Salviolone from Salvia miltiorrhiza Roots Impairs Cell Cycle Progression, Colony Formation, and Metalloproteinase-2 Activity in A375 Melanoma Cells: Involvement of P21(Cip1/Waf1) Expression and STAT3 Phosphorylation
Abstract
:1. Introduction
2. Results
2.1. Isolation of Compounds 1–7 from Salvia miltiorrhiza Roots
2.2. Quantitative Analysis of Compounds 1–7 in S. miltiorrhiza Ethanol Extract
2.3. A375 and MeWo Melanoma Cell Viability was Affected by Tanshinone IIA, Cryptotanshinone, and Salviolone Extracted from Salvia miltiorrhiza Roots
2.4. Salviolone and Cryptotanshinone Did Not Affect NHEM Cell Viability
2.5. Signaling Pathways Involved in the Salviolone and Cryptotanshinone Anti-Melanoma Activity
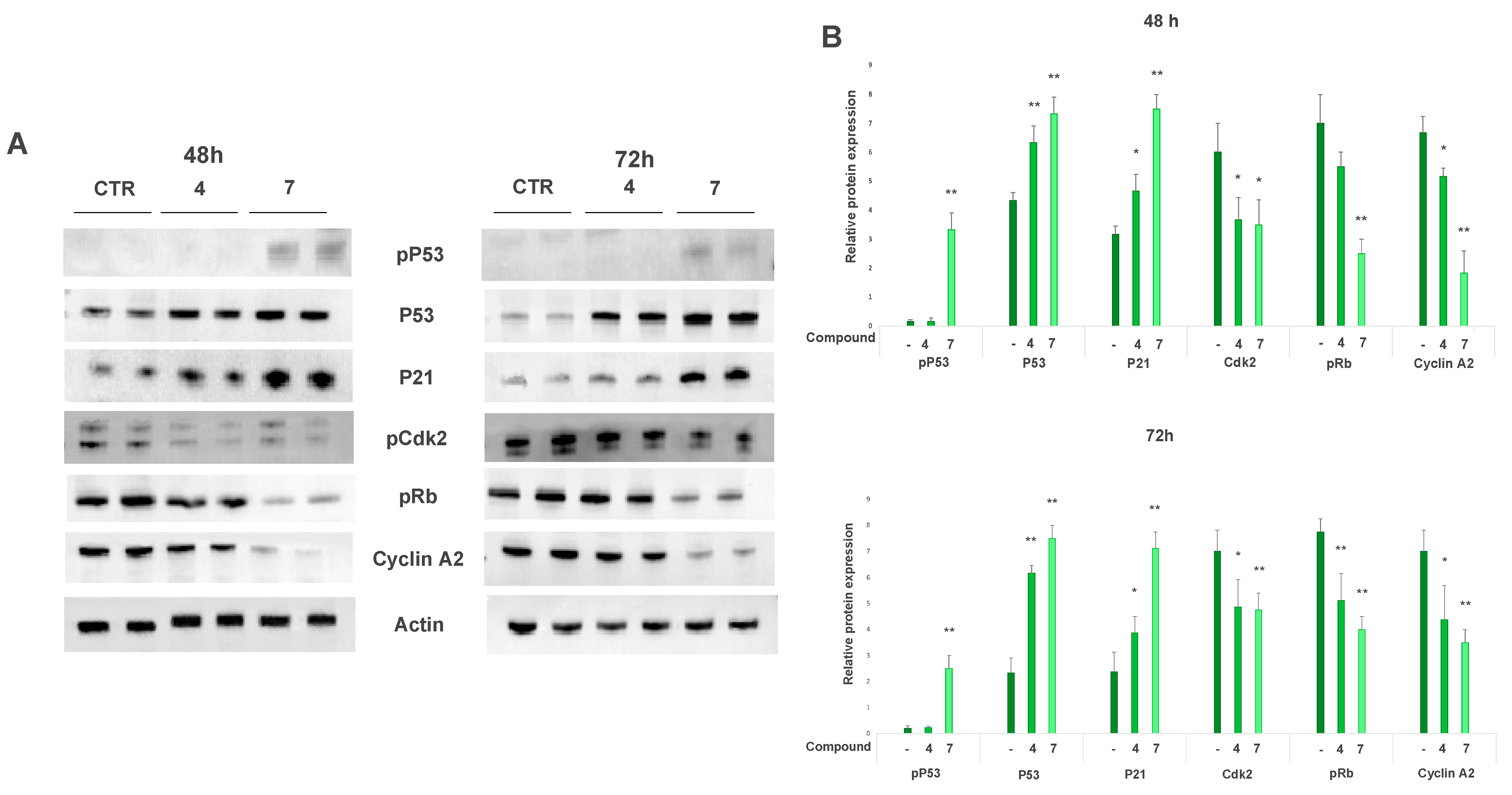
2.5.1. Salviolone and Cryptotanshinone Affect A375 Cell Cycle Progression
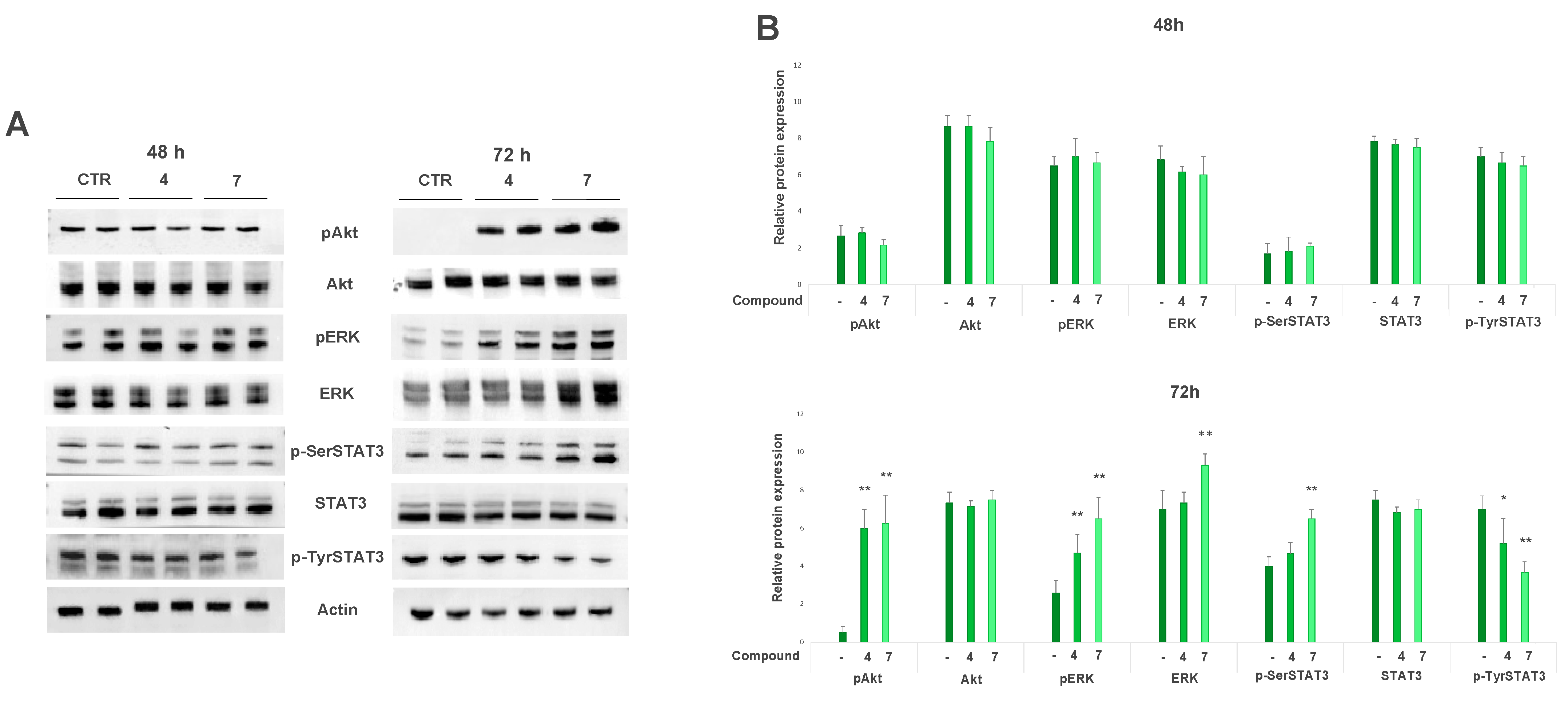
2.5.2. Salviolone and Cryptotanshinone Affect Pro-Survival Pathways in the A375 Cell Line
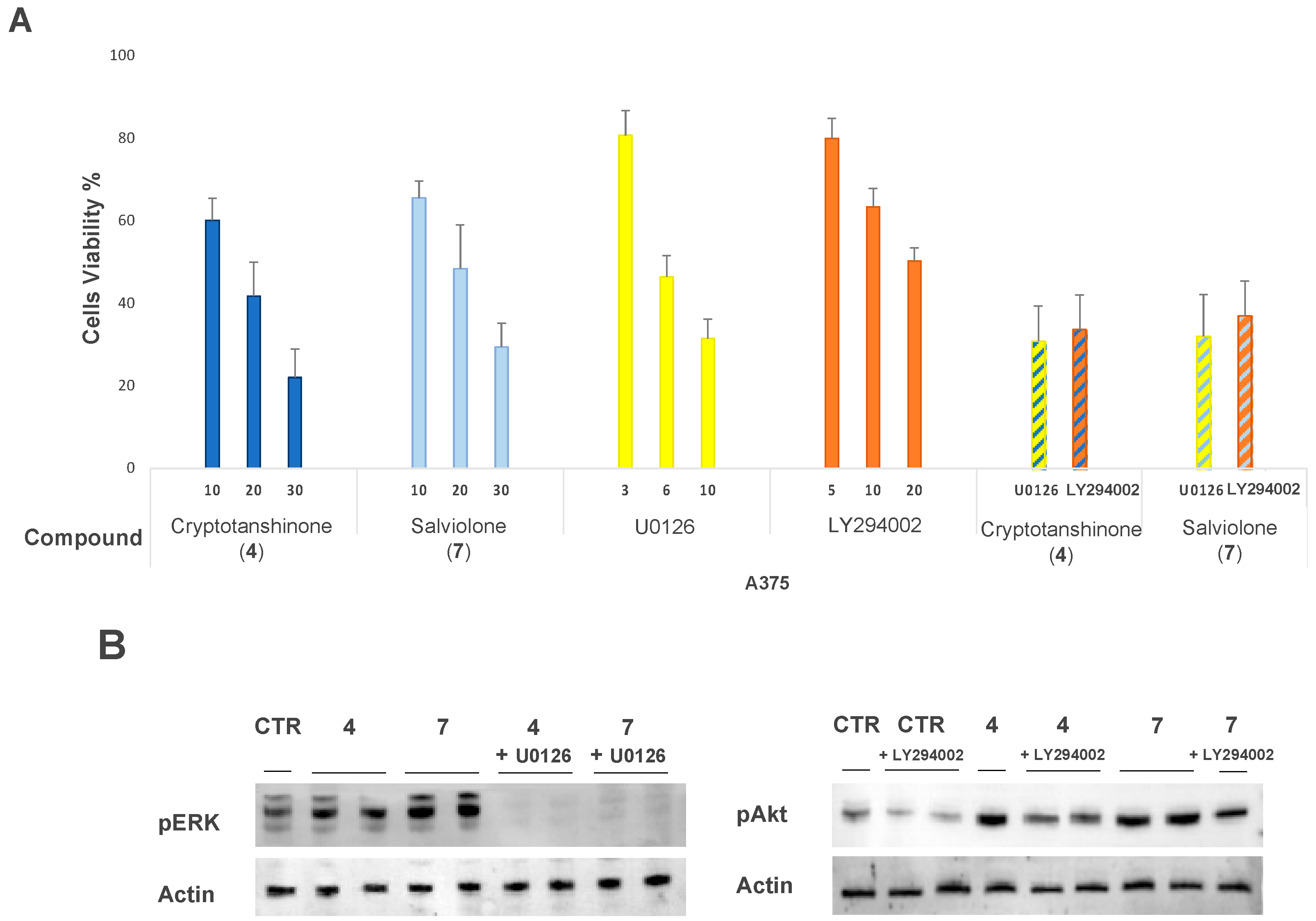
2.6. Effect of MEK1-Inhibitor U0126 and PI3K-Inhibitor LY294002 on A375 Cells Treated with Salviolone or Cryptotanshinone
2.7. Salviolone and Cryptotanshinone Inhibit the Ability of A375 Cells to Form Colonies in Soft Agar
2.8. Salviolone and Cryptotanshinone Inhibit Matrix Metalloproteinase 2 (MMP2) Activity in A375 Cells
2.9. Cryptotanshinone but Not Salviolone Inhibits A375 Cell Migration
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Quantitative Analysis of Compounds 1–7 in S. miltiorrhiza Roots
4.5. Malignant Melanoma and Normal Melanocyte Cell Culture
4.6. Cell Viability Sulforhodamine B Assay
4.7. Total Protein Extracts and Sample Preparation for Immunoblot Analysis
4.8. Immunoblot Analysis
4.9. Soft Agar Colony Formation Assay
4.10. Gelatin Zymography
4.11. Wound Closure Cell Migration Assay
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Indini, A.; Grossi, F.; Mandalà, M.; Taverna, D.; Audrito, V. Metabolic Interplay between the Immune System and Melanoma Cells: Therapeutic Implications. Biomedicines 2021, 9, 607. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614, Corrigendum in Front. Pharmacol. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashed, A.A.; Rathi, D.-N.G. Bioactive Components of Salvia and Their Potential Antidiabetic Properties: A Review. Molecules 2021, 26, 3042. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, S.; Wang, Y.; Dong, S.; Guan, T.; Hou, L.; Xing, X.; Zhang, J.; Li, T. In vitro and in silico perspectives on estrogenicity of tanshinones from Salvia miltiorrhiza. Food Chem. 2019, 270, 281–286. [Google Scholar] [CrossRef]
- Gao, H.; Sun, W.; Zhao, J.; Wu, X.; Lu, J.-J.; Chen, X.; Xu, Q.-M.; Khan, I.A.; Yang, S. Tanshinones and diethyl blechnics with anti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen). Sci. Rep. 2016, 6, 33720. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The Anticancer Properties of Tanshinones and the Pharmacological Effects of Their Active Ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Chu, C.-F.; Wang, C.-N.; Wu, H.-T.; Bi, K.-W.; Pang, J.-H.S.; Huang, S.-T. The anti-atherosclerotic effect of tanshinone IIA is associated with the inhibition of TNF-α-induced VCAM-1, ICAM-1 and CX3CL1 expression. Phytomedicine 2014, 21, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, C.; Liu, C.; Liu, L.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.; Sun, C.; Wu, J. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 2021, 137, 111332. [Google Scholar] [CrossRef]
- Slameňová, D.; Mašterová, I.; Lábaj, J.; Horváthová, E.; Kubala, P.; Jakubikova, J.; Wsolova, L. Cytotoxic and DNA-Damaging Effects of Diterpenoid Quinones from the Roots of Salvia officinalis L. on Colonic and Hepatic Human Cells Cultured in vitro. Basic Clin. Pharmacol. Toxicol. 2004, 94, 282–290. [Google Scholar] [CrossRef]
- Li, Z.; Zou, J.; Cao, D.; Ma, X. Pharmacological basis of tanshinone and new insights into tanshinone as a multitarget natural product for multifaceted diseases. Biomed. Pharmacother. 2020, 130, 110599. [Google Scholar] [CrossRef] [PubMed]
- Fischedick, J.T.; Standiford, M.; Johnson, D.A.; Johnson, J.A. Structure activity relationship of phenolic diterpenes from Salvia officinalis as activators of the nuclear factor E2-related factor 2 pathway. Bioorg. Med. Chem. 2013, 21, 2618–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.A.; Khan, F.B.; Safdari, H.A.; Almatroudi, A.; Alzohairy, M.A.; Safdari, M.; Amirizadeh, M.; Rehman, S.; Equbal, M.J.; Hoque, M. Prospective therapeutic potential of Tanshinone IIA: An updated overview. Pharmacol. Res. 2021, 164, 105364. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Saberifar, S.; Salami, S.; Hushmandi, K.; Najafi, M. Recent advances and future directions in anti-tumor activity of cryptotanshinone: A mechanistic review. Phytother. Res. 2021, 35, 155–179. [Google Scholar] [CrossRef]
- Chiu, T.-L.; Su, C.C. Tanshinone IIA increases protein expression levels of PERK, ATF6, IRE1α, CHOP, caspase-3 and caspase-12 in pancreatic cancer BxPC-3 cell-derived xenograft tumors. Mol. Med. Rep. 2017, 15, 3259–3263. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, R.; Zheng, J.; Chen, C.; Huang, C.; Ma, J.; Xu, C.; Zhai, W.; Zheng, J. Cryptotanshinone inhibits proliferation yet induces apoptosis by suppressing STAT3 signals in renal cell carcinoma. Oncotarget 2017, 8, 50023–50033. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lu, H.-L.; Liu, Y.-D.; Yang, L.-Y.; Jiang, Q.-K.; Zhu, X.-J.; Fan, H.-N.; Qian, Y. Cryptotanshinone sensitizes antitumor effect of paclitaxel on tongue squamous cell carcinoma growth by inhibiting the JAK/STAT3 signaling pathway. Biomed. Pharmacother. 2017, 95, 1388–1396. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Li, X.; Liu, B.; Liu, Z. Mechanisms of Tanshinone II a inhibits malignant melanoma development through blocking autophagy signal transduction in A375 cell. BMC Cancer 2017, 17, 357. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, S.-Z.; Sun, Z.-G.; Wang, A.-Y.; Huang, C.-H.; Punchard, N.A.; Huang, S.-L.; Gao, X.; Lu, Y. Cryptotanshinone has diverse effects on cell cycle events in melanoma cell lines with different metastatic capacity. Cancer Chemother. Pharmacol. 2011, 68, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Ye, T.; Zhu, S.; Zhu, Y.; Feng, Q.; He, B.; Xiong, Y.; Zhao, L.; Zhang, Y.; Yu, L.; Yang, L. Cryptotanshinone induces melanoma cancer cells apoptosis via ROS-mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed. Pharmacother. 2016, 82, 319–326, Erratum in Biomed. Pharmacother. 2019, 120, 109510. [Google Scholar] [CrossRef]
- Saraf, R.S.; Datta, A.; Sima, C.; Hua, J.; Lopes, R.; Bittner, M. An in-silico study examining the induction of apoptosis by Cryptotanshinone in metastatic melanoma cell lines. BMC Cancer 2018, 18, 855. [Google Scholar] [CrossRef]
- Ueda, Y.; Richmond, A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006, 19, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Niu, G.; Bowman, T.; Huang, M.; Shivers, S.; Reintgen, D.; Daud, A.; Chang, A.; Kraker, A.; Jove, R.; Yu, H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002, 21, 7001–7010. [Google Scholar] [CrossRef] [Green Version]
- Masullo, M.; Montoro, P.; Autore, G.; Marzocco, S.; Pizza, C.; Piacente, S. Quali-quantitative determination of triterpenic acids of Ziziphus jujuba fruits and evaluation of their capability to interfere in macrophages activation inhibiting NO release and iNOS expression. Food Res. Int. 2015, 77, 109–117. [Google Scholar] [CrossRef]
- Lin, X.; Qureshi, M.Z.; Romero, M.A.; Khalid, S.; Aras, A.; Ozbey, U.; Farooqi, A.A. Regulation of signaling pathways by tanshinones in different cancers. Cell. Mol. Biol. 2017, 63, 53–58. [Google Scholar] [CrossRef]
- Roskoski, R. Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacol. Res. 2019, 139, 471–488. [Google Scholar] [CrossRef]
- Starostina, N.G.; Kipreos, E.T. Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol. 2012, 22, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Bitar, S.; Gali-Muhtasib, H. The Role of the Cyclin Dependent Kinase Inhibitor p21cip1/waf1 in Targeting Cancer: Molecular Mechanisms and Novel Therapeutics. Cancers 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logotheti, S.; Pützer, B.M. STAT3 and STAT5 Targeting for Simultaneous Management of Melanoma and Autoimmune Diseases. Cancers 2019, 11, 1448. [Google Scholar] [CrossRef] [Green Version]
- Tesoriere, A.; Dinarello, A.; Argenton, F. The Roles of Post-Translational Modifications in STAT3 Biological Activities and Functions. Biomedicines 2021, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Decker, T.; Kovarik, P. Serine phosphorylation of STATs. Oncogene 2000, 19, 2628–2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, M.; Oka, M.; Iwasaki, T.; Fukami, Y.; Nishigori, C. Role and Regulation of STAT3 Phosphorylation at Ser727 in Melanocytes and Melanoma Cells. J. Investig. Dermatol. 2012, 132, 1877–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-C.; Pan, M.; Liu, N.; Xiao, J.-G.; Chen, H.-Q. Effects of nuclear factor-κB and ERK signaling transduction pathway inhibitors on human melanoma cell proliferation in vitro. Oncol. Lett. 2015, 10, 3233–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlova, N.I.; Morozevich, G.E.; Ushakova, N.A.; Berman, A.E. Implication of Integrin α2β1 in Proliferation and Invasion of Human Breast Carcinoma and Melanoma Cells: Noncanonical Function of Akt Protein Kinase. Biochemistry 2018, 83, 738–745. [Google Scholar] [CrossRef]
- Frankowski, H.; Gu, Y.-H.; Heo, J.H.; Milner, R.; del Zoppo, G.J. Use of Gel Zymography to Examine Matrix Metalloproteinase (Gelatinase) Expression in Brain Tissue or in Primary Glial Cultures. In Astrocytes; Milner, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 814, pp. 221–233. ISBN 978-1-61779-451-3. [Google Scholar] [CrossRef] [Green Version]
- Ginda, H.; Kusumi, T.; Ishitsuka, M.O.; Kakisawa, H.; Weijie, Z.; Jun, C.; Tian, G.Y. Salviolone, a cytotoxic bisnorditerpene with a benzotropolone chromophore from a chinese drug dan-shen (salvia miltiorrhiza). Tetrahedron Lett. 1988, 29, 4603–4606. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, T.-S.; Chien, H.-J.; Juang, Y.-M.; Chen, C.-J.; Wang, C.-S.; Lai, C.-C. A rapid, simple, and high-throughput UPLC-MS/MS method for simultaneous determination of bioactive constituents in Salvia miltiorrhiza with positive/negative ionization switching. J. Pharm. Biomed. Anal. 2018, 161, 94–100. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Xing, M. Activities of multiple cancer-related pathways are associated withBRAFmutation and predict the resistance to BRAF/MEK inhibitors in melanoma cells. Cell Cycle 2014, 13, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Kaoud, T.S.; Mohassab, A.M.; Hassan, H.A.; Yan, C.; Van Ravenstein, S.X.; Abdelhamid, D.; Dalby, K.N.; Abdel-Aziz, M. NO-releasing STAT3 inhibitors suppress BRAF-mutant melanoma growth. Eur. J. Med. Chem. 2020, 186, 111885. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yamauchi, H. p27-Associated G1 arrest induced by hinokitiol in human malignant melanoma cells is mediated via down-regulation of pRb, Skp2 ubiquitin ligase, and impairment of Cdk2 function. Cancer Lett. 2009, 286, 240–249. [Google Scholar] [CrossRef]
- Park, I.-J.; Yang, W.K.; Nam, S.-H.; Hong, J.; Yang, K.R.; Kim, J.; Kim, S.S.; Choe, W.; Kang, I.; Ha, J. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis 2014, 19, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Ji, C. Inhibition of human cervical cancer cell growth by Salviolone is mediated via autophagy induction, cell migration and cell invasion suppression, G2/M cell cycle arrest and downregulation of Nf-kB/m-TOR/PI3K/AKT pathway. JBUON 2018, 23, 1739–1744. [Google Scholar]
- Liu, C.; Sun, H.-N.; Luo, Y.-H.; Piao, X.-J.; Wu, D.-D.; Meng, L.-Q.; Wang, Y.; Zhang, Y.; Wang, J.-R.; Wang, H.; et al. Cryptotanshinone induces ROS-mediated apoptosis in human gastric cancer cells. Oncotarget 2017, 8, 115398–115412. [Google Scholar] [CrossRef] [Green Version]
- Ehexige, E.; Bao, M.; Bazarjav, P.; Yu, X.; Xiao, H.; Han, S.; Baigude, H. Silencing of STAT3 via Peptidomimetic LNP-Mediated Systemic Delivery of RNAi Downregulates PD-L1 and Inhibits Melanoma Growth. Biomolecules 2020, 10, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohassab, A.M.; Hassan, H.A.; Abdelhamid, D.; Abdel-Aziz, M. STAT3 transcription factor as target for anti-cancer therapy. Pharmacol. Rep. 2020, 72, 1101–1124. [Google Scholar] [CrossRef]
- Momtaz, S.; Niaz, K.; Maqbool, F.; Abdollahi, M.; Rastrelli, L.; Nabavi, S.M. STAT3 targeting by polyphenols: Novel therapeutic strategy for melanoma. BioFactors 2017, 43, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kunnumakkara, A.B.; Harikumar, K.B.; Gupta, S.R.; Tharakan, S.T.; Koca, C.; Dey, S.; Sung, B. Signal Transducer and Activator of Transcription-3, Inflammation, and Cancer: How Intimate Is the Relationship? Ann. N. Y. Acad. Sci. 2009, 1171, 59–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, J.; Uchida, E.; Grammer, T.C.; Blenis, J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 1997, 17, 6508–6516. [Google Scholar] [CrossRef] [Green Version]
- Venkatasubbarao, K.; Choudary, A.; Freeman, J.W. Farnesyl Transferase Inhibitor (R115777)–Induced Inhibition of STAT3(Tyr705)Phosphorylation in Human Pancreatic Cancer Cell Lines Require Extracellular Signal-Regulated Kinases. Cancer Res. 2005, 65, 2861–2871. [Google Scholar] [CrossRef] [Green Version]
- Krasil’Nikov, M.; Ivanov, V.N.; Dong, J.; Ronai, Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: Implications towards sensitization to apoptosis. Oncogene 2003, 22, 4092–4101. [Google Scholar] [CrossRef] [Green Version]
- Vultur, A.; Villanueva, J.A.; Krepler, C.; Rajan, G.; Chen, Q.; Xiao, M.; Li, L.; Gimotty, P.A.; Wilson, M.; Hayden, J.; et al. MEK inhibition affects STAT3 signaling and invasion in human melanoma cell lines. Oncogene 2014, 33, 1850–1861. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Baik, C.; Kirkwood, J.M. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer 2020, 6, 797–810. [Google Scholar] [CrossRef]
- Haugh, A.M.; Salama, A.K.S.; Johnson, D.B. Advanced Melanoma: Resistance Mechanisms to Current Therapies. Hematol. Oncol. Clin. North Am. 2021, 35, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, Y.-I.; Miyazawa, K.; Kitamura, N. High Intensity ERK Signal Mediates Hepatocyte Growth Factor-induced Proliferation Inhibition of the Human Hepatocellular Carcinoma Cell Line HepG2. J. Biol. Chem. 2001, 276, 40968–40976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kaur, C.D.; Jangdey, M.; Saraf, S. Matrix metalloproteinase enzymes and their naturally derived inhibitors: Novel targets in photocarcinoma therapy. Ageing Res. Rev. 2014, 13, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kortylewski, M.; Jove, R.; Yu, H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005, 24, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and In Vitro Preliminary Anti-Inflammatory Activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) Burs, Leaves and Chestnuts Extracts and their Metabolite Profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Raineri, A.; Fasoli, S.; Campagnari, R.; Gotte, G.; Menegazzi, M. Onconase Restores Cytotoxicity in Dabrafenib-Resistant A375 Human Melanoma Cells and Affects Cell Migration, Invasion and Colony Formation Capability. Int. J. Mol. Sci. 2019, 20, 5980. [Google Scholar] [CrossRef] [Green Version]
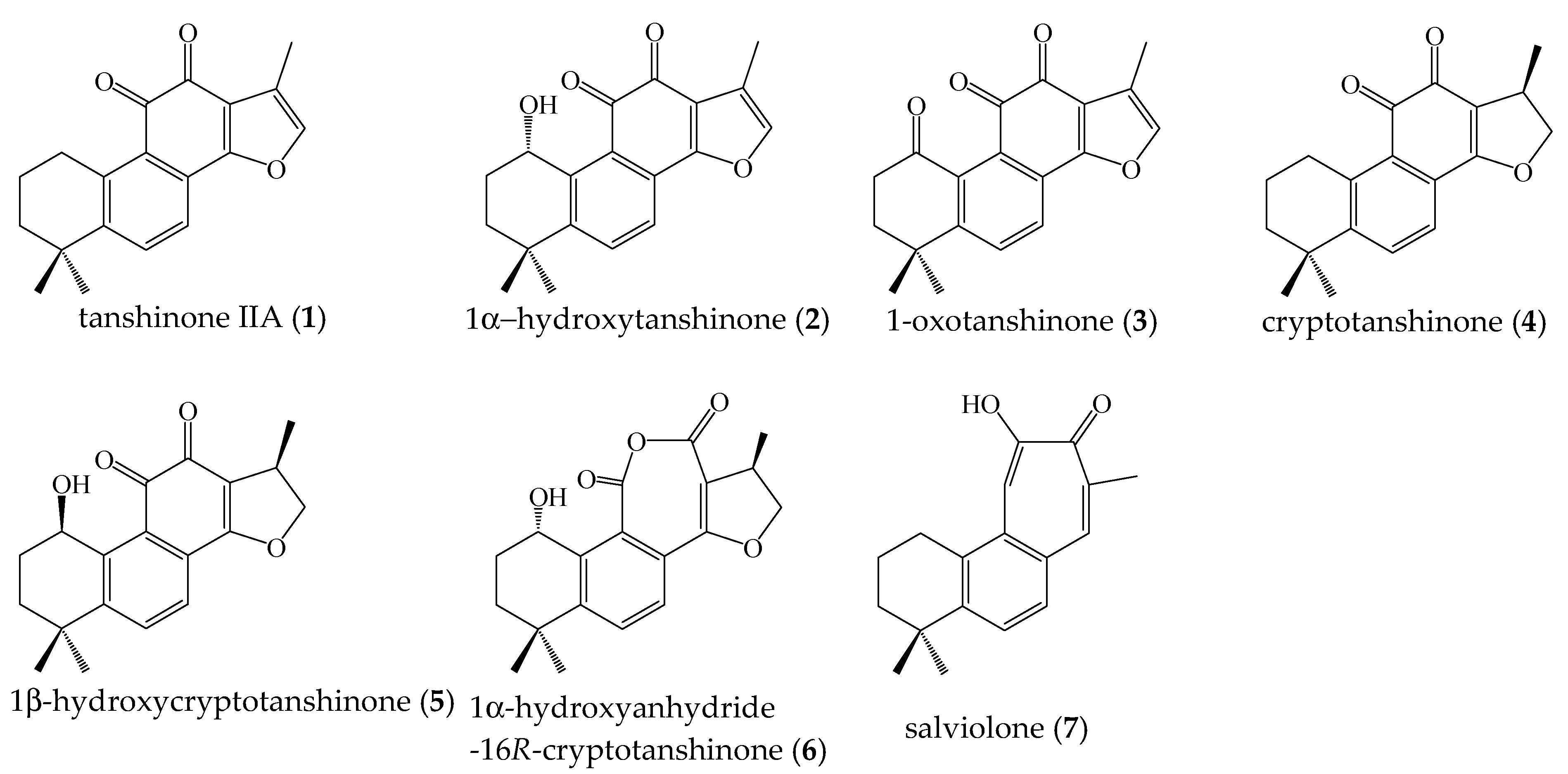


| Compound | MRM Transition | R2 | Regression Line | mg/100 g Roots ± SD |
|---|---|---|---|---|
| Tanshinone IIA (1) | 295 → 277 | 0.992 | y = 3.50 × 103x + 1.15 × 106 | 1361.51 ± 33.39 |
| 1α-Hydroxytanshinone (2) | 311 → 293 | 0.995 | y = 103x − 4.91 × 104 | 83.00 ± 1.82 |
| 1-oxotanshinone (3) | 309 → 281 | 0.996 | y = 170x − 5.12 × 104 | 353.65 ± 11.08 |
| Cryptotanshinone (4) | 297 → 251 | 0.995 | y = 4.75 × 103x + 2.30 × 106 | 1379.00 ± 55.72 |
| 1β-Hydroxycryptotanshinone (5) | 313 → 267 | 0.994 | y = 2.72 × 103x + 6.63 × 105 | N.D. |
| 1α-Hydroxyanhydride-16R-cryptotanshinone (6) | 329 → 267 | 0.991 | y = 1.73 × 103x + 2.26 × 104 | 20.44 ± 0.89 |
| Salviolone (7) | 269 → 251 | 0.992 | y = 96.2x + 3.675 × 104 | 31.60 ± 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanrè, V.; Campagnari, R.; Cerulli, A.; Masullo, M.; Cardile, A.; Piacente, S.; Menegazzi, M. Salviolone from Salvia miltiorrhiza Roots Impairs Cell Cycle Progression, Colony Formation, and Metalloproteinase-2 Activity in A375 Melanoma Cells: Involvement of P21(Cip1/Waf1) Expression and STAT3 Phosphorylation. Int. J. Mol. Sci. 2022, 23, 1121. https://doi.org/10.3390/ijms23031121
Zanrè V, Campagnari R, Cerulli A, Masullo M, Cardile A, Piacente S, Menegazzi M. Salviolone from Salvia miltiorrhiza Roots Impairs Cell Cycle Progression, Colony Formation, and Metalloproteinase-2 Activity in A375 Melanoma Cells: Involvement of P21(Cip1/Waf1) Expression and STAT3 Phosphorylation. International Journal of Molecular Sciences. 2022; 23(3):1121. https://doi.org/10.3390/ijms23031121
Chicago/Turabian StyleZanrè, Valentina, Rachele Campagnari, Antonietta Cerulli, Milena Masullo, Alessia Cardile, Sonia Piacente, and Marta Menegazzi. 2022. "Salviolone from Salvia miltiorrhiza Roots Impairs Cell Cycle Progression, Colony Formation, and Metalloproteinase-2 Activity in A375 Melanoma Cells: Involvement of P21(Cip1/Waf1) Expression and STAT3 Phosphorylation" International Journal of Molecular Sciences 23, no. 3: 1121. https://doi.org/10.3390/ijms23031121
APA StyleZanrè, V., Campagnari, R., Cerulli, A., Masullo, M., Cardile, A., Piacente, S., & Menegazzi, M. (2022). Salviolone from Salvia miltiorrhiza Roots Impairs Cell Cycle Progression, Colony Formation, and Metalloproteinase-2 Activity in A375 Melanoma Cells: Involvement of P21(Cip1/Waf1) Expression and STAT3 Phosphorylation. International Journal of Molecular Sciences, 23(3), 1121. https://doi.org/10.3390/ijms23031121









