Fat and Protein Combat Triggers Immunological Weapons of Innate and Adaptive Immune Systems to Launch Neuroinflammation in Parkinson’s Disease
Abstract
1. Introduction
2. Triglyceride-Induced α-syn Abnormalities in PD
3. Glycerophosphoethanolamine (GPE)-Induced α-syn Abnormalities in PD
4. Diglyceride (DG)-Induced α-syn Abnormalities in PD
5. Polyunsaturated-Fatty-Acid (PUFA)-Induced α-syn Abnormalities in PD
6. Sphingolipid-Induced α-syn Abnormalities in PD
7. Ganglioside-Induced α-syn Abnormalities in PD
8. Glycerophospholipid-Induced α-syn Abnormalities in PD
9. Cholesterol-Induced α-syn Abnormalities in PD
| Family | Types | PD Mouse Model’s Alpha-Synuclein Abnormalities | Source | Levels | Reference |
|---|---|---|---|---|---|
| Glycerolipid | TAG | A53T mutant | Serum | Low | [272] |
| NEFA | A53T mutant | Serum | Low | [272] | |
| PUFA | α-LA | Transgeneic alpha-synuclein mice | MES Neurons | Induces | [81] |
| alpha-synuclein null C57Bl6 background | MES Neurons | High | [135] | ||
| EPA | Transgeneic alpha-synuclein mice | MES Neurons | Induces | [81] | |
| ETA | a-syn null C57Bl6 background | MES Neurons | High | [135] | |
| ARA | alpha-synuclein null C57Bl6 background | MES Neurons | High | [135] | |
| Fabp3KO mice with MPTP treatment | PC12 cells | High | [274] | ||
| MUFA | OA | A53T overexpressing cells | MES Neurons | Low | [135] |
| Glycerophospholipid | CL | PINK1 KO | Embryonic fibroblasts | Low | [275] |
| Sphingolipids | Sphingosine | GbaL444P/KO SNCAT and GbaKO/KO SNCAT | Neuron | Induce | [173] |
| Ceramide | Lrrk2KO mice | Brain | High | [176] | |
| PINK1 KO | Olfactory bulb | High | [177] | ||
| Sterol | Cholesterol | DJ1 KO | Embryonic fibroblasts | Low | [273] |
| DJ1 KO | Astrocyte | Low | [273] | ||
| GBA1 KO | Fibroblasts | Low | [271] | ||
| PRKN KO | MEF cells | High | [276] | ||
| A53T mutant | Serum | Low | [272] | ||
| alpha-synuclein gene-ablated mice | Astrocyte | High | [77] |
| Family | Types | Source | Level | Reference |
|---|---|---|---|---|
| Glycerolipid | TAG | Plasma | Low | [217,236,277] |
| PUFA | DHA | MES | High | [135] |
| Brain | High | [135] | ||
| LA | MES | High | [135] | |
| Brain | Low | [135] | ||
| DSA | MES | High | [135] | |
| Brain | High | [135] | ||
| EPA | MES | High | [135] | |
| OBA | MES | High | [135] | |
| Eicosanoids | PGL J2 | SK-N-SH cells | Induces | [278] |
| Glycerophospholipids | PA | Plasma | Low | [235] |
| PC | Frontal cortex | Low | [110] | |
| PE | SN | Low | [235] | |
| Visual cortex | Low | [178] | ||
| Plasma | Low | [236] | ||
| Frontal cortex | High | [140] | ||
| Plasma | Low | [175] | ||
| PS | Plasma | Low | [236] | |
| Fibroblast | High | [214] | ||
| Frontal cortex | High | [110] | ||
| Visual cortex | High | [178] | ||
| PI | Fibroblast | High | [214] | |
| Frontal cortex | High | [140] | ||
| SN | Low | [179] | ||
| PG | Frontal cortex | High | [110] | |
| APG (acyl PG) | Plasma | Low | [175] | |
| Sphingolipid | Sphingosine | Neurons | Induce | [173] |
| Ceramide | Plasma | High | [173] | |
| Plasma | Low | [170] | ||
| Plasma | Low | [175] | ||
| Frontal cortex | Low | [110] | ||
| ACC | Low | [39] | ||
| PVC | High | [279] | ||
| SM | Plasma | High | [175] | |
| ACC | Low | [279] | ||
| ACC | High | [279] | ||
| PVC | High | [178] | ||
| SN | High | [179] | ||
| Cerebroside | SN | High | [179] | |
| Plasma | High | [175] | ||
| Frontal cortex | Low | [140] | ||
| GlcCer | Plasma | High | [217] | |
| GM3 | Plasma | High | [217] | |
| GM2 | Fibroblast | High | [214] | |
| Brain | High | [218] | ||
| GM1 | SN | Low | [213] | |
| Sulfatide | Frontal cortex | High | [110] | |
| Sterol | Cholesterol | Plasma | Low | [267] |
| Plasma | High | [266] | ||
| PVC | High | [178] | ||
| Phospholipids | CP | Frontal cortex | High | [110] |
| EP | Frontal cortex | High | [110] | |
| Plasma | Low | [175] |
10. Lipid-α-syn-Proinflammatory Cytokine Pathway in PD
11. Lipid-α-syn-ROS Pathway in PD
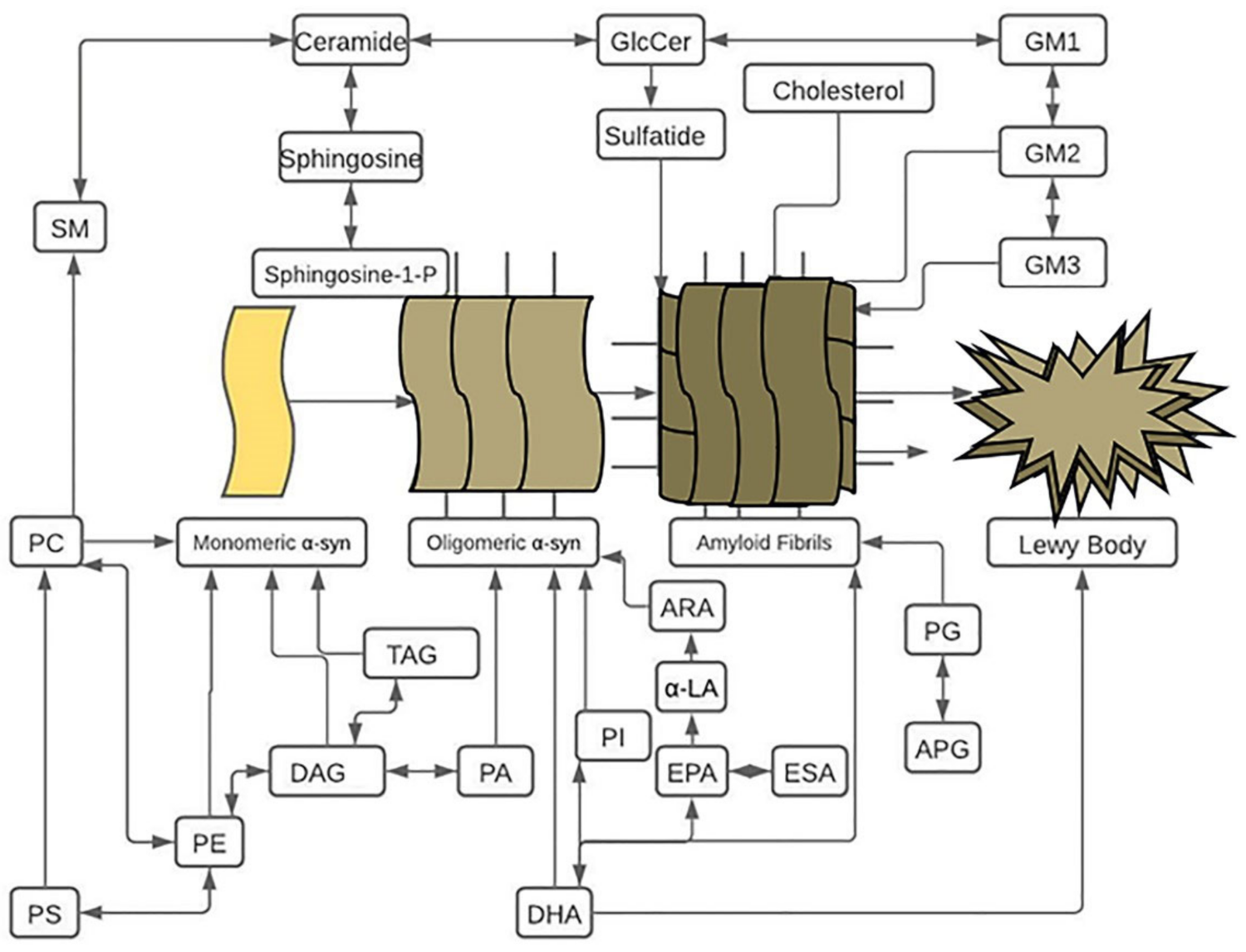
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Tanji, K.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 2007, 27, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol. Neurobiol. 2012, 47, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, W.; Ou, R.; Liu, J.; Yang, J.; Fu, J.; Cao, B.; Chen, Y.; Wei, Q.; Shang, H. Evidence for Peripheral Immune Activation in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13. [Google Scholar] [CrossRef]
- Cao, J.-J.; Li, K.-S.; Shen, Y.-Q. Activated Immune Cells in Parkinson’s Disease. J. Neuroimmune Pharmacol. 2011, 6, 323–329. [Google Scholar] [CrossRef]
- Clark, L.F.; Kodadek, T. The Immune System and Neuroinflammation as Potential Sources of Blood-Based Biomarkers for Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. ACS Chem. Neurosci. 2016, 7, 520–527. [Google Scholar] [CrossRef]
- Jayaram, S.; Krishnamurthy, P.T. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of Parkinson’s disease: The therapeutic role of Nrf2 activators. Neurochem. Int. 2021, 145, 105014. [Google Scholar] [CrossRef]
- Qian, L.; Flood, P.M. Microglial cells and Parkinson’s disease. Immunol. Res. 2008, 41, 155–164. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. S3), S26–S38. [Google Scholar] [CrossRef]
- Wei, Z.; Li, X.; Li, X.; Liu, Q.; Cheng, Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 236. [Google Scholar] [CrossRef]
- Jiang, T.; Li, G.; Xu, J.; Gao, S.; Chen, X. The Challenge of the Pathogenesis of Parkinson’s Disease: Is Autoimmunity the Culprit? Front. Immunol. 2018, 9, 2047. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Muller, S. Parkinson’s disease is an autoimmune disease: A reappraisal. Autoimmun. Rev. 2020, 19, 102684. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s disease. Park. Relat. Disord. 2012, 18, S210–S212. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Mao, Z.-H. Progression of motor symptoms in Parkinson’s disease. Neurosci. Bull. 2012, 28, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 (Suppl. S1), 14–20. [Google Scholar] [CrossRef]
- Magnusen, A.F.; Hatton, S.L.; Rani, R.; Pandey, M.K. Genetic Defects and Pro-inflammatory Cytokines in Parkinson’s Disease. Front. Neurol. 2021, 12, Review. [Google Scholar] [CrossRef]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef]
- Sidransky, E.; Lopez, G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012, 11, 986–998. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Liang, H.; Zhao, Z.; Hashimoto, M.; Wei, J. Gaucher-Associated Parkinsonism. Cell. Mol. Neurobiol. 2015, 35, 755–761. [Google Scholar] [CrossRef][Green Version]
- Westbroek, W.; Gustafson, A.M.; Sidransky, E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol. Med. 2011, 17, 485–493. [Google Scholar] [CrossRef]
- Migdalska-Richards, A.; Schapira, A.H.V. The relationship between glucocerebrosidase mutations and Parkinson disease. J. Neurochem. 2016, 139, 77–90. [Google Scholar] [CrossRef]
- Gispert, S.; del Turco, D.; Garrett, L.; Chen, A.; Bernard, D.J.; Hamm-Clement, J.; Korf, H.-W.; Deller, T.; Braak, H.; Auburger, G.; et al. Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol. Cell. Neurosci. 2003, 24. [Google Scholar] [CrossRef]
- Murphy, K.E.; Gysbers, A.M.; Abbott, S.K.; Tayebi, N.; Kim, W.S.; Sidransky, E.; Cooper, A.; Garner, B.; Halliday, G.M. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 2014, 137, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.-J.; Yang, N.Y.; Lee, C.; Lee, H.-J.; Kim, S.; Sardi, S.P.; Lee, S.-J. Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and α-synuclein aggregation. Exp. Mol. Med. 2015, 47, e188. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Yang, T.; Gu, J.W.; Kuang, Y.Q.; Cheng, L.; Yang, W.T.; Yang, X.K.; Xia, X.; Cheng, J.M.; Ma, Y.; et al. The association between lysosomal protein glucocerebrosidase and Parkinson’s disease. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 143–151. [Google Scholar]
- Parnetti, L.; Chiasserini, D.; Persichetti, E.; Eusebi, P.; Varghese, S.; Msc, M.M.Q.; Dardis, A.; Deganuto, M.; de Carlo, C.; Castrioto, A.; et al. Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov. Disord. 2014, 29, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Zunke, F.; Mazzulli, J.R.; Schweizer, M.; Altmeppen, H.; Lüllmann-Rauch, R.; Kallemeijn, W.W.; Gaspar, P.J.M.D.S.; Aerts, J.; Glatzel, M.; et al. LIMP-2 expression is critical for -glucocerebrosidase activity and alpha-synuclein clearance. Proc. Natl. Acad. Sci. USA 2014, 111, 15573–15578. [Google Scholar] [CrossRef]
- Senkevich, K.A.; Kopytova, A.E.; Usenko, T.S.; Emelyanov, A.K.; Pchelina, S.N. Parkinson’s Disease Associated with GBA Gene Mutations: Molecular Aspects and Potential Treatment Approaches. Acta Naturae 2021, 13, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, K.; Cuvelier, E.; Bonte, M.-A.; Desplanque, M.; Gressier, B.; Devos, D.; Chartier-Harlin, M.-C. Glycosphingolipids and neuroinflammation in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 1–16. [Google Scholar] [CrossRef]
- Avisar, H.; Guardia-Laguarta, C.; Area-Gomez, E.; Surface, M.; Chan, A.K.; Alcalay, R.N.; Lerner, B. Lipidomics Prediction of Parkinson’s Disease Severity: A Machine-Learning Analysis. J. Park. Dis. 2021, 11, 1141–1155. [Google Scholar] [CrossRef]
- Fanning, S.; Selkoe, D.; Dettmer, U. Parkinson’s disease: Proteinopathy or lipidopathy? Npj Park. Dis. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Castellanos, D.B.; Martín-Jiménez, C.A.; Rojas-Rodríguez, F.; Barreto, G.E.; González, J. Brain lipidomics as a rising field in neurodegenerative contexts: Perspectives with Machine Learning approaches. Front. Neuroendocr. 2021, 61, 100899. [Google Scholar] [CrossRef]
- Klemann, C.J.H.M.; Martens, G.J.M.; Sharma, M.; Martens, M.B.; Isacson, O.; Gasser, T.; Visser, J.E.; Poelmans, G. Integrated molecular landscape of Parkinson’s disease. Npj Park. Dis. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Divane, A.; Goedert, M. Assignment of Human α-Synuclein (SNCA) and β-Synuclein (SNCB) Genes to Chromosomes 4q21 and 5q35. Genomics 1995, 27, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef]
- Murphy, D.D.; Rueter, S.M.; Trojanowski, J.Q.; Lee, V.M.-Y. Synucleins Are Developmentally Expressed, and α-Synuclein Regulates the Size of the Presynaptic Vesicular Pool in Primary Hippocampal Neurons. J. Neurosci. 2000, 20, 3214–3220. [Google Scholar] [CrossRef]
- Scott, D.; Roy, S. α-Synuclein Inhibits Intersynaptic Vesicle Mobility and Maintains Recycling-Pool Homeostasis. J. Neurosci. 2012, 32, 10129–10135. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. α-Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased Expression of α-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; McLaurin, J.; Yip, C.M.; George-Hyslop, P.S.; Fraser, P.E. α-Synuclein Membrane Interactions and Lipid Specificity. J. Biol. Chem. 2000, 275, 34328–34334. [Google Scholar] [CrossRef]
- Van Rooijen, B.D.; van Leijenhorst-Groener, K.A.; Claessens, M.M.; Subramaniam, V. Tryptophan Fluorescence Reveals Structural Features of α-Synuclein Oligomers. J. Mol. Biol. 2009, 394, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Desplats, P.; Sigurdson, C.; Tsigelny, I.; Masliah, E. Cell-to-cell transmission of non-prion protein aggregates. Nat. Rev. Neurol. 2010, 6, 702–706. [Google Scholar] [CrossRef]
- McCann, H.; Stevens, C.H.; Cartwright, H.; Halliday, G.M. alpha-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 2014, 20 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef]
- Li, J.-Y.; Englund, E.; Holton, J.L.; Soulet, D.; Hagell, P.; Lees, A.J.; Lashley, T.; Quinn, N.P.; Rehncrona, S.; Björklund, A.; et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008, 14, 501–503. [Google Scholar] [CrossRef]
- Kordower, J.H.; Chu, Y.; Hauser, R.A.; Freeman, T.B.; Olanow, C.W. Lewy body–like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008, 14, 504–506. [Google Scholar] [CrossRef]
- Gorodinsky, A.; Harris, D.A. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J. Cell Biol. 1995, 129, 619–627. [Google Scholar] [CrossRef]
- Puig, B.; Altmeppen, H.; Glatzel, M. The GPI-anchoring of PrP. Prion 2014, 8, 11–18. [Google Scholar] [CrossRef]
- Baldwin, M.A. Analysis of Glycosylphosphatidylinositol Protein Anchors: The Prion Protein. Methods Enzymol. 2005, 405, 172–187. [Google Scholar] [CrossRef]
- Latarjet, R.; Muel, B.; Haig, D.A.; Clarke, M.C.; Alper, T. Inactivation of the Scrapie Agent by Near Monochromatic Ultraviolet Light. Nature 1970, 227, 1341–1343. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J. Role of lipid in forming an infectious prion? Acta Biochim. Biophys. Sin. 2013, 45, 485–493. [Google Scholar] [CrossRef]
- Kim, C.; Xiao, X.; Chen, S.; Haldiman, T.; Smirnovas, V.; Kofskey, D.; Warren, M.; Surewicz, K.; Maurer, N.R.; Kong, Q.; et al. Artificial strain of human prions created in vitro. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Taraboulos, A.; Scott, M.; Semenov, A.; Avrahami, D.; Laszlo, L.; Prusiner, S.B. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform [published erratum appears in J Cell Biol 1995 Jul;130(2):501]. J. Cell Biol. 1995, 129, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Deleault, N.R.; Kascsak, R.; Geoghegan, J.C.; Supattapone, S. Species-Dependent Differences in Cofactor Utilization for Formation of the Protease-Resistant Prion Protein in Vitro. Biochemistry 2010, 49, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Aulic, S.; Masperone, L.; Narkiewicz, J.; Isopi, E.; Bistaffa, E.; Ambrosetti, E.; Pastore, B.; de Cecco, E.; Scaini, D.; Zago, P.; et al. α-Synuclein Amyloids Hijack Prion Protein to Gain Cell Entry, Facilitate Cell-to-Cell Spreading and Block Prion Replication. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.; Lee, H.-J.; Bae, E.-J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.-J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Song, C.; O’Brien, P.; Stieber, A.; Branch, J.R.; Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M.-Y. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20051–20056. [Google Scholar] [CrossRef]
- Prusiner, S.B.; Woerman, A.L.; Mordes, D.A.; Watts, J.C.; Rampersaud, R.; Berry, D.B.; Patel, S.; Oehler, A.; Lowe, J.K.; Kravitz, S.N.; et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. USA 2015, 112, E5308–E5317. [Google Scholar] [CrossRef]
- Van den Berge, N.; Ferreira, N.; Gram, H.; Mikkelsen, T.W.; Alstrup, A.K.O.; Casadei, N.; Tsung-Pin, P.; Riess, O.; Nyengaard, J.R.; Tamgüney, G.; et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019, 138, 535–550. [Google Scholar] [CrossRef]
- Ferreira, N.; Gram, H.; Sorrentino, Z.A.; Gregersen, E.; Schmidt, S.I.; Reimer, L.; Betzer, C.; Perez-Gozalbo, C.; Beltoja, M.; Nagaraj, M.; et al. Multiple system atrophy-associated oligodendroglial protein p25α stimulates formation of novel α-synuclein strain with enhanced neurodegenerative potential. Acta Neuropathol. 2021, 142, 87–115. [Google Scholar] [CrossRef]
- Irwin, D.; Lee, V.M.-Y.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; So, R.W.L.; Lau, H.H.C.; Sang, J.C.; Ruiz-Riquelme, A.; Fleck, S.C.; Stuart, E.; Menon, S.; Visanji, N.P.; Meisl, G.; et al. α-Synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2019, 23, 21–31. [Google Scholar] [CrossRef]
- Jan, A.; Gonçalves, N.P.; Vaegter, C.B.; Jensen, P.H.; Ferreira, N. The Prion-Like Spreading of Alpha-Synuclein in Parkinson’s Disease: Update on Models and Hypotheses. Int. J. Mol. Sci. 2021, 22, 8338. [Google Scholar] [CrossRef]
- Ferreira, N.; Gonçalves, N.P.; Jan, A.; Jensen, N.M.; van der Laan, A.; Mohseni, S.; Vægter, C.B.; Jensen, P.H. Trans-synaptic spreading of alpha-synuclein pathology through sensory afferents leads to sensory nerve degeneration and neuropathic pain. Acta Neuropathol. Commun. 2021, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Berge, N.V.D.; Ferreira, N.; Mikkelsen, T.W.; Alstrup, A.K.O.; Tamgüney, G.; Karlsson, P.; Terkelsen, A.J.; Nyengaard, J.R.; Jensen, P.H.; Borghammer, P. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain 2021, 144, 1853–1868. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gao, J.; Wang, J.; Xie, A. Prion-Like Mechanisms in Parkinson’s Disease. Front. Neurosci. 2019, 13, 552. [Google Scholar] [CrossRef]
- Uversky, V.N. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J. Neurochem. 2007, 103, 17–37. [Google Scholar] [CrossRef]
- Varkey, J.; Isas, J.M.; Mizuno, N.; Jensen, M.B.; Bhatia, V.K.; Jao, C.C.; Petrlova, J.; Voss, J.C.; Stamou, D.G.; Steven, A.C.; et al. Membrane Curvature Induction and Tubulation Are Common Features of Synucleins and Apolipoproteins. J. Biol. Chem. 2010, 285, 32486–32493. [Google Scholar] [CrossRef]
- Mori, A.; Imai, Y.; Hattori, N. Lipids: Key Players That Modulate α-Synuclein Toxicity and Neurodegeneration in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3301. [Google Scholar] [CrossRef]
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73. [Google Scholar] [CrossRef]
- Esposito, E.; di Matteo, V.; di Giovanni, G. Death in the substantia nigra: A motor tragedy. Expert Rev. Neurother. 2007, 7, 677–697. [Google Scholar] [CrossRef]
- Reish, H.E.A.; Standaert, D.G. Role of α-Synuclein in Inducing Innate and Adaptive Immunity in Parkinson Disease. J. Park. Dis. 2015, 5, 1–19. [Google Scholar] [CrossRef]
- Jenco, J.M.; Rawlingson, A.; Daniels, A.B.; Morris, A.J. Regulation of Phospholipase D2: Selective Inhibition of Mammalian Phospholipase D Isoenzymes by α- and β-Synucleins. Biochemistry 1998, 37, 4901–4909. [Google Scholar] [CrossRef]
- Payton, J.E.; Perrin, R.J.; Woods, W.S.; George, J.M. Structural Determinants of PLD2 Inhibition by α-Synuclein. J. Mol. Biol. 2004, 337, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Castagnet, P.I.; Golovko, M.Y.; Barceló-Coblijn, G.C.; Nussbaum, R.L.; Murphy, E.J. Fatty acid incorporation is decreased in astrocytes cultured from α-synuclein gene-ablated mice. J. Neurochem. 2005, 94, 839–849. [Google Scholar] [CrossRef]
- Golovko, M.; Faergeman, .N.J.; Cole, .N.B.; Castagnet, .P.I.; Nussbaum, .A.R.L.; Murphy, .E.J. α-Synuclein Gene Deletion Decreases Brain Palmitate Uptake and Alters the Palmitate Metabolism in the Absence of α-Synuclein Palmitate Binding. Biochemistry 2005, 44, 8251–8259. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.; Guo, Y.; Scarlata, S. Fluorescence Studies Suggest a Role for α-Synuclein in the Phosphatidylinositol Lipid Signaling Pathway. Biochemistry 2004, 44, 462–470. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Golovko, M.; Weinhofer, I.; Berger, J.; Murphy, E.J. Brain neutral lipids mass is increased in α-synuclein gene-ablated mice. J. Neurochem. 2006, 101, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Sharon, R.; Bar-Joseph, I.; Frosch, M.P.; Walsh, D.M.; Hamilton, J.A.; Selkoe, D.J. The Formation of Highly Soluble Oligomers of α-Synuclein Is Regulated by Fatty Acids and Enhanced in Parkinson’s Disease. Neuron 2003, 37, 583–595. [Google Scholar] [CrossRef]
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A Role for α-Synuclein in the Regulation of Dopamine Biosynthesis. J. Neurosci. 2002, 22, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Pfefferkorn, C.M.; Jiang, Z.; Lee, J.C. Biophysics of α-synuclein membrane interactions. Biochim. Biophys. Acta (BBA)—Biomembr. 2012, 1818, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ostrerova-Golts, N.; Petrucelli, L.; Hardy, J.; Lee, J.M.; Farrer, M.; Wolozin, B. The A53T α-Synuclein Mutation Increases Iron-Dependent Aggregation and Toxicity. J. Neurosci. 2000, 20, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, K.; Dickson, D.W.; McComb, R.D.; Wszolek, Z.K.; Katechalidou, L.; Avery, L.; Stansbury, M.S.; Chase, B.A. Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson’s disease. Variability in familial Parkinson’s disease. Acta Neuropathol. 2008, 116, 25–35. [Google Scholar] [CrossRef]
- Gispert, S.; Brehm, N.; Weil, J.; Seidel, K.; Rüb, U.; Kern, B.; Walter, M.; Roeper, J.; Auburger, G. Potentiation of neurotoxicity in double-mutant mice with Pink1 ablation and A53T-SNCA overexpression. Hum. Mol. Genet. 2014, 24, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Brunzell, J.D.; Goldberg, A.C.; Goldberg, I.J.; Sacks, F.M.; Murad, M.H.; Stalenhoef, A.F.H. Evaluation and Treatment of Hypertriglyceridemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2969–2989. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Mokdad, A.H. Hypertriglyceridemia and Its Pharmacologic Treatment Among US Adults. Arch. Intern. Med. 2009, 169, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Ness, R.B.; Harger, G.F.; Roberts, J.M. Inflammation and Triglycerides Partially Mediate the Effect of Prepregnancy Body Mass Index on the Risk of Preeclampsia. Am. J. Epidemiol. 2005, 162, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.; Vedel-Krogh, S.; Nielsen, S.F.; Afzal, S.; Smith, G.D.; Nordestgaard, B.G. Per-Particle Triglyceride-Rich Lipoproteins Imply Higher Myocardial Infarction Risk Than Low-Density Lipoproteins: Copenhagen General Population Study. Arter. Thromb. Vasc. Biol. 2021, 41, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. High Triglycerides and Low HDL Cholesterol and Blood Pressure and Risk of Ischemic Heart Disease. Hypertension 2000, 36, 226–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scherer, J.; Singh, V.P.; Pitchumoni, C.S.; Yadav, D. Issues in Hypertriglyceridemic Pancreatitis: An Up-date. J. Clin. Gastroenterol. 2014, 48, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yan, J.; Ma, J.; Na Kang, A.; Kang, S.Y.; Zhang, Q.; Lyu, C.; Park, Y.-K.; Jung, H.W.; Zhang, S. Effects of Jowiseungki-tang on high fat diet-induced obesity in mice and functional analysis on network pharmacology and metabolomics analysis. J. Ethnopharmacol. 2021, 283, 114700. [Google Scholar] [CrossRef]
- Park, B.; Lee, H.S.; Lee, Y.-J. Triglyceride glucose (TyG) index as a predictor of incident type 2 diabetes among nonobese adults: A 12-year longitudinal study of the Korean Genome and Epidemiology Study cohort. Transl. Res. 2020, 228, 42–51. [Google Scholar] [CrossRef]
- Cole, N.B.; Murphy, D.D.; Grider, T.; Rueter, S.; Brasaemle, D.; Nussbaum, R.L. Lipid Droplet Binding and Oligomerization Properties of the Parkinson’s Disease Protein α-Synuclein. J. Biol. Chem. 2002, 277, 6344–6352. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.S.; Alza, N.P.; Salvador, G.A. Lipid metabolism alterations in the neuronal response to A53T α-synuclein and Fe-induced injury. Arch. Biochem. Biophys. 2018, 655, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Sääksjärvi, K.; Knekt, P.; Männistö, S.; Lyytinen, J.; Heliövaara, M. Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease. Park. Relat. Disord. 2015, 21, 1148–1155. [Google Scholar] [CrossRef]
- He, Q.; Wang, M.; Petucci, C.; Gardell, S.J.; Han, X. Rotenone induces reductive stress and triacylglycerol deposition in C2C12 cells. Int. J. Biochem. Cell Biol. 2013, 45, 2749–2755. [Google Scholar] [CrossRef]
- Taylor-Robinson, S.D.; Sargentoni, J.; Bell, J.D.; Thomas, E.L.; Marcus, C.D.; Changani, K.K.; Saeed, N.; Hodgson, H.J.F.; Davidson, B.R.; Burroughs, A.K.; et al. In vivo and in vitro hepatic phosphorus-31 magnetic resonance spectroscopy and electron microscopy in chronic ductopenic rejection of human liver allografts. Gut 1998, 42, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kemp, W.J.M.; Stehouwer, B.L.; Runge, J.H.; Wijnen, J.P.; Nederveen, A.J.; Luijten, P.R.; Klomp, D.W.J. Glycerophosphocholine and Glycerophosphoethanolamine Are Not the Main Sources of the In Vivo31P MRS Phosphodiester Signals from Healthy Fibroglandular Breast Tissue at 7 T. Front. Oncol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Gonzalez-Coviella, I.L.; Logue, M.; Growdon, J.H.; Wurtman, R.J. Levels of phospholipid catabolic intermediates, glycerophosphocholine and glycerophosphoethanolamine, are elevated in brains of Alzheimer’s disease but not of Down’s syndrome patients. Brain Res. 1990, 536, 240–244. [Google Scholar] [CrossRef]
- Zakharov, S.D.; Hulleman, J.D.; Dutseva, E.A.; Antonenko, Y.N.; Rochet, J.-C.; Cramer, W.A. Helical α-Synuclein Forms Highly Conductive Ion Channels. Biochemistry 2007, 46, 14369–14379. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (IRS-1)-associated Phosphatidylinositol 3-Kinase Activity in Muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef]
- Griffin, M.E.; Marcucci, M.J.; Cline, G.W.; Bell, K.; Barucci, N.; Lee, D.; Goodyear, L.J.; Kraegen, E.W.; White, M.F.; Shulman, G.I. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999, 48, 1270–1274. [Google Scholar] [CrossRef]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef]
- Evangelisti, C.; Bortul, R.; Tabellini, G.; Papa, V.; Cocco, L.; Martelli, A.M. Nuclear expression of diacyl-glycerol kinases: Possible involvement in DNA replication. Eur. J. Histochem. 2006, 50, 9–13. [Google Scholar]
- Soste, M.; Charmpi, K.; Lampert, F.; Gerez, J.A.; van Oostrum, M.; Malinovska, L.; Boersema, P.J.; Prymaczok, N.C.; Riek, R.; Peter, M.; et al. Proteomics-Based Monitoring of Pathway Activity Reveals that Blocking Diacylglycerol Biosynthesis Rescues from Alpha-Synuclein Toxicity. Cell Syst. 2019, 9, 309–320.e8. [Google Scholar] [CrossRef]
- Wood, P.L.; Tippireddy, S.; Feriante, J.; Woltjer, R.L. Augmented frontal cortex diacylglycerol levels in Parkinson’s disease and Lewy Body Disease. PLoS ONE 2018, 13, e0191815. [Google Scholar] [CrossRef]
- Buckley, M.T.; Racimo, F.; Allentoft, M.E.; Jensen, M.K.; Jonsson, A.; Huang, H.; Hormozdiari, F.; Sikora, M.; Marnetto, D.; Eskin, E.; et al. Selection in Europeans on Fatty Acid Desaturases Associated with Dietary Changes. Mol. Biol. Evol. 2017, 34, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, C.; Palazzi, L.; de Laureto, P.P. α-Synuclein and Polyunsaturated Fatty Acids: Molecular Basis of the Interaction and Implication in Neurodegeneration. Molecules 2018, 23, 1531. [Google Scholar] [CrossRef] [PubMed]
- Marszalek, J.R.; Lodish, H.F. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: Breastmilk and Fish Are Good for You. Annu. Rev. Cell Dev. Biol. 2005, 21, 633–657. [Google Scholar] [CrossRef]
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 2006, 440, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.A.; Fu, W.; Akey, J.M.; Ainsworth, H.C.; Torgerson, D.G.; Ruczinski, I.; Sergeant, S.; Barnes, K.C.; Chilton, F.H. Adaptive Evolution of the FADS Gene Cluster within Africa. PLoS ONE 2012, 7, e44926. [Google Scholar] [CrossRef]
- Hester, A.G.; Murphy, R.C.; Uhlson, C.J.; Ivester, P.; Lee, T.C.; Sergeant, S.; Miller, L.R.; Howard, T.D.; Mathias, R.A.; Chilton, F.H. Relationship between a Common Variant in the Fatty Acid Desaturase (FADS) Cluster and Eicosanoid Generation in Humans. J. Biol. Chem. 2014, 289, 22482–22489. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is docosahexaenoic acid, an n−3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005, 82, 281–295. [Google Scholar] [CrossRef]
- Patel, J.V.; Tracey, I.; Hughes, E.A.; Lip, G.Y. Omega-3 polyunsaturated acids and cardiovascular disease: Notable ethnic differences or unfulfilled promise? J. Thromb. Haemost. 2010, 8, 2095–2104. [Google Scholar] [CrossRef]
- Gu, Y.; Vorburger, R.S.; Gazes, Y.; Habeck, C.G.; Stern, Y.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Brickman, A.M. White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Ann. Neurol. 2016, 79, 1014–1025. [Google Scholar] [CrossRef]
- Pottala, J.V.; Yaffe, K.; Robinson, J.G.; Espeland, M.A.; Wallace, R.; Harris, W.S. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI Study. Neurology 2014, 82, 435–442. [Google Scholar] [CrossRef]
- Titova, O.E.; Sjögren, P.; Brooks, S.; Kullberg, J.; Ax, E.; Kilander, L.; Riserus, U.; Cederholm, T.; Larsson, E.-M.; Johansson, L.; et al. Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. AGE 2012, 35, 1495–1505. [Google Scholar] [CrossRef]
- Samieri, C.; Maillard, P.; Crivello, F.; Proust-Lima, C.; Peuchant, E.; Helmer, C.; Amieva, H.; Allard, M.; Dartigues, J.-F.; Cunnane, S.C.; et al. Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 2012, 79, 642–650. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Albers, R.; Antoine, J.-M.; Blum, S.; Bourdet-Sicard, R.; Ferns, G.A.; Folkerts, G.; Friedmann, P.S.; Frost, G.S.; Guarner, F.; et al. Inflammatory Disease Processes and Interactions with Nutrition. Br. J. Nutr. 2009, 101, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Ben Gedalya, T.; Loeb, V.; Israeli, E.; Altschuler, Y.; Selkoe, D.J.; Sharon, R. α-Synuclein and Polyunsaturated Fatty Acids Promote Clathrin-Mediated Endocytosis and Synaptic Vesicle Recycling. Traffic 2009, 10, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Assayag, K.; Yakunin, E.; Loeb, V.; Selkoe, D.J.; Sharon, R. Polyunsaturated Fatty Acids Induce α-Synuclein-Related Pathogenic Changes in Neuronal Cells. Am. J. Pathol. 2007, 171, 2000–2011. [Google Scholar] [CrossRef]
- De Franceschi, G.; Frare, E.; Pivato, M.; Relini, A.; Penco, A.; Greggio, E.; Bubacco, L.; Fontana, A.; de Laureto, P.P. Structural and Morphological Characterization of Aggregated Species of α-Synuclein Induced by Docosahexaenoic Acid. J. Biol. Chem. 2011, 286, 22262–22274. [Google Scholar] [CrossRef]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Exposure to Long Chain Polyunsaturated Fatty Acids Triggers Rapid Multimerization of Synucleins. J. Biol. Chem. 2001, 276, 41958–41962. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, D.; Han, S.; Reaney, S.H.; di Monte, D.; Fink, A.L. Effect of 4-Hydroxy-2-nonenal Modification on α-Synuclein Aggregation. J. Biol. Chem. 2007, 282, 5862–5870. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.-W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Shamoto-Nagai, M.; Hisaka, S.; Naoi, M.; Maruyama, W. Modification of α-synuclein by lipid peroxidation products derived from polyunsaturated fatty acids promotes toxic oligomerization: Its relevance to Parkinson disease. J. Clin. Biochem. Nutr. 2018, 62, 207–212. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Park. Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef]
- De Franceschi, G.; Fecchio, C.; Sharon, R.; Schapira, A.H.V.; Proukakis, C.; Bellotti, V.; de Laureto, P.P. α-Synuclein structural features inhibit harmful polyunsaturated fatty acid oxidation, suggesting roles in neuroprotection. J. Biol. Chem. 2017, 292, 6927–6937. [Google Scholar] [CrossRef]
- Sharon, R.; Bar-Joseph, I.; Mirick, G.E.; Serhan, C.N.; Selkoe, D.J. Altered Fatty Acid Composition of Dopaminergic Neurons Expressing α-Synuclein and Human Brains with α-Synucleinopathies. J. Biol. Chem. 2003, 278, 49874–49881. [Google Scholar] [CrossRef] [PubMed]
- Broersen, K.; Brink, D.V.D.; Fraser, G.; Goedert, M.; Davletov, B. α-Synuclein Adopts an α-Helical Conformation in the Presence of Polyunsaturated Fatty Acids to Hinder Micelle Formation. Biochemistry 2006, 45, 15610–15616. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, G.; Frare, E.; Bubacco, L.; Mammi, S.; Fontana, A.; de Laureto, P.P. Molecular Insights into the Interaction between α-Synuclein and Docosahexaenoic Acid. J. Mol. Biol. 2009, 394, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Ruipérez, V.; Darios, F.; Davletov, B. Alpha-synuclein, lipids and Parkinson’s disease. Prog. Lipid Res. 2010, 49, 420–428. [Google Scholar] [CrossRef]
- Yakunin, E.; Loeb, V.; Kisos, H.; Biala, Y.; Yehuda, S.; Yaari, Y.; Selkoe, D.J.; Sharon, R. α-Synuclein Neuropathology is Controlled by Nuclear Hormone Receptors and Enhanced by Docosahexaenoic Acid in A Mouse Model for Parkinson’s Disease. Brain Pathol. 2011, 22, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe Alterations in Lipid Composition of Frontal Cortex Lipid Rafts from Parkinson’s Disease and Incidental Parkinson’s Disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef]
- Meng, Q.; Luchtman, D.W.; el Bahh, B.; Zidichouski, J.A.; Yang, J.; Song, C. Ethyl-eicosapentaenoate modulates changes in neurochemistry and brain lipids induced by parkinsonian neurotoxin 1-methyl-4-phenylpyridinium in mouse brain slices. Eur. J. Pharmacol. 2010, 649, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Chalimoniuk, M.; Stolecka-Warzecha, A.; Zieminska, E.; Stępień, A.; Langfort, J.; Strosznajder, J. Involvement of multiple protein kinases in cPLA2phosphorylation, arachidonic acid release, and cell death inin vivoandin vitromodels of 1-methyl-4-phenylpyridinium-induced parkinsonism—The possible key role of PKG. J. Neurochem. 2009, 110, 307–317. [Google Scholar] [CrossRef]
- Fecchio, C.; de Franceschi, G.; Relini, A.; Greggio, E.; Serra, M.D.; Bubacco, L.; de Laureto, P.P. α-Synuclein Oligomers Induced by Docosahexaenoic Acid Affect Membrane Integrity. PLoS ONE 2013, 8, e82732. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.; Goldbaum, O.; Wille, M.; Richter-Landsberg, C. Membrane Lipid Modification by Docosahexaenoic Acid (DHA) Promotes the Formation of α-Synuclein Inclusion Bodies Immunopositive for SUMO-1 in Oligodendroglial Cells After Oxidative Stress. J. Mol. Neurosci. 2010, 43, 290–302. [Google Scholar] [CrossRef]
- Ilan, Y. Compounds of the sphingomyelin-ceramide-glycosphingolipid pathways as secondary messenger molecules: New targets for novel therapies for fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 310, G1102–G1117. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 19, 175–191. [Google Scholar] [CrossRef]
- Quinville, B.M.; Deschenes, N.M.; Ryckman, A.E.; Walia, J.S. A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. Int. J. Mol. Sci. 2021, 22, 5793. [Google Scholar] [CrossRef]
- Ventura, A.E.; Mestre, B.; Silva, L.C. Ceramide Domains in Health and Disease: A Biophysical Perspective. Adv. Exp. Med. Biol. 2019, 1159, 79–108. [Google Scholar] [CrossRef]
- Sukocheva, O.A.; Furuya, H.; Ng, M.L.; Friedemann, M.; Menschikowski, M.; Tarasov, V.V.; Chubarev, V.N.; Klochkov, S.G.; Neganova, M.E.; Mangoni, A.A.; et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol. Ther. 2020, 207, 107464. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G.; Zheng, X.; et al. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2018, 195, 85–99. [Google Scholar] [CrossRef]
- Watson, L.; Tullus, K.; Marks, S.D.; Holt, R.C.L.; Pilkington, C.; Beresford, M.W. Increased Serum Concentration of Sphingosine-1-phosphate in Juvenile-onset Systemic Lupus Erythematosus. J. Clin. Immunol. 2012, 32, 1019–1025. [Google Scholar] [CrossRef]
- Mike, E.V.; Makinde, H.M.; Der, E.; Stock, A.; Gulinello, M.; Gadhvi, G.T.; Winter, D.R.; Cuda, C.M.; Putterman, C. Neuropsychiatric Systemic Lupus Erythematosus Is Dependent on Sphingosine-1-Phosphate Signaling. Front. Immunol. 2018, 9, 2189. [Google Scholar] [CrossRef]
- Snider, A.J. Sphingosine kinase and sphingosine-1-phosphate: Regulators in autoimmune and inflammatory disease. Int. J. Clin. Rheumatol. 2013, 8, 453–463. [Google Scholar] [CrossRef]
- Nagahashi, M.; Abe, M.; Sakimura, K.; Takabe, K.; Wakai, T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer Sci. 2018, 109, 3671–3678. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nejabati, H.R.; Fattahi, A.; Latifi, Z.; Pezeshkian, M.; Afrasiabi, A.; Safaie, N.; Jodati, A.R.; Nouri, M. The role of sphingosine 1 phosphate in coronary artery disease and ischemia reperfusion injury. J. Cell. Physiol. 2018, 234, 2083–2094. [Google Scholar] [CrossRef]
- Wang, E.; He, X.; Zeng, M. The Role of S1P and the Related Signaling Pathway in the Development of Tissue Fibrosis. Front. Pharmacol. 2019, 9, 1504. [Google Scholar] [CrossRef]
- Ishay, Y.; Nachman, D.; Khoury, T.; Ilan, Y. The role of the sphingolipid pathway in liver fibrosis: An emerging new potential target for novel therapies. Am. J. Physiol. Physiol. 2020, 318, C1055–C1064. [Google Scholar] [CrossRef]
- Taha, T.A.; Mullen, T.D.; Obeid, L.M. A house divided: Ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Yamada, A.; Katsuta, E.; Aoyagi, T.; Huang, W.-C.; Terracina, K.P.; Hait, N.C.; Allegood, J.C.; Tsuchida, J.; Yuza, K.; et al. Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis. Cancer Res. 2018, 78, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.; Pallbo, J.; Weininger, U.; Linse, S.; Sparr, E. Ganglioside lipids accelerate α-synuclein amyloid formation. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2018, 1866, 1062–1072. [Google Scholar] [CrossRef]
- Clark, L.N.; Kisselev, S.; Park, N.; Ross, B.; Verbitsky, M.; Rios, E.; Alcalay, R.N.; Lee, J.H.; Louis, E.D. Mutations in the Parkinson’s disease genes, Leucine Rich Repeat Kinase 2 (LRRK2) and Glucocerebrosidase (GBA), are not associated with essential tremor. Park. Relat. Disord. 2010, 16, 132–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pandey, M.K.; Burrow, T.A.; Rani, R.; Martin, L.J.; Witte, D.; Setchell, K.D.; McKay, M.A.; Magnusen, A.F.; Zhang, K.D.S.W.; Liou, B.; et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 2017, 543, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Grabowski, G.A. Cytology of Gaucher disease. In Advances in Gaucher Disease: Basic and Clinical Perspectives; Future Medicine: London, UK, 2013; pp. 78–93. [Google Scholar]
- Cullen, V.; Sardi, S.P.; Ng, J.; Xu, Y.H.; Sun, Y.; Tomlinson, J.J.; Kolodziej, P.; Kahn, I.; Saftig, P.; Woulfe, J.; et al. Acid β-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter α-synuclein processing. Ann. Neurol. 2011, 69, 940–953. [Google Scholar] [CrossRef]
- Pandey, M.K.; Grabowski, G.A. Immunological Cells and Functions in Gaucher Disease. Crit. Rev. Oncog. 2013, 18, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Rani, R.; Zhang, W.; Setchell, K.; Grabowski, G.A. Immunological cell type characterization and Th1–Th17 cytokine production in a mouse model of Gaucher disease. Mol. Genet. Metab. 2012, 106, 310–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abbott, S.K.; Li, H.; Muñoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2013, 29, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Du, T.-T.; Wang, L.; Duan, C.-L.; Lu, L.-L.; Zhang, J.-L.; Gao, G.; Qiu, X.-B.; Wang, X.-M.; Yang, H. GBA deficiency promotes SNCA/α-synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy 2015, 11, 1803–1820. [Google Scholar] [CrossRef]
- Atashrazm, F.; Hammond, D.; Perera, G.; Dobson-Stone, C.; Mueller, N.; Pickford, R.; Kim, W.S.; Kwok, J.B.; Lewis, S.; Halliday, G.M.; et al. Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Hughes, L.P.; Pereira, M.M.; Hammond, D.A.; Kwok, J.B.; Halliday, G.M.; Lewis, S.J.; Dzamko, N. Glucocerebrosidase Activity is Reduced in Cryopreserved Parkinson’s Disease Patient Monocytes and Inversely Correlates with Motor Severity. J. Park. Dis. 2021, 11, 1157–1165. [Google Scholar] [CrossRef]
- Gündner, A.L.; Duran-Pacheco, G.; Zimmermann, S.; Ruf, I.; Moors, T.; Baumann, K.; Jagasia, R.; van de Berg, W.; Kremer, T. Path mediation analysis reveals GBA impacts Lewy body disease status by increasing α-synuclein levels. Neurobiol. Dis. 2018, 121, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.V.; Liu, J.; Ruan, J.; Pacheco, J.; Zhang, X.; Abbasi, J.; Keutzer, J.; Mistry, P.K.; Chandra, S.S. Glucosylsphingosine Promotes α-Synuclein Pathology in Mutant GBA-Associated Parkinson’s Disease. J. Neurosci. 2017, 37, 9617–9631. [Google Scholar] [CrossRef] [PubMed]
- Fonteh, A.N.; Chiang, A.J.; Arakaki, X.; Edminster, S.P.; Harrington, M.G. Accumulation of Cerebrospinal Fluid Glycerophospholipids and Sphingolipids in Cognitively Healthy Participants with Alzheimer’s Biomarkers Precedes Lipolysis in the Dementia Stage. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Guedes, L.C.; Chan, R.B.; Gomes, M.A.; Conceição, V.A.; Machado, R.B.; Soares, T.; Xu, Y.; Gaspar, P.; Carriço, J.A.; Alcalay, R.N.; et al. Serum lipid alterations in GBA-associated Parkinson’s disease. Park. Relat. Disord. 2017, 44, 58–65. [Google Scholar] [CrossRef]
- Ferrazza, R.; Cogo, S.; Melrose, H.; Bubacco, L.; Greggio, E.; Guella, G.; Civiero, L.; Plotegher, N. LRRK2 deficiency impacts ceramide metabolism in brain. Biochem. Biophys. Res. Commun. 2016, 478, 1141–1146. [Google Scholar] [CrossRef]
- Torres-Odio, S.; Key, J.; Hoepken, H.-H.; Canet-Pons, J.; Valek, L.; Roller, B.; Walter, M.; Morales-Gordo, B.; Meierhofer, D.; Harter, P.N.; et al. Progression of pathology in PINK1-deficient mouse brain from splicing via ubiquitination, ER stress, and mitophagy changes to neuroinflammation. J. Neuroinflammation 2017, 14, 1–26. [Google Scholar] [CrossRef]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Halliday, G.M.; et al. Lipid Pathway Alterations in Parkinson’s Disease Primary Visual Cortex. PLoS ONE 2011, 6, e17299. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Choi, H.; Chevalier, A.; Hogan, D.; Akgoc, Z.; Schneider, J.S. Sex-Related Abnormalities in Substantia Nigra Lipids in Parkinson’s Disease. ASN Neuro 2018, 10, 1759091418781889. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Halliday, G.M. Changes in Sphingomyelin Level Affect Alpha-Synuclein and ABCA5 Expression. J. Park. Dis. 2012, 2, 41–46. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Luberto, C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000, 10, 73–80. [Google Scholar] [CrossRef]
- Lucki, N.C.; Sewer, M.B. Nuclear Sphingolipid Metabolism. Annu. Rev. Physiol. 2012, 74, 131–151. [Google Scholar] [CrossRef]
- Ledeen, R.W.; Yu, R.K. [10] Gangliosides: Structure, isolation, and analysis. Methods Enzymol. 1982, 83, 139–191. [Google Scholar] [CrossRef]
- Ariga, T. The Pathogenic Role of Ganglioside Metabolism in Alzheimer’s Disease-Cholinergic Neuron-Specific Gangliosides and Neurogenesis. Mol Neurobiol. 2017, 54, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009, 50, S440–S445. [Google Scholar] [CrossRef]
- Schnaar, R.L. Glycolipid-mediated cell–cell recognition in inflammation and nerve regeneration. Arch. Biochem. Biophys. 2004, 426, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Van Echten-Deckert, G.; Guravi, G.V.E.-D.A.M. Golgi Localization of Glycosyltransferases Involved in Ganglioside Biosynthesis. Curr. Drug Targets 2008, 9, 282–291. [Google Scholar] [CrossRef]
- Yamaji, T.; Hanada, K. Sphingolipid Metabolism and Interorganellar Transport: Localization of Sphingolipid Enzymes and Lipid Transfer Proteins. Traffic 2014, 16, 101–122. [Google Scholar] [CrossRef]
- Möbius, W.; Herzog, V.; Sandhoff, K.; Schwarzmann, G. Intracellular Distribution of a Biotin-labeled Ganglioside, GM1, by Immunoelectron Microscopy after Endocytosis in Fibroblasts. J. Histochem. Cytochem. 1999, 47, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Chinnapen, D.J.-F.; Hsieh, W.-T.; Welscher, Y.M.T.; Saslowsky, D.E.; Kaoutzani, L.; Brandsma, E.; D’Auria, L.; Park, H.; Wagner, J.S.; Drake, K.R.; et al. Lipid Sorting by Ceramide Structure from Plasma Membrane to ER for the Cholera Toxin Receptor Ganglioside GM1. Dev. Cell 2012, 23, 573–586. [Google Scholar] [CrossRef]
- Sano, R.; Annunziata, I.; Patterson, A.; Moshiach, S.; Gomero, E.; Opferman, J.; Forte, M.; D’Azzo, A. GM1-Ganglioside Accumulation at the Mitochondria-Associated ER Membranes Links ER Stress to Ca2+-Dependent Mitochondrial Apoptosis. Mol. Cell 2009, 36, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Sorice, M.; Garofalo, T.; Misasi, R.; Manganelli, V.; Vona, R.; Malorni, W. Ganglioside GD3 as a Raft Component in Cell Death Regulation. Anti-Cancer Agents Med. Chem. 2012, 12, 376–382. [Google Scholar] [CrossRef]
- Xie, X.; Wu, G.; Lu, Z.-H.; Ledeen, R.W. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J. Neurochem. 2002, 81, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-T.; Itokazu, Y.; Yu, R.K. GM1 Ganglioside is Involved in Epigenetic Activation Loci of Neuronal Cells. Neurochem. Res. 2015, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.A.; Cross, H.; Proukakis, C.; Priestman, D.A.; Neville, D.C.A.; Reinkensmeier, G.; Wang, H.; Wiznitzer, M.; Gurtz, K.; Verganelaki, A.; et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat. Genet. 2004, 36, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, A.; Schüle-Freyer, R.; Loureiro, J.L.; Lourenço, C.M.; Mundwiller, E.; Gonzalez, M.A.; Charles, P.; Gauthier, J.; Rekik, I.; Lebrigio, R.F.A.; et al. Alteration of Ganglioside Biosynthesis Responsible for Complex Hereditary Spastic Paraplegia. Am. J. Hum. Genet. 2013, 93, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Fragaki, K.; Ait-El-Mkadem, S.; Chaussenot, A.; Gire, C.; Mengual, R.; Bonesso, L.; Bénéteau, M.; Ricci, J.E.; Desquiret-Dumas, V.; Procaccio, V.; et al. Refractory epilepsy and mitochondrial dysfunction due to GM3 synthase deficiency. Eur. J. Hum. Genet. 2012, 21, 528–534. [Google Scholar] [CrossRef]
- Harlalka, G.V.; Lehman, A.; Chioza, B.; Baple, E.L.; Maroofian, R.; Cross, H.; Sreekantan-Nair, A.; Priestman, D.A.; Al-Turki, S.; McEntagart, M.E.; et al. Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain 2013, 136, 3618–3624. [Google Scholar] [CrossRef]
- Boccuto, L.; Aoki, K.; Flanagan-Steet, H.; Chen, C.-F.; Fan, X.; Bartel, F.; Petukh, M.; Pittman, A.; Saul, R.A.; Chaubey, A.; et al. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum. Mol. Genet. 2013, 23, 418–433. [Google Scholar] [CrossRef]
- Wakil, S.M.; Monies, D.M.; Ramzan, K.; Hagos, S.; Bastaki, L.; Meyer, B.; Bohlega, S. NovelB4GALNT1mutations in a complicated form of hereditary spastic paraplegia. Clin. Genet. 2013, 86, 500–501. [Google Scholar] [CrossRef]
- Allende, M.L.; Proia, R.L. Simplifying complexity: Genetically resculpting glycosphingolipid synthesis pathways in mice to reveal function. Glycoconj. J. 2014, 31, 613–622. [Google Scholar] [CrossRef]
- Segler-Stahl, K.; Webster, J.C.; Brunngraber, E.G. Changes in the Concentration and Composition of Human Brain Gangliosides with Aging. Gerontology 1983, 29, 161–168. [Google Scholar] [CrossRef]
- Palestini, P.; Masserini, M.; Sonnino, S.; Giuliani, A.; Tettamanti, G. Changes in the Ceramide Composition of Rat Forebrain Gangliosides with Age. J. Neurochem. 1990, 54, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kracun, I.; Rosner, H.; Drnovsek, V.; Vukelic, Z.; Cosovic, C.; Trbojevic-Cepe, M.; Kubat, M. Gangliosides in the human brain development and aging. Neurochem. Int. 1992, 20, 421–431. [Google Scholar] [CrossRef]
- Mo, L.; Ren, Q.; Duchemin, A.-M.; Neff, N.H.; Hadjiconstantinou, M. GM1 and ERK signaling in the aged brain. Brain Res. 2005, 1054, 125–134. [Google Scholar] [CrossRef]
- Blennow, K.; Davidsson, P.; Wallin, A.; Fredman, P.; Gottfries, C.-G.; Karlsson, I.; Månsson, J.-E.; Svennerholm, L. Gangliosides in Cerebrospinal Fluid in “Probable Alzheimer’s Disease”. Arch. Neurol. 1991, 48, 1032–1035. [Google Scholar] [CrossRef]
- Blennow, K.; Davidsson, P.; Wallin, A.; Frcdman, P.; Gottfries, C.G.; Mansson, J.; Svennerholm, L. Differences in cerebrospinal fluid gangliosides between “probable Alzheimer’s disease” and normal aging. Aging Clin. Exp. Res. 1992, 4, 301–306. [Google Scholar] [CrossRef]
- Desplats, P.; Denny, C.A.; Kass, K.E.; Gilmartin, T.; Head, S.R.; Sutcliffe, J.G.; Seyfried, T.; Thomas, E.A. Glycolipid and ganglioside metabolism imbalances in Huntington’s disease. Neurobiol. Dis. 2007, 27, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Maglione, V.; Marchi, P.; di Pardo, A.; Lingrell, S.; Horkey, M.; Tidmarsh, E.; Sipione, S. Impaired Ganglioside Metabolism in Huntington’s Disease and Neuroprotective Role of GM1. J. Neurosci. 2010, 30, 4072–4080. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.-H.; Kulkarni, N.; Ledeen, R.W. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 2012, 90, 1997–2008. [Google Scholar] [CrossRef]
- Di Pasquale, E.; Fantini, J.; Chahinian, H.; Maresca, M.; Taïeb, N.; Yahi, N. Altered Ion Channel Formation by the Parkinson’s-Disease-Linked E46K Mutant of α-Synuclein Is Corrected by GM3 but Not by GM1 Gangliosides. J. Mol. Biol. 2010, 397, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.; Dunning, C.J.; Gaspar, R.; Grey, C.; Brundin, P.; Sparr, E.; Linse, S. Acceleration of α-Synuclein Aggregation by Exosomes. J. Biol. Chem. 2015, 290, 2969–2982. [Google Scholar] [CrossRef]
- Schneider, J.S. Altered expression of genes involved in ganglioside biosynthesis in substantia nigra neurons in Parkinson’s disease. PLoS ONE 2018, 13, e0199189. [Google Scholar] [CrossRef] [PubMed]
- Lobasso, S.; Tanzarella, P.; Vergara, D.; Maffia, M.; Cocco, T.; Corcelli, A. Lipid profiling of parkin -mutant human skin fibroblasts. J. Cell. Physiol. 2017, 232, 3540–3551. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Odaka, A.; Suzuki, N.; Ihara, Y. GM1 ganglioside–bound amyloid β–protein (Aβ): A possible form of preamyloid in Alzheimer’s disease. Nat. Med. 1995, 1, 1062–1066. [Google Scholar] [CrossRef]
- Hoshino, T.; Mahmood, I.; Mori, K.; Matsuzaki, K. Binding and Aggregation Mechanism of Amyloid β-Peptides onto the GM1 Ganglioside-Containing Lipid Membrane. J. Phys. Chem. B 2013, 117, 8085–8094. [Google Scholar] [CrossRef]
- Chan, R.B.; Perotte, A.; Zhou, B.; Liong, C.; Shorr, E.J.; Marder, K.S.; Kang, U.J.; Waters, C.H.; Levy, O.A.; Xu, Y.; et al. Elevated GM3 plasma concentration in idiopathic Parkinson’s disease: A lipidomic analysis. PLoS ONE 2017, 12, e0172348. [Google Scholar] [CrossRef]
- Suzuki, K.; Iseki, E.; Togo, T.; Yamaguchi, A.; Katsuse, O.; Katsuyama, K.; Kanzaki, S.; Shiozaki, K.; Kawanishi, C.; Yamashita, S.; et al. Neuronal and glial accumulation of α- and β-synucleins in human lipidoses. Acta Neuropathol. 2007, 114, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Distefano, L. Response of the damaged dopamine system to gm1 and semisynthetic gangliosides: Effects of dose and extent of lesion. Neuropharmacology 1995, 34, 489–493. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, K.S.; Lee, S.-B.; Ryu, J.-S.; Chung, K.C.; Choo, Y.-K.; Jou, I.; Kim, J.; Park, S.M. On the mechanism of internalization of α-synuclein into microglia: Roles of ganglioside GM1 and lipid raft. J. Neurochem. 2009, 110, 400–411. [Google Scholar] [CrossRef]
- Reddan, J.M.; White, D.J.; Macpherson, H.; Scholey, A.; Pipingas, A. Glycerophospholipid Supplementation as a Potential Intervention for Supporting Cerebral Structure in Older Adults. Front. Aging Neurosci. 2018, 10, 49. [Google Scholar] [CrossRef]
- Glomset, J.A. Role of docosahexaenoic acid in neuronal plasma membranes. Sci STKE. 2006. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A. Excitotoxicity and Neurological Disorders: Involvement of Membrane Phospholipids. In International Review of Neurobiology; Bradley, R.J., Harris, R.A., Eds.; Academic Press: Cambridge, MA, USA, 1994; pp. 267–323. [Google Scholar]
- Farooqui, A.A.; Horrocks, L.A. Plasmalogen-selective phospholipase A2 and its involvement in Alzheimer’s disease. Biochem. Soc. Trans. 1998, 26, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Gattaz, W.F.; Maras, A.; Cairns, N.J.; Levy, R.; Förstl, H. Decreased phospholipase A2 activity in Alzheimer brains. Biol. Psychiatry 1995, 37, 13–17. [Google Scholar] [CrossRef]
- Shvadchak, V.; Lockhart, L.J.F.; Yushchenko, D.A.; Jovin, T.M. Specificity and Kinetics of α-Synuclein Binding to Model Membranes Determined with Fluorescent Excited State Intramolecular Proton Transfer (ESIPT) Probe. J. Biol. Chem. 2011, 286, 13023–13032. [Google Scholar] [CrossRef]
- Davidson, W.S.; Jonas, A.; Clayton, D.F.; George, J.M. Stabilization of α-Synuclein Secondary Structure upon Binding to Synthetic Membranes. J. Biol. Chem. 1998, 273, 9443–9449. [Google Scholar] [CrossRef] [PubMed]
- Sztacho, M.; Šalovská, B.; Červenka, J.; Balaban, C.; Hoboth, P.; Hozák, P. Limited Proteolysis-Coupled Mass Spectrometry Identifies Phosphatidylinositol 4,5-Bisphosphate Effectors in Human Nuclear Proteome. Cells 2021, 10, 68. [Google Scholar] [CrossRef]
- Frere, S.G.; Chang-Ileto, B.; di Paolo, G. Role of Phosphoinositides at the Neuronal Synapse. Subcell. Biochem. 2012, 59, 131–175. [Google Scholar] [CrossRef]
- Alza, N.P.; Iglesias González, P.A.; Conde, M.A.; Uranga, R.M.; Salvador, G.A. Lipids at the Crossroad of α-Synuclein Function and Dysfunction: Biological and Pathological Implications. Front. Cell. Neurosci. 2019, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Y.; Wang, X. Phosphatidic Acid-Mediated Signaling. Adv. Exp. Med. Biol. 2013, 991, 159–176. [Google Scholar] [CrossRef]
- Perrin, R.J.; Woods, W.S.; Clayton, D.F.; George, J.M. Interaction of human alpha-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J. Biol. Chem. 2000, 275, 34393–34398. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, C.; Zhao, N.; Li, W.; Yang, Z.; Liu, X.; Le, W.; Zhang, X. Potential biomarkers of Parkinson’s disease revealed by plasma metabolic profiling. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1081–1082, 101–108. [Google Scholar] [CrossRef]
- Jiang, Z.; Flynn, J.D.; Teague, W.E., Jr.; Gawrisch, K.; Lee, J.C. Stimulation of α-synuclein amyloid formation by phosphatidylglycerol micellar tubules. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 1840–1847. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Wang, L.; Yang, C. High Performance Liquid Chromatography-Mass Spectrometry (LC-MS) Based Quantitative Lipidomics Study of Ganglioside-NANA-3 Plasma to Establish Its Association with Parkinson’s Disease Patients. Med Sci. Monit. 2017, 23, 5345–5353. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hancock, J.F. Compartmentalization of Ras proteins. J. Cell Sci. 2001, 114, 1603–1608. [Google Scholar] [CrossRef]
- Simons, M.; Keller, P.; Dichgans, J.; Schulz, J.B. Cholesterol and Alzheimer’s disease: Is there a link? Neurology 2001, 57, 1089–1093. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.-C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Goritz, C.; Mauch, D.H.; Pfrieger, F.W. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol. Cell. Neurosci. 2005, 29, 190–201. [Google Scholar] [CrossRef]
- Russell, D.W.; Setchell, K.D.R. Bile acid biosynthesis. Biochemistry 1992, 31, 4737–4749. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F. Determinants of plasma HDL concentrations and reverse cholesterol transport. Curr. Opin. Cardiol. 2006, 21, 345–352. [Google Scholar] [CrossRef]
- Hampton, R.; Dimster-Denk, D.; Rine, J. The biology of HMG-CoA reductase: The pros of contra-regulation. Trends Biochem. Sci. 1996, 21, 140–145. [Google Scholar] [CrossRef]
- Elshourbagy, N.A.; Meyers, H.V.; Abdel-Meguid, S.S. Cholesterol: The Good, the Bad, and the Ugly—Therapeutic Targets for the Treatment of Dyslipidemia. Med Princ. Pr. 2013, 23, 99–111. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Song, W.-L. Egg yolk, source of bad cholesterol and good lipids? Am. J. Clin. Nutr. 2019, 110, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Porter, F.D.; Herman, G.E. Malformation syndromes caused by disorders of cholesterol synthesis. J. Lipid Res. 2011, 52, 6–34. [Google Scholar] [CrossRef] [PubMed]
- Khosla, P.; Hayes, K.C. Dietary palmitic acid raises plasma LDL cholesterol relative to oleic acid only at a high intake of cholesterol. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1993, 1210, 13–22. [Google Scholar] [CrossRef]
- Pollin, T.I.; Quartuccio, M. What We Know About Diet, Genes, and Dyslipidemia: Is There Potential for Translation? Curr. Nutr. Rep. 2013, 2, 236–242. [Google Scholar] [CrossRef]
- Jira, P. Cholesterol metabolism deficiency. Handb. Clin. Neurol. 2013, 113, 1845–1850. [Google Scholar] [CrossRef]
- Kluck, G.E.G.; Yoo, J.-A.; Sakarya, E.H.; Trigatti, B.L. Good Cholesterol Gone Bad? HDL and COVID-19. Int. J. Mol. Sci. 2021, 22, 10182. [Google Scholar] [CrossRef]
- Feig, J.E.; Hewing, B.; Smith, J.D.; Hazen, S.L.; Fisher, E.A. High-Density Lipoprotein and Atherosclerosis Regression. Circ. Res. 2014, 114, 205–213. [Google Scholar] [CrossRef]
- Kratzer, A.; Giral, H.; Landmesser, U. High-density lipoproteins as modulators of endothelial cell functions: Alterations in patients with coronary artery disease. Cardiovasc. Res. 2014, 103, 350–361. [Google Scholar] [CrossRef]
- Moghadasian, M.H.; Salen, G.; Frohlich, J.J.; Scudamore, C.H. Cerebrotendinous Xanthomatosis: A Rare Disease with Diverse Manifestations. Arch. Neurol. 2002, 59, 527–529. [Google Scholar] [CrossRef]
- Puntoni, M.; Sbrana, F.; Bigazzi, F.; Sampietro, T. Tangier Disease: Epidemiology, pathophysiology, and management. Am. J. Cardiovasc. Drugs 2012, 12. [Google Scholar] [CrossRef]
- Lester, D. Serum cholesterol levels and suicide: A meta-analysis. Suicide Life-Threatening Behav. 2002, 32, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.; Busch, K.A.; Jacobs, D.; Kravitz, H.M.; Fogg, L. Suicide: A four-pathway clinical-biochemical model. Ann. New York Acad. Sci. 1997, 836, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carpio, P.A.; Barba, J.; Campillo, A.B.-D. Relation between cholesterol levels and neuropsychiatric disorders. Rev. Neurol. 2009, 48, 261–264. (In Spanish) [Google Scholar] [PubMed]
- Valenza, M.; Rigamonti, D.; Goffredo, D.; Zuccato, C.; Fenu, S.; Jamot, L.; Strand, A.; Tarditi, A.; Woodman, B.; Racchi, M.; et al. Dysfunction of the Cholesterol Biosynthetic Pathway in Huntington’s Disease. J. Neurosci. 2005, 25, 9932–9939. [Google Scholar] [CrossRef]
- Varghese, M.J. Familial hypercholesterolemia: A review. Ann. Pediatr. Cardiol. 2014, 7, 107–117. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.; Qi, X.; Huang, C.; Hou, L. Cholesterol Levels and Risk of Hemorrhagic Stroke: A systematic review and meta-analysis. Stroke 2013, 44, 1833–1839. [Google Scholar] [CrossRef]
- Ancelin, M.-L.; Carriere, I.; Boulenger, J.-P.; Malafosse, A.; Stewart, R.; Cristol, J.-P.; Ritchie, K.; Chaudieu, I.; Dupuy, A.M. Gender and Genotype Modulation of the Association Between Lipid Levels and Depressive Symptomatology in Community-Dwelling Elderly (The ESPRIT Study). Biol. Psychiatry 2010, 68, 125–132. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Stevens, M.V.; Akter, M.H.; Rusk, S.E.; Huang, R.J.; Cohen, A.; Noguchi, A.; Springer, D.; Bocharov, A.V.; Eggerman, T.L.; et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Investig. 2011, 121, 3701–3712. [Google Scholar] [CrossRef]
- Eriksson, I.; Nath, S.; Bornefall, P.; Giraldo, A.M.V.; Öllinger, K. Impact of high cholesterol in a Parkinson’s disease model: Prevention of lysosomal leakage versus stimulation of α-synuclein aggregation. Eur. J. Cell Biol. 2017, 96, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kamp, F.; Beyer, K. Binding of α-Synuclein Affects the Lipid Packing in Bilayers of Small Vesicles. J. Biol. Chem. 2006, 281, 9251–9259. [Google Scholar] [CrossRef]
- Bar-On, P.; Rockenstein, E.; Adame, A.; Ho, G.; Hashimoto, M.; Masliah, E. Effects of the cholesterol-lowering compound methyl-β-cyclodextrin in models of α-synucleinopathy. J. Neurochem. 2006, 98, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Gregório, M.; Pinhel, M.A.S.; Sado, C.L.; Longo, G.S.; Oliveira, F.N.; Amorim, G.S.; Nakazone, M.A.; Florim, G.M.; Mazeti, C.M.; Martins, D.P.; et al. Impact of Genetic Variants of Apolipoprotein E on Lipid Profile in Patients with Parkinson’s Disease. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Wang, M.; Sterling, N.W.; Du, G.; Lewis, M.M.; Yao, T.; Mailman, R.B.; Li, R.; Huang, X. Circulating Cholesterol Levels May Link to the Factors Influencing Parkinson’s Risk. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Alonso, A.; Guo, X.; Umbach, D.M.; Lichtenstein, M.L.; Ballantyne, C.M.; Mailman, R.; Mosley, T.H.; Chen, H. Statins, plasma cholesterol, and risk of Parkinson’s disease: A prospective study. Mov. Disord. 2015, 30, 552–559. [Google Scholar] [CrossRef]
- Ma, V.R.; Gurevich, T.; Giladi, N.; El-Ad, B.; Tsamir, J.; Hemo, B.; Peretz, C. Higher serum cholesterol and decreased Parkinson’s disease risk: A statin-free cohort study. Mov. Disord. 2018, 33, 1298–1305. [Google Scholar] [CrossRef]
- Gudala, K.; Bansal, D.; Muthyala, H. Role of Serum Cholesterol in Parkinson’s Disease: A Meta-Analysis of Evidence. J. Park. Dis. 2013, 3, 363–370. [Google Scholar] [CrossRef]
- Magalhaes, J.; Gegg, M.E.; Migdalska-Richards, A.; Doherty, M.K.; Whitfield, P.D.; Schapira, A.H. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: Relevance to Parkinson disease. Hum. Mol. Genet. 2016, 25, 3432–3445. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, P.; Coelho, J.; Sousa-Lima, I.; Macedo, M.P.; Lopes, L.V.; Outeiro, T.F.; Pais, T.F. Mutant A53T α-Synuclein Improves Rotarod Performance Before Motor Deficits and Affects Metabolic Pathways. NeuroMolecular Med. 2016, 19, 113–121. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yamane, T.; Takahashi-Niki, K.; Kato, I.; Niki, T.; Goldberg, M.S.; Shen, J.; Ishimoto, K.; Doi, T.; Iguchi-Ariga, S.M.M.; et al. Transcriptional Activation of Low-Density Lipoprotein Receptor Gene by DJ-1 and Effect of DJ-1 on Cholesterol Homeostasis. PLoS ONE 2012, 7, e38144. [Google Scholar] [CrossRef]
- Shioda, N.; Yabuki, Y.; Kobayashi, Y.; Onozato, M.; Owada, Y.; Fukunaga, K. FABP3 Protein Promotes α-Synuclein Oligomerization Associated with 1-Methyl-1,2,3,6-tetrahydropiridine-induced Neurotoxicity. J. Biol. Chem. 2014, 289, 18957–18965. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Geens, A.; Böhm, C.; Deaulmerie, L.; Swerts, J.; Rossi, M.; Craessaerts, K.; Leites, E.; Seibler, P.; Rakovic, A.; et al. Cardiolipin promotes electron transport between ubiquinone and complex I to rescue PINK1 deficiency. J. Cell Biol. 2017, 216, 695–708. [Google Scholar] [CrossRef]
- Cha, S.-H.; Choi, Y.R.; Heo, C.-H.; Kang, S.-J.; Joe, E.-H.; Jou, I.; Kim, H.-M.; Park, S.M. Loss of parkin promotes lipid rafts-dependent endocytosis through accumulating caveolin-1: Implications for Parkinson’s disease. Mol. Neurodegener. 2015, 10, 63. [Google Scholar] [CrossRef]
- Guo, X.; Song, W.; Chen, K.; Chen, X.; Zheng, Z.; Cao, B.; Huang, R.; Zhao, B.; Wu, Y.; Shang, H.-F. The serum lipid profile of Parkinson’s disease patients: A study from China. Int. J. Neurosci. 2014, 125, 838–844. [Google Scholar] [CrossRef]
- Ogburn, K.D.; Figueiredo-Pereira, M.E. Cytoskeleton/Endoplasmic Reticulum Collapse Induced by Prostaglandin J2 Parallels Centrosomal Deposition of Ubiquitinated Protein Aggregates. J. Biol. Chem. 2006, 281, 23274–23284. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Jenner, A.M.; Spiro, A.S.; Batterham, M.; Halliday, G.M.; Garner, B. Fatty Acid Composition of the Anterior Cingulate Cortex Indicates a High Susceptibility to Lipid Peroxidation in Parkinson’s Disease. J. Park. Dis. 2015, 5, 175–185. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L. Microglia and Beyond: Innate Immune Cells as Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef]
- Guerrini, V.; Gennaro, M.L. Foam Cells: One Size Doesn’t Fit All. Trends Immunol. 2019, 40, 1163–1179. [Google Scholar] [CrossRef]
- Thiam, A.R.; Farese, R.V.F., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.-C.; Everts, B.; Ivanova, Y.; O’Sullivan, D.; Nascimento, M.; Smith, A.M.; Beatty, W.; Love-Gregory, L.; Lam, W.Y.; O’Neill, C.M.; et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 2014, 15, 846–855. [Google Scholar] [CrossRef]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef]
- Castoldi, A.; Monteiro, L.B.; Bakker, N.V.T.; Sanin, D.E.; Rana, N.; Corrado, M.; Cameron, A.M.; Hässler, F.; Matsushita, M.; Caputa, G.; et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Grabowski, G.A.; Köhl, J. An unexpected player in Gaucher disease: The multiple roles of complement in disease development. Semin. Immunol. 2018, 37, 30–42. [Google Scholar] [CrossRef]
- Van Eijk, M.; Aerts, J.M. The Unique Phenotype of Lipid-Laden Macrophages. Int. J. Mol. Sci. 2021, 22, 4039. [Google Scholar] [CrossRef] [PubMed]
- Serfecz, J.C.; Saadin, A.; Santiago, C.P.; Zhang, Y.; Bentzen, S.M.; Vogel, S.N.; Feldman, R.A. C5a Activates a Pro-Inflammatory Gene Expression Profile in Human Gaucher iPSC-Derived Macrophages. Int. J. Mol. Sci. 2021, 22, 9912. [Google Scholar] [CrossRef]
- Magnusen, A.F.; Rani, R.; McKay, M.A.; Hatton, S.L.; Nyamajenjere, T.C.; Magnusen, D.N.A.; Köhl, J.; Grabowski, G.A.; Pandey, M.K. C-X-C Motif Chemokine Ligand 9 and Its CXCR3 Receptor Are the Salt and Pepper for T Cells Trafficking in a Mouse Model of Gaucher Disease. Int. J. Mol. Sci. 2021, 22, 12712. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Zhang, S.-P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, C.-C.; Chiang, H.-L.; Liou, J.-M.; Chang, C.-M.; Lu, T.-P.; Chuang, E.Y.; Tai, Y.-C.; Cheng, C.; Lin, H.-Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflammation 2019, 16, 1–9. [Google Scholar] [CrossRef]
- Chao, Y.; Wong, S.C.; Tan, E.K. Evidence of Inflammatory System Involvement in Parkinson’s Disease. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Dri, P.; Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1990, 39, 151–170. [Google Scholar] [CrossRef]
- Ho, M.S. Microglia in Parkinson’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 335–353. [Google Scholar] [CrossRef]
- Lecours, C.; Bordeleau, M.; Cantin, L.; Parent, M.; di Paolo, T.; Tremblay, M. Microglial Implication in Parkinson’s Disease: Loss of Beneficial Physiological Roles or Gain of Inflammatory Functions? Front. Cell. Neurosci. 2018, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. Role of Cytokines in Inducing Hyperlipidemia. Diabetes 1992, 41 (Suppl 2), 97–101. [Google Scholar] [CrossRef]
- Tavares, F.L.; Seelaender, M.C.L. Hepatic denervation impairs the assembly and secretion of VLDL-TAG. Cell Biochem. Funct. 2008, 26, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hohjoh, H.; Inazumi, T.; Tsuchiya, S.; Sugimoto, Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1851, 414–421. [Google Scholar] [CrossRef]
- Geng, Y.; Fang, M.; Wang, J.; Yu, H.; Hu, Z.; Yew, D.T.; Chen, W. Triptolide Down-regulates COX-2 Expression and PGE2 Release by Suppressing the Activity of NF-κB and MAP kinases in Lipopolysaccharide-treated PC12 Cells. Phytotherapy Res. 2011, 26, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.-W.; Zhang, T.; Fu, H.; Liu, G.-X.; Wang, X.-M. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur. J. Pharmacol. 2012, 692, 29–37. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Wang, Y.; Fong, H.; Murray, A.T.M.; Zhang, J. Identification of Proteins Involved in Microglial Endocytosis of α-Synuclein. J. Proteome Res. 2007, 6, 3614–3627. [Google Scholar] [CrossRef]
- Dey, I.; Lejeune, M.; Chadee, K. Prostaglandin E2receptor distribution and function in the gastrointestinal tract. J. Cereb. Blood Flow Metab. 2006, 149, 611–623. [Google Scholar] [CrossRef]
- Yu, S.-Y.; Zuo, L.-J.; Wang, F.; Chen, Z.-J.; Hu, Y.; Wang, Y.-J.; Wang, X.-M.; Zhang, W. Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: A cross-sectional study. BMC Neurol. 2014, 14, 113. [Google Scholar] [CrossRef]
- Ashley, A.K.; Hinds, A.I.; Hanneman, W.H.; Tjalkens, R.B.; Legare, M.E. DJ-1 mutation decreases astroglial release of inflammatory mediators. NeuroToxicology 2016, 52, 198–203. [Google Scholar] [CrossRef]
- Ahmad, A.S.; Maruyama, T.; Narumiya, S.; Doré, S. PGE2 EP1 Receptor Deletion Attenuates 6-OHDA-Induced Parkinsonism in Mice: Old Switch, New Target. Neurotox. Res. 2013, 23, 260–266. [Google Scholar] [CrossRef]
- Carrasco, E.; Werner, P.; Casper, D. Prostaglandin receptor EP2 protects dopaminergic neurons against 6-OHDA-mediated low oxidative stress. Neurosci. Lett. 2008, 441, 44–49. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Salinas, K.; Garduno, A.C.; Johansson, J.U.; Wang, Q.; Manning-Bog, A.; Andreasson, K.I. Anti-Inflammatory and Neuroprotective Effects of PGE2 EP4 Signaling in Models of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2016, 12, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Roeske-Nielsen, A.; Fredman, P.; Mansson, J.E.; Bendtzen, K.; Buschard, K. Beta-galactosylceramide increases and sulfatide decreases cytokine and chemokine production in whole blood cells. Immunol. Lett. 2004, 91, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Buschard, K.; Josefsen, K.; Horn, T.; Larsen, S.; Fredman, P. Sulphatide antigen in islets of Langerhans and in diabetic glomeruli, and anti-sulphatide antibodies in Type 1 diabetes mellitus. APMIS 1993, 101, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Fredman, P.; Månsson, J.-E.; Rynmark, B.-M.; Josefsen, K.; Ekblond, A.; Halldner, L.; Osterbye, T.; Horn, T.; Buschard, K. The glycosphingolipid sulfatide in the islets of Langerhans in rat pancreas is processed through recycling: Possible involvement in insulin trafficking. Glycobiology 2000, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Bendtzen, K.; Mandrup-Poulsen, T.; Nerup, J.; Nielsen, J.H.; Dinarello, C.A.; Svenson, M. Cytotoxicity of Human p I 7 Interleukin-1 for Pancreatic Islets of Langerhans. Science 1986, 232, 1545–1547. [Google Scholar] [CrossRef]
- Buschard, K.; Schloot, N.C.; Kaas, A.; Bock, T.; Horn, T.; Fredman, P.; Roep, B.O. Inhibition of insulin-specific autoreactive T-cells by sulphatide which is variably expressed in beta cells. Diabetologia 1999, 42, 1212–1218. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, G.; Nie, H.; Li, S.; He, S.; Yang, J. Sulfatide-activated type II NKT cells suppress immunogenic maturation of lung dendritic cells in murine models of asthma. Am. J. Physiol. Cell. Mol. Physiol. 2019, 317, L578–L590. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. RIG-I-like antiviral protein in flies. Nat. Immunol. 2008, 9, 1327–1328. [Google Scholar] [CrossRef]
- Matzinger, P. Friendly and dangerous signals: Is the tissue in control? Nat. Immunol. 2007, 8, 11–13. [Google Scholar] [CrossRef]
- Alisi, A.; Carsetti, R.; Nobili, V. Pathogen- or damage-associated molecular patterns during nonalcoholic fatty liver disease development. Hepatology 2011, 54, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Gauley, J.; Ullal, A.J. HMGB1 and Microparticles as Mediators of the Immune Response to Cell Death. Antioxidants Redox Signal. 2011, 15, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Ochiel, D.O.; Rossoll, R.M.; Schaefer, T.M.; Wira, C.R. Effect of oestradiol and pathogen-associated molecular patterns (PAMP) on class II-mediated antigen presentation and immunomodulatory molecule expression in the mouse female reproductive tract. Immunology 2011, 135, 51–62. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Lu, G.-H.; Zhang, S.-Z.; Wang, B.-Y.; Ye, Y.-Y.; Qian, C.; Zhang, H.-B.; Mao, H.-X.; Yao, L.-P.; Sun, X. Stress increases MHC-I expression in dopaminergic neurons and induces autoimmune activation in Parkinson’s disease. Neural Regen. Res. 2021, 16, 2521–2527. [Google Scholar] [CrossRef]
- Sulzer, D.; Alcalay, R.N.; Garretti, F.; Cote, L.; Kanter, E.; Agin-Liebes, J.; Liong, C.; McMurtrey, C.; Hildebrand, W.H.; Mao, X.; et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 2017, 546, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ferrer, I.; Bäckström, F.; Dueñas-Rey, A.; Jewett, M.; Boza-Serrano, A.; Luk, K.C.; Deierborg, T.; Swanberg, M. The MHC class II transactivator modulates seeded alpha-synuclein pathology and dopaminergic neurodegeneration in an in vivo rat model of Parkinson’s disease. Brain Behav. Immun. 2021, 91, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Pamer, E.; Cresswell, P. Mechanisms of Mhc Class I–Restricted Antigen Processing. Annu. Rev. Immunol. 1998, 16, 323–358. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, P. Assembly, Transport, and Function of MHC Class II Molecules. Annu. Rev. Immunol. 1994, 12, 259–291. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huseby, E.; Scott-Browne, J.; Rubtsova, K.; Pinilla, C.; Crawford, F.; Marrack, P.; Dai, S.; Kappler, J.W. A Single T Cell Receptor Bound to Major Histocompatibility Complex Class I and Class II Glycoproteins Reveals Switchable TCR Conformers. Immunity 2011, 35, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, P.A.; Balk, S.P.; Hagen, S.J.; Blumberg, R.S.; Flotte, T.J.; Terhorst, C. Expression of Murine CD1 on Gastrointestinal Epithelium. Science 1990, 250, 679–682. [Google Scholar] [CrossRef]
- Porcelli, S.A.; Modlin, R.L. THE CD1 SYSTEM: Antigen-Presenting Molecules for T Cell Recognition of Lipids and Glycolipids. Annu. Rev. Immunol. 1999, 17, 297–329. [Google Scholar] [CrossRef]
- Vincent, M.S.; Xiong, X.; Grant, E.P.; Peng, W.; Brenner, M.B. CD1a-, b-, and c-Restricted TCRs Recognize Both Self and Foreign Antigens. J. Immunol. 2005, 175, 6344–6351. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.P.; Degano, M.; Rosat, J.-P.; Stenger, S.; Modlin, R.; Wilson, I.A.; Porcelli, S.A.; Brenner, M.B. Molecular Recognition of Lipid Antigens by T Cell Receptors. J. Exp. Med. 1999, 189, 195–205. [Google Scholar] [CrossRef]
- Shamshiev, A.; Donda, A.; Carena, I.; Mori, L.; Kappos, L.; de Libero, G. Self glycolipids as T-cell auto-antigens. Eur. J. Immunol. 1999, 29, 1667–1675. [Google Scholar] [CrossRef]
- Mattner, J.; DeBord, K.L.; Ismail, N.; Goff, R.D.; Cantu, C.; Zhou, D.; Saint-Mezard, P.; Wang, V.; Gao, Y.; Yin, N.; et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 2005, 434, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.R.; Garg, S.; Brenner, M.B. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv. Immunol. 2009, 102, 1–94. [Google Scholar] [CrossRef]
- Brigl, M.; Brenner, M.B. CD1: Antigen Presentation and T Cell Function (Review). Annu. Rev. Immunol. 2004, 22, 817–890. [Google Scholar] [CrossRef]
- Albutti, A.; Longet, S.; McEntee, C.; Quinn, S.; Liddicoat, A.; Rîmniceanu, C.; Lycke, N.; Lynch, L.; Cardell, S.; Lavelle, E. Type II NKT Cell Agonist, Sulfatide, Is an Effective Adjuvant for Oral Heat-Killed Cholera Vaccines. Vaccines 2021, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Kumar, V. Type II NKT cells: A distinct CD1d-restricted immune regulatory NKT cell subset. Immunogenetics 2016, 68, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Masuda, S.; Tomaru, U.; Ishizu, A. CD1d-Restricted Type II NKT Cells Reactive with Endogenous Hydrophobic Peptides. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Stax, A.M.; Tuengel, J.; Girardi, E.; Kitano, N.; Allan, L.L.; Liu, V.; Zheng, D.; Panenka, W.J.; Guillaume, J.; Wong, C.-H.; et al. Autoreactivity to Sulfatide by Human Invariant NKT Cells. J. Immunol. 2017, 199, 97–106. [Google Scholar] [CrossRef]
- Roy, K.C.; Maricic, I.; Khurana, A.; Smith, T.R.F.; Halder, R.C.; Kumar, V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J. Immunol. 2008, 180, 2942–2950. [Google Scholar] [CrossRef] [PubMed]
- Barral, D.C.; Brenner, M.B. CD1 antigen presentation: How it works. Nat. Rev. Immunol. 2007, 7, 929–941. [Google Scholar] [CrossRef]
- De la Salle, H.; Mariotti, S.; Angenieux, C.; Gilleron, M.; Garcia-Alles, L.F.; Malm, D.; Berg, T.; Paoletti, S.; Maître, B.; Mourey, L.; et al. Assistance of microbial glycolipid antigen pro-cessing by CD1e. Science 2005, 310, 1321–1324. [Google Scholar] [CrossRef]
- Ichikawa, S.; Sakiyama, H.; Suzuki, G.; Hidari, K.I.; Hirabayashi, Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc. Natl. Acad. Sci. USA 1996, 93, 4638–4643. [Google Scholar] [CrossRef]
- Yuan, W.; Qi, X.; Tsang, P.; Kang, S.-J.; Illarionov, P.A.; Besra, G.; Gumperz, J.; Cresswell, P. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc. Natl. Acad. Sci. USA 2007, 104, 5551–5556. [Google Scholar] [CrossRef]
- Zhou, D.; Cantu, C.; Sagiv, Y.; Schrantz, N.; Kulkarni, A.B.; Qi, X.; Mahuran, D.J.; Morales, C.R.; Grabowski, G.A.; Benlagha, K.; et al. Editing of CD1d-Bound Lipid Antigens by Endosomal Lipid Transfer Proteins. Science 2004, 303, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-J.; Cresswell, P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat. Immunol. 2004, 5, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gerlini, G.; Tun-Kyi, A.; Dudli, C.; Burg, G.; Pimpinelli, N.; Nestle, F.O. Metastatic Melanoma Secreted IL-10 Down-Regulates CD1 Molecules on Dendritic Cells in Metastatic Tumor Lesions. Am. J. Pathol. 2004, 165, 1853–1863. [Google Scholar] [CrossRef]
- Vogt, A.B.; Kropshofer, H.; Moldenhauer, G.; Hammerling, G.J. Kinetic analysis of peptide loading onto HLA-DR molecules mediated by HLA-DM. Proc. Natl. Acad. Sci. USA 1996, 93, 9724–9729. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, Y.; Wu, D.; Kim, G.; Xing, G.-W.; Poles, M.; Ho, D.D.; Tsuji, M.; Kawahara, K.; Wong, C.-H.; Kronenberg, M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005, 434, 520–525. [Google Scholar] [CrossRef]
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef]
- Michel, M.L.; Keller, A.C.; Paget, C.; Fujio, M.; Trottein, F.; Savage, P.B.; Wong, C.H.; Schneider, E. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007, 204, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Coquet, J.M.; Kyparissoudis, K.; Pellicci, D.; Besra, G.; Berzins, S.P.; Smyth, M.J.; Godfrey, D.I. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 2007, 178, 2827–2834. [Google Scholar] [CrossRef]
- Beckman, E.M.; Porcelli, S.A.; Morita, C.T.; Behar, S.; Furlong, S.T.; Brenner, M.B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 1994, 372, 691–694. [Google Scholar] [CrossRef]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-Restricted and TCR-Mediated Activation of V α 14 NKT Cells by Glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, E.E.; Matsumoto, T.; Exley, M.; Schleipman, R.A.; Glickman, J.; Bailey, D.T.; Corazza, N.; Colgan, S.P.; Onderdonk, A.B.; Blumberg, R.S. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 2002, 8, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.S.; Siomos, M.-A.; Buckingham, L.; Scalzo, A.A.; Schofield, L. Regulation of Murine Cerebral Malaria Pathogenesis by CD1d-Restricted NKT Cells and the Natural Killer Complex. Immunity 2003, 18, 391–402. [Google Scholar] [CrossRef]
- Kawakami, K.; Kinjo, Y.; Uezu, K.; Yara, S.; Miyagi, K.; Koguchi, Y.; Nakayama, T.; Taniguchi, M.; Saito, A. Monocyte chemoattractant protein-1-dependent increase of V alpha 14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J. Immunol. 2001, 167, 6525–6532. [Google Scholar] [CrossRef]
- Hong, S.; Wilson, M.T.; Serizawa, I.; Wu, L.; Singh, N.; Naidenko, O.V.; Miura, T.; Haba, T.; Scherer, D.C.; Wei, J.; et al. The natural killer T-cell ligand α-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat. Med. 2001, 7, 1052–1056. [Google Scholar] [CrossRef]
- Iba, M.; Kim, C.; Sallin, M.; Kwon, S.; Verma, A.; Overk, C.; Rissman, R.A.; Sen, R.; Sen, J.M.; Masliah, E. Neuroinflammation is associated with infiltration of T cells in Lewy body disease and α-synuclein transgenic models. J. Neuroinflammation 2020, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.C.; Anderson, P.; Widner, H.; Korsgrent, O. Enhanced survival of porcine neural xenografts in mice lacking CD1d1, but no effect of NK1.1 depletion. Cell Transplant. 2001, 10, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Dahlgren, C. Assembly and Activation of the Neutrophil NADPH Oxidase in Granule Membranes. Antioxidants Redox Signal. 2002, 4, 49–60. [Google Scholar] [CrossRef]
- Kopprasch, S.; Pietzsch, J.; Graessler, J. Validation of different chemilumigenic substrates for detecting extracellular generation of reactive oxygen species by phagocytes and endothelial cells. Luminescence 2003, 18, 268–273. [Google Scholar] [CrossRef]
- Duval, C.; Cantero, A.V.; Auge, N.; Mabile, L.; Thiers, J.C.; Negre-Salvayre, A.; Salvayre, R. Proliferation and wound healing of vascular cells trigger the generation of extracellular reactive oxygen species and LDL oxidation. Free. Radic. Biol. Med. 2003, 35, 1589–1598. [Google Scholar] [CrossRef]
- Harris, G.K.; Shi, X. Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat. Res. Mol. Mech. Mutagen. 2003, 533, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans. Am. Ophthalmol. Soc. 1990, 88, 797–850. [Google Scholar]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Truscott, R.J. Age-Related Nuclear Cataract: A Lens Transport Problem. Ophthalmic Res. 2000, 32, 185–194. [Google Scholar] [CrossRef]
- Yoshida, Y.; Umeno, A.; Shichiri, M. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. J. Clin. Biochem. Nutr. 2013, 52, 9–16. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Lipid-Mediated Oxidative Stress and Inflammation in the Pathogenesis of Parkinson’s Disease. Park. Dis. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trappe, T.A.; Liu, S.Z. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J. Appl. Physiol. 2013, 115, 909–919. [Google Scholar] [CrossRef]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.J.; Lees, P. The acute inflammatory process, arachidonic acid metabolism and the mode of action of anti-inflammatory drugs. Equine Veter J. 1984, 16, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Glycerophospholipids in brain: Their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef]
- Smink, W.; Gerrits, W.J.; Gloaguen, M.; Ruiter, A.; van Baal, J. Linoleic and α-linolenic acid as precursor and inhibitor for the synthesis of long-chain polyunsaturated fatty acids in liver and brain of growing pigs. Animal 2012, 6, 262–270. [Google Scholar] [CrossRef]
- Wang, Y.; Plastina, P.; Vincken, J.-P.; Jansen, R.; Balvers, M.; Klooster, J.P.T.; Gruppen, H.; Witkamp, R.; Meijerink, J. N-Docosahexaenoyl Dopamine, an Endocannabinoid-like Conjugate of Dopamine and the n-3 Fatty Acid Docosahexaenoic Acid, Attenuates Lipopolysaccharide-Induced Activation of Microglia and Macrophages via COX-2. ACS Chem. Neurosci. 2016, 8, 548–557. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Armida, M.; Carnevale, D.; Ajmone-Cat, M.A.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Cassano, T.; Minghetti, L.; et al. Striatal 6-OHDA lesion in mice: Investigating early neurochemical changes underlying Parkinson’s disease. Behav. Brain Res. 2010, 208, 137–143. [Google Scholar] [CrossRef]
- Dudek, J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front. Cell Dev. Biol. 2017, 5, 90. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef] [PubMed]
- Pope, S.; Land, J.M.; Heales, S.J. Oxidative stress and mitochondrial dysfunction in neurodegeneration; cardiolipin a critical target? Biochim. Biophys. Acta (BBA)—Bioenerg. 2008, 1777, 794–799. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Ostrander, D.B.; Sparagna, G.C.; Amoscato, A.A.; McMillin, J.B.; Dowhan, W. Decreased Cardiolipin Synthesis Corresponds with Cytochromec Release in Palmitate-induced Cardiomyocyte Apoptosis. J. Biol. Chem. 2001, 276, 38061–38067. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Agents Actions 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Robotta, M.; Gerding, H.R.; Vogel, A.; Hauser, K.; Schildknecht, S.; Karreman, C.; Leist, M.; Subramaniam, V.; Drescher, M. Alpha-Synuclein Binds to the Inner Membrane of Mitochondria in an α-Helical Conformation. ChemBioChem 2014, 15, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.; Bamm, V.V.; Stykel, M.; Coackley, C.L.; Humphries, K.M.; Jamieson-Williams, R.; Ambasudhan, R.; Mosser, D.D.; Lipton, S.A.; Harauz, G.; et al. Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Z.; Lu, L.; Duan, C.; Wang, X.; Yang, H. Morphological analysis of mitochondria for evaluating the toxicity of α-synuclein in transgenic mice and isolated preparations by atomic force microscopy. Biomed. Pharmacother. 2017, 96, 1380–1388. [Google Scholar] [CrossRef]
- Clementi, M.E.; Lazzarino, G.; Sampaolese, B.; Brancato, A.; Tringali, G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019, 43, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Beyaert, R.; Vandevoorde, V.; Haegeman, G.; Fiers, W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993, 12, 3095–3104. [Google Scholar] [CrossRef]
- Corda, S.; LaPlace, C.; Vicaut, E.; Duranteau, J. Rapid Reactive Oxygen Species Production by Mitochondria in Endothelial Cells Exposed to Tumor Necrosis Factor- α Is Mediated by Ceramide. Am. J. Respir. Cell Mol. Biol. 2001, 24, 762–768. [Google Scholar] [CrossRef]
- Mendes, A.F.; Caramona, M.M.; Carvalho, A.P.; Lopes, M.C. Hydrogen peroxide mediates interleukin-1β-induced AP-1 activation in articular chondrocytes: Implications for the regulation of iNOS expression. Cell Biol. Toxicol. 2003, 19, 203–214. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Banning, A.; Kny, M.; Böl, G.-F. Redox events in interleukin-1 signaling. Arch. Biochem. Biophys. 2004, 423, 66–73. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Jeong, M.; Park, J.S.; Kim, M.H.; Lee, D.B.; Shin, B.A.; Mukaida, N.; Ellis, L.M.; Kim, H.R.; Ahn, B.W.; et al. Interleukin-1β stimulates IL-8 expression through MAP kinase and ROS signaling in human gastric carcinoma cells. Oncogene 2004, 23, 6603–6611. [Google Scholar] [CrossRef]
- Kaur, J.; Turner, R.B.; Dhaunsi, G.S. Interleukin-1 and Nitric Oxide Increase NADPH Oxidase Activity in Human Coronary Artery Smooth Muscle Cells. Med. Princ. Pr. 2003, 13, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Pearl-Yafe, M.; Halperin, D.; Halevy, A.; Kalir, H.; Bielorai, B.; Fabian, I. An oxidative mechanism of interferon induced priming of the Fas pathway in Fanconi anemia cells. Biochem. Pharmacol. 2003, 65, 833–842. [Google Scholar] [CrossRef]
- Watanabe, Y.; Suzuki, O.; Haruyama, T.; Akaike, T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell. Biochem. 2003, 89, 244–253. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-kappa B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Shin, W.H.; Kim, J.; Joe, E.H.; Lee, Y.B.; Cho, K.G.; Oh, Y.J.; Kim, S.U.; Jin, B.K. Trisialoganglioside GT1b induces in vivo degeneration of nigral dopaminergic neurons: Role of microglia. Glia 2002, 38, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Erard, F.; Wild, M.T.; Garcia-Sanz, J.A.; le Gros, G. Switch of CD8 T Cells to Noncytolytic CD8—CD4—Cells that Make T H 2 Cytokines and Help B Cells. Science 1993, 260, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.A.; Schmidlin, H.; Nagasawa, M.; Blom, B.; Spits, H. IL-6 Triggers IL-21 production by human CD4 + T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol. Cell Biol. 2012, 90, 802–811. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Baumjohann, D.; Craft, J.; Fazilleau, N.; Ma, C.S.; Tangye, S.G.; Vinuesa, C.G.; Linterman, M.A. CD4+ T cells that help B cells—A proposal for uniform nomenclature. Trends Immunol. 2021, 42, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Dornmair, K.; Meinl, E.; Wekerle, H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 2015, 15, 317–331. [Google Scholar] [CrossRef]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef]
- Pandey, M.K. Molecular Basis for Downregulation of C5a-Mediated Inflammation by IgG1 Immune Complexes in Allergy and Asthma. Curr. Allergy Asthma Rep. 2013, 13, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M.; Kouli, A.; Yeoh, S.L.; Clatworthy, M.R.; Williams-Gray, C.H. A Systematic Review and Meta-Analysis of Alpha Synuclein Auto-Antibodies in Parkinson’s Disease. Front. Neurol. 2018, 9, 815. [Google Scholar] [CrossRef]
- Smith, L.M.; Schiess, M.C.; Coffey, M.P.; Klaver, A.C.; Loeffler, D.A. α-Synuclein and Anti-α-Synuclein Antibodies in Parkinson’s Disease, Atypical Parkinson Syndromes, REM Sleep Behavior Disorder, and Healthy Controls. PLoS ONE 2012, 7, e52285. [Google Scholar] [CrossRef]
- Papachroni, K.K.; Ninkina, N.; Papapanagiotou, A.; Hadjigeorgiou, G.M.; Xiromerisiou, G.; Papadimitriou, A.; Kalofoutis, A.; Buchman, V.L. Autoantibodies to alpha-synuclein in inherited Parkinson’s disease. J. Neurochem. 2006, 101, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.S.; Licata, J.P.; Luk, K.C.; Shaw, L.M.; Trojanowski, J.Q.; Lee, V.M.Y. Measurements of auto-antibodies to α-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease. J. Neurochem. 2018, 145, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Alibhai, J.D.; Manson, J. The role of the immune system in prion infection. Handb. Clin. Neurol. 2018, 153, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Prigione, A.; Piazza, F.; Brighina, L.; Begni, B.; Galbussera, A.; DiFrancesco, J.C.; Andreoni, S.; Piolti, R.; Ferrarese, C. Alpha-synuclein nitration and autophagy response are induced in peripheral blood cells from patients with Parkinson disease. Neurosci. Lett. 2010, 477, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Killinger, B.; Labrie, V. The Appendix in Parkinson’s Disease: From Vestigial Remnant to Vital Organ? J. Parkinsons Dis. 2019, 9, S345–S358. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, B.S.; Heo, C.; Im, P.S.; Ahn, T.; Seo, J.; Kim, H.; Park, C.H.; Choi, S.H.; Cho, S.; et al. α-Synuclein induces apoptosis by altered expression in human peripheral lymphocytes in Parkinson’s disease. FASEB J. 2004, 18, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Grozdanov, V.; Danzer, K.M. Intracellular Alpha-Synuclein and Immune Cell Function. Front. Cell Dev. Biol. 2020, 8, 562692. [Google Scholar] [CrossRef] [PubMed]
- Gardai, S.J.; Mao, W.; Schuele, B.; Babcock, M.; Schoebel, S.; Lorenzana, C.; Alexander, J.; Kim, S.; Glick, H.; Hilton, K.; et al. Elevated Alpha-Synuclein Impairs Innate Immune Cell Function and Provides a Potential Peripheral Biomarker for Parkinson’s Disease. PLoS ONE 2013, 8, e71634. [Google Scholar] [CrossRef]
- Magnusen, D.; Nyamajenjere, T.; Rapien, J.; Mckay, M.; Magnusen, A.; Pandey, M. Dendritic cells, CD4+ T cells, and α synuclein triangle fuels neuroinflammation in Parkinson’s disease (P3.053). Neurology 2018, 90, P3.053. [Google Scholar]
- Pandey, M.K.; Gross, C.; Caballero, M.; McKay, M.A.; Tiwari, D.; Schroeder, L.M.; Gurtler, M.A.; Burrow, T.A. Immune cells encounter with α-synuclein fuels neuro-degeneration in Parkinson’s disease. J. Immunol. 2016, 196, 51.5. [Google Scholar]
- Rajput, A.H.; Rozdilsky, B.; Rajput, A.; Ang, L. Levodopa Efficacy and Pathological Basis of Parkinson Syndrome. Clin. Neuropharmacol. 1990, 13, 553–558. [Google Scholar] [CrossRef]
- Stoker, T.B.; Barker, R.A. Recent developments in the treatment of Parkinson’s Disease. F1000Research 2020, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Ntetsika, T.; Papathoma, P.-E.; Markaki, I. Novel targeted therapies for Parkinson’s disease. Mol. Med. 2021, 27, 1–20. [Google Scholar] [CrossRef]
- Marsden, C.D. Problems with long-term levodopa therapy for Parkinson’s disease. Clin. Neuropharmacol. 1994, 17 (Suppl. S2), S32–S44. [Google Scholar] [CrossRef]
| Class of Lipid | Lipids | Mouse Models and Human PD Patients | Source and Level of Lipid | Cytokine | Source and Level of Cytokine or Inflammatory Marker (m for mRNA and p for Protein) | References |
|---|---|---|---|---|---|---|
| Glycerolipids | NEFA | A53T mutant mice | Serum- | TNF α | Brainm+ | [272] |
| TAG | A53T mutant mice | Serum- | TNFα | Brainm+ | [272] | |
| Eicosanoids | PGL E2 | DJ KO mice | Astrocyte- | TNFα | Astrocytesm− | [305] |
| PD patients with cognitive impairment and dementia | CSF+ | TNFα IL1β IL6 INFγ | CSFp− CSFp+ CSFp+ CSFp− | [304] | ||
| Sphingolipid | β -GalCer | PHA- and LPS-stimulated blood cells | PB+ | TNFα IL1β IL6 IL8 MIP1α | PBp+ PBp+ PBp+ PBp+ PBp+ | [309] |
| Sulfatides | PHA- and LPS-stimulated blood cells | PB+ | TNFα IL1β IL6 IL8 IL12 MIP1α | PBp− PBp- PBp− PBp− PBp- PBp- | [309] | |
| Sterol | Cholesterol | A53T mutant mice | Serum- | TNFα | Brainm+ | [272] |
| Class of Lipid | Lipids | Mouse Models and Human Patients with PD | Source and Level of Lipid | Reactive Oxygen Species | Source and Level of Reactive Oxygen Species | References |
|---|---|---|---|---|---|---|
| Eicosanoids | PGL E2 | 6-OHDA treated mice | Striatum− Hipp− Cortex− | 15-F2t-IsoP | Striatump− Cortexp− | [377] |
| DJKO mice | Astrocyte− | NOS2 COX2 | Astrocytesm+ Astrocytesm+ | [305] | ||
| Glyercophospholipids | CL | PINK1 KO mice | Embryonic fibroblast− | Palmitate | Embryonic fibroblast− | [275] |
| Thy1α-syn mice | Brain− | n/a | n/a | [386] | ||
| Sterol | Cholesterol | DJ1 KO | Embryonic fibroblasts− | H2O2 | D2 Cells+ | [273] |
| DJ1 KO | Astrocyte− | H2O2 | D2 Cells+ | [273] | ||
| PUFA | α-LA | MPP+ treated mice on E-EPA diet | Frontal cortex+ | cPLA2 COX2 | Striatumm+ Striatumm+ | [141] |
| MPP+ treated mice on E-EPA diet | Striatum+ | cPLA2 COX2 | Striatumm+ Striatumm+ | [141] | ||
| ARA | MPP+ treated mice on E-EPA diet | Frontal cortex+ | cPLA2 COX2 | Striatumm+ Striatumm+ | [141] | |
| MPP+ treated mice on E-EPA diet | Striatum+ | cPLA2 COX2 | Striatumm+ Striatumm+ | [141] | ||
| MPTP-treated mice | Striatal synaptoneurosomes+ | cPLA2 nNOS | Striatump+ Midbrainp+ Striatump+ Midbrainp+ | [142] | ||
| MPTP-treated mice | Midbrain synaptoneurosomes+ | cPLA2 nNOS | Striatump+ Midbrainp+ Striatump+ Midbrainp+ | [142] | ||
| DHA | PD postmortem tissue | ACC+ | F2-IsoP | ACCp+ | [279] | |
| DPA | PD postmortem tissue | ACC+ | F2-IsoP | ACCp+ | [279] | |
| Sphingolipid | GT1b | Sprague-Dawley rats | SN− | iNOS | SN+ | [398] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatton, S.L.; Pandey, M.K. Fat and Protein Combat Triggers Immunological Weapons of Innate and Adaptive Immune Systems to Launch Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 1089. https://doi.org/10.3390/ijms23031089
Hatton SL, Pandey MK. Fat and Protein Combat Triggers Immunological Weapons of Innate and Adaptive Immune Systems to Launch Neuroinflammation in Parkinson’s Disease. International Journal of Molecular Sciences. 2022; 23(3):1089. https://doi.org/10.3390/ijms23031089
Chicago/Turabian StyleHatton, Shelby Loraine, and Manoj Kumar Pandey. 2022. "Fat and Protein Combat Triggers Immunological Weapons of Innate and Adaptive Immune Systems to Launch Neuroinflammation in Parkinson’s Disease" International Journal of Molecular Sciences 23, no. 3: 1089. https://doi.org/10.3390/ijms23031089
APA StyleHatton, S. L., & Pandey, M. K. (2022). Fat and Protein Combat Triggers Immunological Weapons of Innate and Adaptive Immune Systems to Launch Neuroinflammation in Parkinson’s Disease. International Journal of Molecular Sciences, 23(3), 1089. https://doi.org/10.3390/ijms23031089







