Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
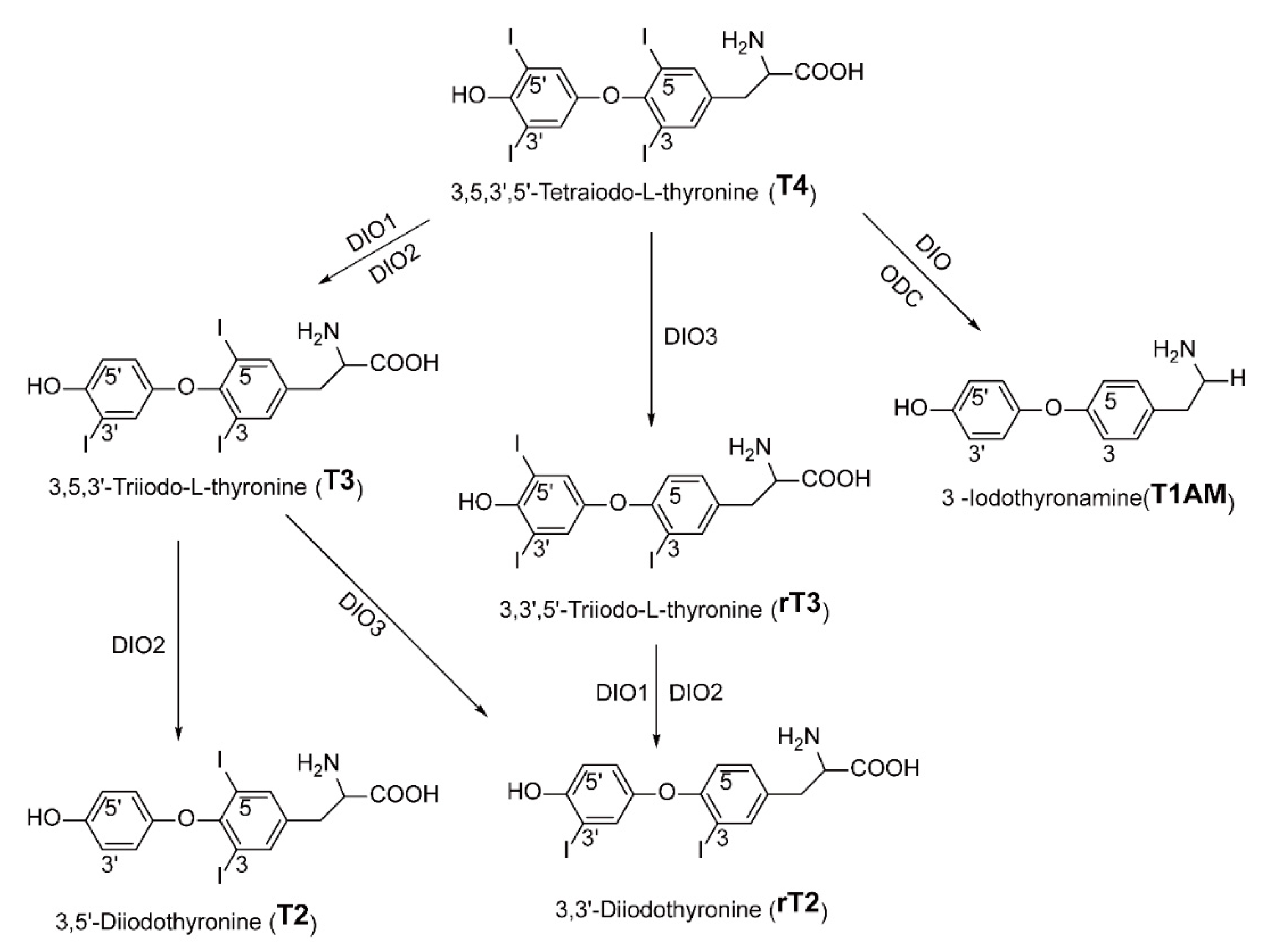
2. Thyroid Hormones and Their Metabolites
2.1. T4/T3
2.2. T2
2.3. T1AM
3. Synthetic Thyromimetics
3.1. GC-1, GC-24
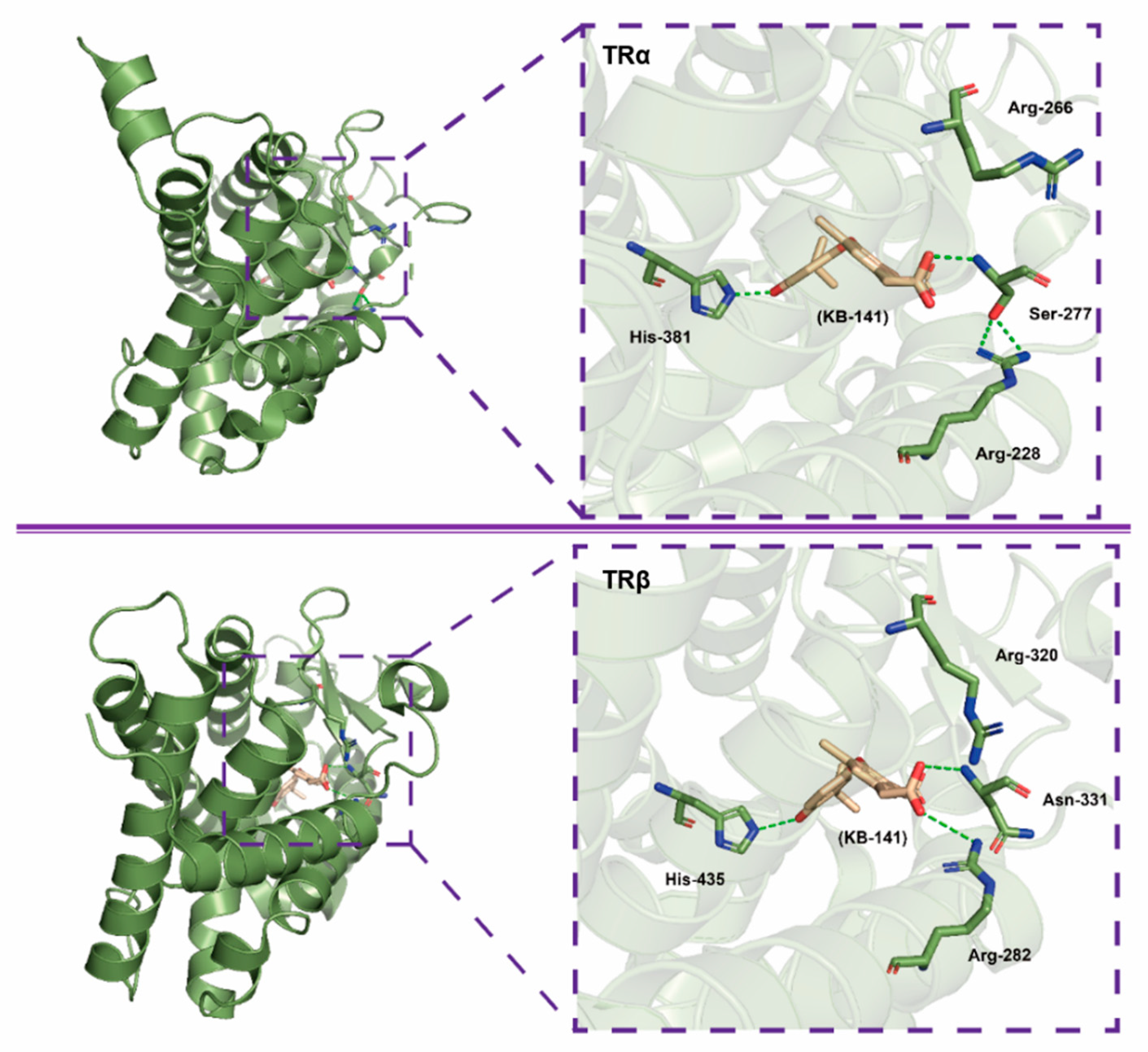
3.2. KB141, KB2115
3.3. MB07811(VK2809), MB07344
3.4. MGL-3196
4. Conclusions and Perspective
Funding
Conflicts of Interest
Abbreviations
References
- Brunt, E.M. Nonalcoholic Steatohepatitis: Definition and Pathology. Semin. Liver Dis. 2001, 21, 3–16. [Google Scholar] [CrossRef]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic fatty liver disease: Pathology and pathogenesis. Annu. Rev. Pathol. 2010, 5, 145–171. [Google Scholar] [CrossRef]
- Angulo, P. Medical progress: Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Brunt, E.M. Pathology of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef]
- Yoon, H.J.; Cha, B.S. Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 800–811. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Hardy, T.; Henderson, E.; Burt, A.D.; Day, C.P.; Anstee, Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015, 62, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic fatty liver disease: Pathogenesis and disease spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef]
- Diehl, A.M.; Longo, D.L.; Day, C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e3. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; Sauver, J.S.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J.; American Gastroenterological Association; American Association for the Study of Liver Diseases; et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Younossi, Z.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Barshop, N.J.; Sirlin, C.B.; Schwimmer, J.B.; Lavine, J.E. Review article: Epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2008, 28, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Alisi, A.; Manco, M.; Vania, A.; Nobili, V. Pediatric nonalcoholic fatty liver disease in 2009. J. Pediatr. 2009, 155, 469–474. [Google Scholar] [CrossRef]
- Alisi, A.; Locatelli, M.; Nobili, V. Nonalcoholic fatty liver disease in children. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 397–402. [Google Scholar] [CrossRef]
- Alisi, A.; Feldstein, A.E.; Villani, A.; Raponi, M.; Nobili, V. Pediatric nonalcoholic fatty liver disease: A multidisciplinary approach. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 152–161. [Google Scholar] [CrossRef]
- Wong, V.W.; Wong, G.L.; Chan, R.S.; Shu, S.S.; Cheung, B.H.; Li, L.S.; Chim, A.M.; Chan, C.K.; Leung, J.K.; Chu, W.C.; et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 1349–1356. [Google Scholar] [CrossRef]
- Hohenester, S.; Christiansen, S.; Nagel, J.; Wimmer, R.; Artmann, R.; Denk, G.; Bischoff, M.; Bischoff, G.; Rust, C. Lifestyle intervention for morbid obesity: Effects on liver steatosis, inflammation, and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G329–G338. [Google Scholar] [CrossRef] [PubMed]
- Huber, Y.; Pfirrmann, D.; Gebhardt, I.; Labenz, C.; Gehrke, N.; Straub, B.K.; Ruckes, C.; Bantel, H.; Belda, E.; Clement, K.; et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment. Pharmacol. Ther. 2019, 50, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Schattenberg, J.M. Effectiveness of lifestyle interventions in NAFLD (nonalcoholic fatty liver disease)-how are clinical trials affected? Expert Opin. Investig. Drugs 2020, 29, 93–97. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015, 149, 379–388. [Google Scholar] [CrossRef]
- Bower, G.; Toma, T.; Harling, L.; Jiao, L.R.; Efthimiou, E.; Darzi, A.; Athanasiou, T.; Ashrafian, H. Bariatric surgery and non-alcoholic fatty liver disease: A systematic review of liver biochemistry and histology. Obes. Surg. 2015, 25, 2280–2289. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.J.; Weiner, R.; Yashkov, Y.; Fruhbeck, G. Interdisciplinary European Guidelines on metabolic and bariatric surgery. Obes. Facts. 2013, 6, 449–468. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Targher, G.; Day, C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Nonalcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Ballestri, S.; Mantovani, A.; Nascimbeni, F.; Lugari, S.; Lonardo, A. Extra-hepatic manifestations and complications of nonalcoholic fatty liver disease. Future Med. Chem. 2019, 11, 2171–2192. [Google Scholar] [CrossRef]
- Mantovani, A.; Sani, E.; Fassio, A.; Colecchia, A.; Viapiana, O.; Gatti, D.; Idolazzi, L.; Rossini, M.; Salvagno, G.; Lippi, G.; et al. Association between non-alcoholic fatty liver disease and bone turnover biomarkers in post-menopausal women with type 2 diabetes. Diabetes Metab. 2019, 45, 347–355. [Google Scholar] [CrossRef]
- Huber, Y.; Galle, P.R.; Schattenberg, J.M. What is the (right) target for non-alcoholic fatty liver disease (NAFLD)? Z. Gastroenterol. 2020, 58, 68–73. [Google Scholar]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Younossi, Z.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.; et al. GS-06-positive results from REGENERATE: A phase 3 international, randomized, placebo-controlled study evaluating obeticholic acid treatment for NASH. J. Hepatol. 2019, 114, S546. [Google Scholar] [CrossRef]
- Adorini, L.; Pruzanski, M.; Shapiro, D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov. Today 2012, 17, 988–997. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Lawitz, E.; Charlton, M.; Loomba, R.; Myers, R.P.; Subramanian, M.; McHutchison, J.G.; Goodman, Z. Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib. Liver Int. 2018, 38, 1849–1859. [Google Scholar] [CrossRef]
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.F.; Aithal, G.P.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767. [Google Scholar] [CrossRef]
- Ratziu, V.; Giral, P.; Jacqueminet, S.; Charlotte, F.; Hartemann-Heurtier, A.; Serfaty, L.; Podevin, P.; Lacorte, J.M.; Bernhardt, C.; Bruckert, E.; et al. Rosiglitazone for nonalcoholic steatohepatitis: One-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology 2008, 135, 100–110. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016, 150, 1147–1159.e5. [Google Scholar] [CrossRef]
- Van Meeteren, M.J.W.; Drenth, J.P.H.; Tjwa, E.T.T.L. Elafibranor: A potential drug for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin Investig. Drugs 2019, 29, 117–123. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Abouda, G.; Aldersley, M.A.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- De LaCruz-Villar, L.; Fernandez-Ramos, D.; Lopitz-Otsoa, F.; Iruarrizaga-Lejarreta, M.; Bilbao, J.; Cabrera, D.; Van Liempd, S.M.; Alonso, C.; Lu, S.C.; Gorfine, T.; et al. Aramchol, SCD1 inhibitor, improves liver glucose homeostasis in NASH. Hepatology 2019, 70, 1355A. [Google Scholar]
- Ratziu, V.; Ladron-De-Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Harrison, S.A.; Arrese, M.; Fargion, S.; Ben-Bashat, D.; et al. One-year results of the global phase 2b randomized placebo-controlled arrest trial of aramchol, a stearoyl CoA desaturase inhibitor, in patients with NASH. Hepatology 2018, 68, 1448A–1449A. [Google Scholar]
- Verzijl, C.R.C.; Van De Peppel, I.P.; Struik, D.; Jonker, J.W. Pegbelfermin (BMS-986036): An investigational PEGylated fibroblast growth factor 21 analogue for the treatment of nonalcoholic steatohepatitis. Expert Opin. Investig. Drugs 2020, 29, 125–133. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Taub, R.; Barbone, J.M.; Harrison, S. Hepatic fat reduction due to resmetirom in patients with nonalcoholic steatohepatitis is associated with improvement of quality of life. Clin. Gastroenterol. Hepatol. 2021, in press. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Tudor, R.M.; Garrahy, A.; Woods, C.P.; Crowley, R.K.; Tormey, W.T.; Smith, D.; Hatunic, M.; Thompson, C.J. The prevalence and incidence of thyroid dysfunction in patients with diabetes-a longitudinal follow-up study. Ir. J. Med. Sci. 2020, 189, 171–175. [Google Scholar] [CrossRef]
- Kalra, S.; Aggarwal, S.; Khandelwal, D. Thyroid dysfunction and type 2 diabetes mellitus: Screening strategies and implications for management. Diabetes Ther. 2019, 10, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Aschebrook-Kilfoy, B.; Sabra, M.M.; Brenner, A.; Moore, S.C.; Ron, E.; Schatzkin, A.; Hollenbeck, A.; Ward, M.H. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid 2011, 21, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, H.; Bao, X.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Du, H.; Shi, H.; Xia, Y.; et al. The relationship between thyroid function and the prevalence of type 2 diabetes mellitus in euthyroid subjects. J. Clin. Endocrinol. Metab. 2017, 102, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Sikanderkhel, S.; Gui, J.; Adeniyi, A.R.; O’Dell, K.; Erickson, M.; Malpartida, J.; Mufti, Z.; Khan, T.; Mufti, H.; et al. Thyroid and cardiovascular disease: A focused review on the impact of hyperthyroidism in heart failure. Cardiol. Res. 2020, 11, 68–75. [Google Scholar] [CrossRef]
- Osuna, P.M.; Udovcic, M.; Sharma, M.D. Hyperthyroidism and the heart. Methodist Debakey Cardiovasc. Methodist DeBakey Cardiovasc. J. 2017, 13, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, W.H. Mechanism of Action of Thyroid Hormone on the Cardiac Vascular System. In Thyroid and Heart Failure: From Pathophysiology to Clinics; Iervasi, G., Pingitore, A., Eds.; Springer: Milano, Italy, 2009; pp. 45–54. [Google Scholar]
- Danzi, S.; Klein, I. Thyroid hormone and the cardiovascular system. Med. Clin. N. Am. 2012, 96, 257–268. [Google Scholar] [CrossRef]
- Klein, I.; Ojamaa, K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef]
- Tatar, E.; Kircelli, F.; Asci, G.; Carrero, J.J.; Gungor, O.; Demirci, M.S.; Ozbek, S.S.; Ceylan, N.; Ozkahya, M.; Toz, H.; et al. Associations of triiodothyronine levels with carotid atherosclerosis and arterial stiffness in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2240–2246. [Google Scholar] [CrossRef]
- Tatar, E.; Demirci, M.S.; Kircelli, F.; Gungor, O.; Yaprak, M.; Asci, G.; Basci, A.; Ozkahya, M.; Ok, E. The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. Int. Urol. Nephrol. 2012, 44, 601–606. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, C.Y.; Tsai, M.M.; Tsai, C.Y.; Lin, K.H. Molecular functions of thyroid hormones and their clinical significance in liver-related diseases. Biomed. Res. Int. 2013, 2013, 601361. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Jessica, A.H.; Correa-Medina, M.; Ueta, C.; Kang, H.W.; Cohen, D.E.; Bianco, A.C. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 2011, 60, 1082–1089. [Google Scholar] [CrossRef]
- Carulli, L.; Ballestri, S.; Lonardo, A.; Lami, F.; Violi, E.; Losi, L.; Bonilauri, L.; Verrone, A.M.; Odoardi, M.R.; Scaglioni, F.; et al. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern. Emerg. Med. 2013, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, J.; Jo, K.; Lim, D.J.; Lee, J.M.; Chang, S.A.; Kang, M.I.; Cha, B.Y.; Kim, M.H. Male-specific association between subclinical hypothyroidism and the risk of non-alcoholic fatty liver disease estimated by hepatic steatosis index: Korea National Health and Nutrition Examination Survey 2013 to 2015. Sci. Rep. 2018, 8, 15145. [Google Scholar] [CrossRef] [PubMed]
- Marla, J.B.; Larsen, P.R. The role of selenium in thyroid hormone action. Endocr. Rev. 1992, 13, 207–219. [Google Scholar]
- Dumitrescu, A.M.; Liao, X.-H.; Abdullah, M.S.Y.; Lado-Abeal, J.; Majed, F.A.; Moeller, L.C.; Boran, G.; Schomburg, L.; Weiss, R.E.; Refetoff, S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat. Genet. 2005, 37, 1247–1252. [Google Scholar] [CrossRef]
- Medici, M.; Visser, W.E.; Visser, T.J.; Peeters, R.P. Genetic determination of the hypothalamic-pituitary-thyroid axis: Where do we stand? Endocr. Rev. 2015, 36, 214–244. [Google Scholar] [CrossRef]
- Germain, D.L.S.; Hernandez, A.; Schneider, M.J.; Galton, V.A. Insights into the role of deiodinases from studies of genetically modified animals. Thyroid 2005, 15, 905–916. [Google Scholar] [CrossRef]
- Dentice, M.; Marsili, A.; Zavacki, A.; Larsen, P.R.; Salvatore, D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3937–3945. [Google Scholar] [CrossRef]
- Köhrle, J. Thyroid Hormone Metabolism. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2018; pp. 420–428. [Google Scholar]
- Harvey, C.B.; Williams, G.R. Mechanism of thyroid hormone action. Thyroid 2002, 12, 6. [Google Scholar] [CrossRef]
- Yen, P.M.; Ando, S.; Feng, X.; Liu, Y.; Maruvada, P.; Xia, X. Thyroid hormone action at the cellular, genomic and target gene levels. Mol. Cell. Endocrinol. 2006, 246, 121–127. [Google Scholar] [CrossRef]
- Auwerx, J.; Baulieu, E.; Beato, M.; Becker-Andre, M.; Yamamoto, K. A unified nomenclature system for the nuclear receptor superfamily. Cell 1999, 97, 161–163. [Google Scholar]
- Lazar, M.A. Thyroid hormone receptors: Multiple forms, multiple possibilities. Endocr. Rev. 1993, 14, 184–193. [Google Scholar] [PubMed]
- O’Shea, P.J.; Williams, G.R. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. Endocrinology 2002, 175, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R. Cloning and characterization of two novel thyroid hormone receptor β isoforms. Mol. Cell. Biol. 2000, 20, 8329–8342. [Google Scholar] [CrossRef]
- Krause, C.; Grohs, M.; Gammal, A.T.E.; Wolter, S.; Lehnert, H.; Mann, O.; Mittag, J.; Kirchner, H. Reduced expression of thyroid hormone receptor beta in human nonalcoholic steatohepatitis. Endocr. Connect. 2018, 7, 1448–1456. [Google Scholar] [CrossRef]
- Perra, A.; Simbula, G.; Simbula, M.; Pibiri, M.; Kowalik, M.A.; Sulas, P.; Cocco, M.T.; Ledda-Columbano, G.M.; Columbano, A. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008, 22, 2981–2989. [Google Scholar] [CrossRef]
- Lombardi, B.; Pani, P.; Schlunk, F.F. Choline-deficiency fatty liver: Impaired release of hepatic triglycerides. J. Lipid Res. 1968, 9, 437–446. [Google Scholar] [CrossRef]
- Vetelainen, R.; van Vliet, A.; van Gulik, T.M. Essential pathogenic and metabolic differences in steatosis induced by choline or methione-choline deficient diets in a rat model. J. Gastroenterol. Hepatol. 2007, 22, 1526–1533. [Google Scholar] [CrossRef]
- Packard, C.J.; Shepherd, J.; Lindsay, G.M.; Gaw, A.; Taskinen, M.R. Thyroid replacement therapy and its influence on postheparin plasma lipases and apolipoprotein-B metabolism in hypothyroidism. J. Clin. Endocrinol. Metab. 1993, 76, 1209–1216. [Google Scholar]
- Wiseman, S.A.; Powell, J.T.; Humphries, S.E.; Press, M. The magnitude of the hypercholesterolemia of hypothyroidism is associated with variation in the low density lipoprotein receptor gene. J. Clin. Endocrinol. Metab. 1993, 77, 108–112. [Google Scholar] [PubMed]
- Pazos, F.; Alvarez, J.J.; Rubiés-Prat, J.; Varela, C.; Lasunción, M.A. Long-term thyroid replacement therapy and levels of lipoprotein(a) and other lipoproteins. J. Clin. Endocrinol. Metab. 1995, 80, 562–566. [Google Scholar]
- Hoogwerf, B.J.; Nuttall, F.Q. Long-term weight regulation in treated hyperthyroid and hypothyroid subjects. Am. J. Med. 1984, 76, 963–970. [Google Scholar] [CrossRef]
- Braverman, L.E.; Utiger, R.D. Introduction to thyrotoxicosis. In Werner and Ingbar’s the Thyroid: A Fundamental and Clinical Text; Lewis, E.B., Robert, D.U., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; Volume 9, pp. 453–455. [Google Scholar]
- Drigo, R.A.E.; Fonseca, T.L.; Werneck-de-Castro, J.P.S.; Bianco, A.C. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3956–3964. [Google Scholar] [CrossRef]
- Mollica, M.P.; Lionetti, L.; Moreno, M.; Lombardi, A.; De Lange, P.; Antonelli, A.; Lanni, A.; Cavaliere, G.; Barletta, A.; Goglia, F. 3,5-diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J. Hepatol. 2009, 51, 363–370. [Google Scholar] [CrossRef]
- Ball, S.G.; Sokolov, J.; Chin, W.W. 3,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J. Mol. Endocrinol. 1997, 19, 137–147. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Canesi, L.; De Matteis, R.; Goglia, F.; Cioffi, F.; Fugassa, E.; Gallo, G.; Vergani, L. Direct effects of iodothyronines on excess fat storage in rat hepatocytes. J. Hepatol. 2011, 54, 1230–1236. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Lange, P.D.; Silvestri, E.; Ragni, M.; Farina, P.; Baccari, G.C.; Fallahi, P.; Antonelli, A.; et al. 3,5-Diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005, 19, 1552–1554. [Google Scholar] [CrossRef]
- Thakran, S.; Sharma, P.; Attia, R.R.; Hori, R.T.; Deng, X.; Elam, M.B.; Park, E.A. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J. Biol. Chem. 2013, 288, 807–818. [Google Scholar] [CrossRef]
- Suh, J.H.; Sieglaff, D.H.; Zhang, A.; Xia, X.; Cvoro, A.; Winnier, G.E.; Webb, P. SIRT1 is a direct coactivator of thyroid hormone receptor β1 with gene-specific actions. PLoS ONE 2013, 8, e70097. [Google Scholar] [CrossRef]
- de Lange, P.; Cioffi, F.; Senese, R.; Moreno, M.; Lombardi, A.; Silvestri, E.; De Matteis, R.; Lionetti, L.; Mollica, M.P.; Goglia, F.; et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 2011, 60, 2730–2739. [Google Scholar] [CrossRef]
- Grasselli, E.; Voci, A.; Demori, I.; Canesi, L.; De Matteis, R.; Goglia, F.; Lanni, A.; Gallo, G.; Vergani, L. 3,5-Diiodo-L-thyronine modulates the expression of genes of lipid metabolism in a rat model of fatty liver. J. Endocrinol. 2012, 212, 149–158. [Google Scholar] [CrossRef]
- Damiano, F.; Rochira, A.; Gnoni, A.; Siculella, L. Action of thyroid hormones, T3 and T2, on hepatic fatty acids: Differences in metabolic effects and molecular mechanisms. Int. J. Mol. Sci. 2017, 18, 744. [Google Scholar] [CrossRef]
- Jonas, W.; Lietzow, J.; Wohlgemuth, F.; Hoefig, C.S.; Wiedmer, P.; Schweizer, U.; Kohrle, J.; Schurmann, A. 3,5-Diiodo-L-thyronine (3,5-T2) exerts thyromimetic effects on hypothalamus-pituitary-thyroid axis, body composition, and energy metabolism in male diet-induced obese mice. Endocrinology 2015, 156, 389–399. [Google Scholar] [CrossRef]
- Senese, R.; de Lange, P.; Petito, G.; Moreno, M.; Goglia, F.; Lanni, A. 3,5-Diiodothyronine: A novel thyroid hormone metabolite and potent modulator of energy metabolism. Front Endocrinol. 2018, 9, 427. [Google Scholar] [CrossRef]
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: Biological actions of ‘nonclassical’ thyroid hormones. J. Endocrinol. 2014, 221, R1–R12. [Google Scholar] [CrossRef]
- Scanlan, T.S.; Suchland, K.L.; Hart, M.E.; Chiellini, G.; Huang, Y.; Kruzich, P.J.; Frascarelli, S.; Crossley, D.A.; Bunzow, J.R.; Ronca-Testoni, S.; et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 2004, 10, 638–642. [Google Scholar] [CrossRef]
- Saba, A.; Chiellini, G.; Frascarelli, S.; Marchini, M.; Ghelardoni, S.; Raffaelli, A.; Tonacchera, M.; Vitti, P.; Scanlan, T.S.; Zucchi, R. Tissue distribution and cardiac metabolism of 3-iodothyronamine. Endocrinology 2010, 151, 5063–5073. [Google Scholar] [CrossRef]
- Ghelardoni, S.; Chiellini, G.; Frascarelli, S.; Saba, A.; Zucchi, R. Uptake and metabolic effects of 3-iodothyronamine in hepatocytes. J. Endocrinol. 2014, 221, 101–110. [Google Scholar] [CrossRef]
- Mariotti, V.; Melissari, E.; Iofrida, C.; Righi, M.; Di Russo, M.; Donzelli, R.; Saba, A.; Frascarelli, S.; Chiellini, G.; Zucchi, R.; et al. Modulation of gene expression by 3-iodothyronamine: Genetic evidence for a lipolytic pattern. PLoS ONE 2014, 9, e106923. [Google Scholar] [CrossRef]
- Shahrara, S.; Drvota, V.; Sylven, C. Organ Specific Expression of Thyroid Hormone Receptor mRNA and Protein in Different Human Tissues. Biol. Pharm. Bull. 1999, 22, 1027. [Google Scholar] [CrossRef][Green Version]
- Chiellinil, G.; Aprilettl, J.W.; Yoshiharal, H.A.; Baxter, J.D.; Ribeiro, R.C.; ScanIan, T.S. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem. Biol. 1998, 5, 299–306. [Google Scholar] [CrossRef]
- Alkhouri, N. Thyromimetics as emerging therapeutic agents for nonalcoholic steatohepatitis: Rationale for the development of resmetirom (MGL-3196). Expert Opin. Investig. Drugs 2020, 29, 99–101. [Google Scholar] [CrossRef]
- Trost, S.U.; Eric, S.; Bernd, G.; Wang-Iverson, D.B.; Zhang, H.J.; Tanya, V. The thyroid hormone Receptor-β-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 2000, 141, 3057–3064. [Google Scholar] [CrossRef]
- Grover, G.J.; Egan, D.M.; Sleph, P.G.; Beehler, B.C.; Chiellini, G.; Nguyen, N.H.; Baxter, J.D.; Scanlan, T.S. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: Selective actions relative to 3,5,3′-triiodo-L-thyronine. Endocrinology 2004, 145, 1656–1661. [Google Scholar] [CrossRef]
- Vatner, D.F.; Weismann, D.; Beddow, S.A.; Kumashiro, N.; Erion, D.M.; Liao, X.H.; Grover, G.J.; Webb, P.; Phillips, K.J.; Weiss, R.E.; et al. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E89–E100. [Google Scholar] [CrossRef]
- Martagon, A.J.; Lin, J.Z.; Cimini, S.L.; Webb, P.; Phillips, K.J. The amelioration of hepatic steatosis by thyroid hormone receptor agonists is insufficient to restore insulin sensitivity in ob/ob mice. PLoS ONE 2015, 10, e0122987. [Google Scholar] [CrossRef]
- Puliga, E.; Min, Q.; Tao, J.; Zhang, R.; Pradhan-Sundd, T.; Poddar, M.; Singh, S.; Columbano, A.; Yu, J.; Monga, S.P. Thyroid hormone receptor-beta agonist GC-1 Inhibits Met-β-catenin-driven hepatocellular cancer. Am. J. Pathol. 2017, 187, 2473–2485. [Google Scholar] [CrossRef]
- Frau, C.; Loi, R.; Petrelli, A.; Perra, A.; Menegon, S.; Kowalik, M.A.; Pinna, S.; Leoni, V.P.; Fornari, F.; Gramantieri, L.; et al. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology 2015, 61, 249–259. [Google Scholar] [CrossRef]
- Tao, J.; Xu, E.; Zhao, Y.; Singh, S.; Li, X.; Couchy, G.; Chen, X.; Zucman-Rossi, J.; Chikina, M.; Monga, S.P. Modeling a human hepatocellular carcinoma subset in mice through coexpression of met and point-mutant β-catenin. Hepatology 2016, 64, 1587–1605. [Google Scholar] [CrossRef]
- Perra, A.; Kowalik, M.A.; Pibiri, M.; Ledda-Columbano, G.M.; Columbano, A. Thyroid hormone receptor ligands induce regression of rat preneoplastic liver lesions causing their reversion to a differentiated phenotype. Hepatology 2009, 49, 1287–1296. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Perra, A.; Pibiri, M.; Cocco, M.T.; Samarut, J.; Plateroti, M.; Ledda-Columbano, G.M.; Columbano, A. TRβ is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J. Hepatol. 2010, 53, 686–692. [Google Scholar] [CrossRef]
- Alvarado, T.F.; Puliga, E.; Preziosi, M.; Poddar, M.; Singh, S.; Columbano, A.; Nejak-Bowen, K.; Monga, S.P. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: Implications in hepatic regeneration. Gene Expr. 2016, 17, 19–34. [Google Scholar] [CrossRef]
- Borngraeber, S.; Budny, M.-J.; Chiellini, G.T.; Cunha-Lima, S.; Togashi, M.; Webb, P.; Baxter, J.D.; Scanlan, T.S.; Fletterick, R.J. Ligand selectivity by seeking hydrophobicity in thyroid hormone receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 15358–15363. [Google Scholar] [CrossRef]
- Amorim, B.S.; Ueta, C.B.; Freitas, B.C.; Nassif, R.J.; Gouveia, C.H.; Christoffolete, M.A.; Moriscot, A.S.; Lancelloti, C.L.; Llimona, F.; Barbeiro, H.V.; et al. A TRβ-selective agonist confers resistance to diet-induced obesity. J. Endocrinol. 2009, 203, 291–299. [Google Scholar] [CrossRef]
- Grijota-Martinez, C.; Samarut, E.; Scanlan, T.S.; Morte, B.; Bernal, J. In vivo activity of the thyroid hormone receptor β- and α-selective agonists GC-24 and CO23 on rat liver, heart, and brain. Endocrinology 2011, 152, 1136–1142. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.-L.; Mellstrom, K.; Mellin, C.; Lars-Goran, B.; Koehler, K.; Neeraj, G.; Ana, M.G.C.; Chris, L.; Husman, B.; et al. Thyroid receptor ligands. 1. agonist ligands selective for the thyroid receptor β1. J. Med. Chem. 2003, 46, 1580–1588. [Google Scholar]
- Bryzgalova, G.; Effendic, S.; Khan, A.; Rehnmark, S.; Barbounis, P.; Boulet, J.; Dong, G.; Singh, R.; Shapses, S.; Malm, J.; et al. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor β subtype selective agonist KB-141. J. Steroid Biochem. Mol. Biol. 2008, 111, 262–267. [Google Scholar] [CrossRef]
- Grover, G.J.; Mellstrom, K.; Liu, Y.; Malm, J.; Li, Y.-L.; Bladh, L.-G.; Sleph, P.G.; Smith, M.A.; George, R.; Vennstrom, B.; et al. Selective thyroid hormone receptor-β activation: A strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc. Natl. Acad. Sci. USA 2003, 100, 10067–10072. [Google Scholar] [CrossRef]
- Grover, G.J.; Mellstrom, K.; Malm, J. Development of the thyroid hormone receptor β-subtype agonist KB-141: A strategy for body weight reduction and lipid lowering with minimal cardiac side effects. Cardiovasc. Drug Rev. 2005, 23, 133–148. [Google Scholar] [CrossRef]
- Baxter, J.D.; Webb, P. Thyroid hormone mimetics: Potential applications in atherosclerosis, obesity and type 2 diabetes. Nat. Rev. Drug Discov. 2009, 8, 308–320. [Google Scholar] [CrossRef]
- Berkenstam, A.; Kristensen, J.; Mellstrom, K.; Carlsson, B.; Malm, J.; Rehnmark, S.; Garg, N.; Andersson, C.M.; Rudling, M.; Sjoberg, F.; et al. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 663–667. [Google Scholar] [CrossRef]
- Ladenson, P.W.; Kristensen, J.D.; Ridgway, E.C.; Olsson, A.G.; Carlsson, B.; Klein, I.; Baxter, J.D.; Angelin, B. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N. Engl. J. Med. 2010, 362, 906–916. [Google Scholar] [CrossRef]
- Szydlowska, M.; Pibiri, M.; Perra, A.; Puliga, E.; Mattu, S.; Ledda-Columbano, G.M.; Columbano, A.; Leoni, V.P. The thyromimetic KB2115 (Eprotirome) induces rat hepatocyte proliferation. Gene Expr. 2017, 17, 207–218. [Google Scholar] [CrossRef]
- Erion, M.D.; Cable, E.E.; Bruce, R.I.; Jiang, H.J.; Fujitaki, J.M.; Finn, P.D.; Zhang, B.H.; Hou, J.Z.; Boyer, S.H.; Poelje, P.D.; et al. Targeting thyroid hormone receptor-β agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc. Natl. Acad. Sci. USA 2007, 104, 15490–15495. [Google Scholar] [CrossRef]
- Cable, E.E.; Finn, P.D.; Stebbins, J.W.; Hou, J.; Ito, B.R.; van Poelje, P.D.; Linemeyer, D.L.; Erion, M.D. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 2009, 49, 407–417. [Google Scholar] [CrossRef]
- Linemeyer, D.L.; Cable, E.E.; Yang, X.; Ito, B.R.; van Poelje, P.D.; Erion, M.D. MB07811, a liver-targeted prodrug of a novel thyroid hormone receptor agonist, does not cause hyperglycaemia in Sprague Dawley rats or diet-induced obese mice. Diabetologia 2007, 50, S489–S490. [Google Scholar]
- Zhou, J.; Waskowicz, L.R.; Lim, A.; Liao, X.-H.; Lian, B.; Masamune, H.; Refetoff, S.; Tran, B.; Koeberl, D.D.; Yen, P.M. A liver-specific thyromimetic, VK2809, decreases hepatosteatosis in glycogen storage disease type Ia. Thyroid 2019, 29, 1158–1167. [Google Scholar] [CrossRef]
- Loomba, R.; Neutel, J.; Mohseni, R.; Bernard, D.; Severance, R.; Dao, M.; Saini, S.; Margaritescu, C.; Homer, K.; Tran, B.; et al. LBP-20-VK2809, a novel liver-directed thyroid receptor β agonist, significantly reduces liver fat with both low and high doses in patients with non-alcoholic fatty liver disease: A phase 2 randomized, placebo-controlled trial. J. Hepatol. 2019, 70, e150–e151. [Google Scholar] [CrossRef]
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.S.; et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a highly selective thyroid hormone receptor β agonist in clinical trials for the treatment of dyslipidemia. J. Med. Chem. 2014, 57, 3912–3923. [Google Scholar]
- Taub, R.; Chiang, E.; Chabot-Blanchet, M.; Kelly, M.J.; Reeves, R.A.; Guertin, M.C.; Tardif, J.C. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-β agonist. Atherosclerosis 2013, 230, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Taub, R.; Frias, J.P.; Baum, S.J.; Hsia, S.; Harrison, S. In a 36-week placebo-controlled phase 2 trial in patients with non-alcoholic steatohepatitis (NASH), treatment with MGL-3196 (resmetirom) significantly reduces atherogenic lipoprotein particles. Eur. Heart J. 2019, 40, 826. [Google Scholar] [CrossRef]
- Madrigal MAESTRO Phase 3 NASH Trials Continue without Protocol Modifications; New Data Demonstrate That Reductions in Liver Fat Achieved by Resmetirom Predict NASH Resolution and Fibrosis Reduction. Available online: http://www.globenewswire.com/news-release/2020/04/14/2015502/0/en/Madrigal-MAESTRO-Phase-3-NASH-Trials-Continue-without-Protocol-Modifications-New-Data-Demonstrate-that-Reductions-in-Liver-Fat-Achieved-by-Resmetirom-Predict-NASH-Resolution-and-Fi.html (accessed on 12 September 2021).
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Ure, D.R.; Trepanier, D.J.; Mayo, P.R.; Foster, R.T. Cyclophilin inhibition as a potential treatment for nonalcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs. 2019, 29, 163–178. [Google Scholar] [CrossRef]
- Tilg, H.; Effenberger, M.; Adolph, T.E. A role for IL-1 inhibitors in the treatment of non-alcoholic fatty liver disease (NAFLD)? Expert Opin. Investig. Drugs 2020, 29, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef]
- Neumann, T.; Junker, H.D.; Schmidt, K.; Sekul, R. SPR-based fragment screening: Advantages and applications. Curr. Top. Med. Chem. 2007, 7, 1630–1642. [Google Scholar] [CrossRef]

| Compounds | Structure | Beneficial Effects | Deleterious Effects | Clinical Trials |
|---|---|---|---|---|
| Sobetirome (GC-1) |  | (1) 10-fold lower affinity of TRα (2) Reducing cholesterol level (3) Inhibiting HCC development (4) Liver regeneration | Fasting blood sugar and insulin resistance | Ending in phase I |
| GC-24 |  | (1) 40-fold higher affinity of TRβ (2) Lower insulin sensitivity | (1) Low sensitivity for activated-TRβ (2) No hepatic targeting | —— |
| KB-141 | 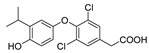 | (1) Metabolic enhancement (2) Weight loss | —— | —— |
| Eprotirome (KB2115) | 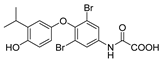 | (1) Reducing triglycerides level markedly (2) Liver targeting (3) Liver regeneration | (1) Increasing fasting blood insulin (2) Adverse effects on dogs’ cartilage of withdrawal | Ending in phase III |
| M07811 (VK2809) /MB07344 | 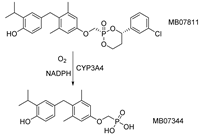 | (1) Reducing cholesterol and triglycerides level (2) Inhibiting hepatic steatosis (3) Promoting hepatocyte proliferation | —— | Phase II ongoing |
| Resmetirom (MGL-3196) | 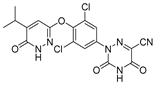 | (1) Reducing cholesterol and triglycerides level (2) Inhibiting hepatic steatosis and fibrosis (3) Reducing hepatic fat markedly (4) Heart protection | —— | Phase III ongoing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Xie, H.; Shan, H.; Zheng, Z.; Li, G.; Li, M.; Hong, L. Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 1102. https://doi.org/10.3390/ijms23031102
Zhao M, Xie H, Shan H, Zheng Z, Li G, Li M, Hong L. Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2022; 23(3):1102. https://doi.org/10.3390/ijms23031102
Chicago/Turabian StyleZhao, Man, Huazhong Xie, Hao Shan, Zhihua Zheng, Guofeng Li, Min Li, and Liang Hong. 2022. "Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 23, no. 3: 1102. https://doi.org/10.3390/ijms23031102
APA StyleZhao, M., Xie, H., Shan, H., Zheng, Z., Li, G., Li, M., & Hong, L. (2022). Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 23(3), 1102. https://doi.org/10.3390/ijms23031102







