the Emergence of Functional Cortical Maps in Mice. Science 2019, 364, 987–990. [Google Scholar] [CrossRef]
- Galli, L.; Maffei, L. Spontaneous Impulse Activity of Rat Retinal Ganglion Cells in Prenatal Life. Science 1988, 242, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Meister, M.; Wong, R.L.; Baylor, D.A.; Shatz, C.J. Action the May. 1991. [Google Scholar]
- Sharma, J.; Angelucci, A.; Sur, M. Induction of Visual Orientation Modules in Auditory Cortex. Nature 2000, 404, 841–847. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, A.; Kurzawski, J.W.; Anobile, G.; Tinelli, F.; Biagi, L.; Morrone, M.C. Normal Retinotopy in Primary Visual Cortex in a Congenital Complete Unilateral Lesion of Lateral Geniculate Nucleus in Human: A Case Study. Int. J. Mol. Sci. 2022, 23, 1055. https://doi.org/10.3390/ijms23031055
Bhat A, Kurzawski JW, Anobile G, Tinelli F, Biagi L, Morrone MC. Normal Retinotopy in Primary Visual Cortex in a Congenital Complete Unilateral Lesion of Lateral Geniculate Nucleus in Human: A Case Study. International Journal of Molecular Sciences. 2022; 23(3):1055. https://doi.org/10.3390/ijms23031055
Chicago/Turabian StyleBhat, Akshatha, Jan W. Kurzawski, Giovanni Anobile, Francesca Tinelli, Laura Biagi, and Maria Concetta Morrone. 2022. "Normal Retinotopy in Primary Visual Cortex in a Congenital Complete Unilateral Lesion of Lateral Geniculate Nucleus in Human: A Case Study" International Journal of Molecular Sciences 23, no. 3: 1055. https://doi.org/10.3390/ijms23031055
APA StyleBhat, A., Kurzawski, J. W., Anobile, G., Tinelli, F., Biagi, L., & Morrone, M. C. (2022). Normal Retinotopy in Primary Visual Cortex in a Congenital Complete Unilateral Lesion of Lateral Geniculate Nucleus in Human: A Case Study. International Journal of Molecular Sciences, 23(3), 1055. https://doi.org/10.3390/ijms23031055






