Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans
Abstract
1. Introduction
2. Simulation of Cardiac Muscle Twitch Contractions
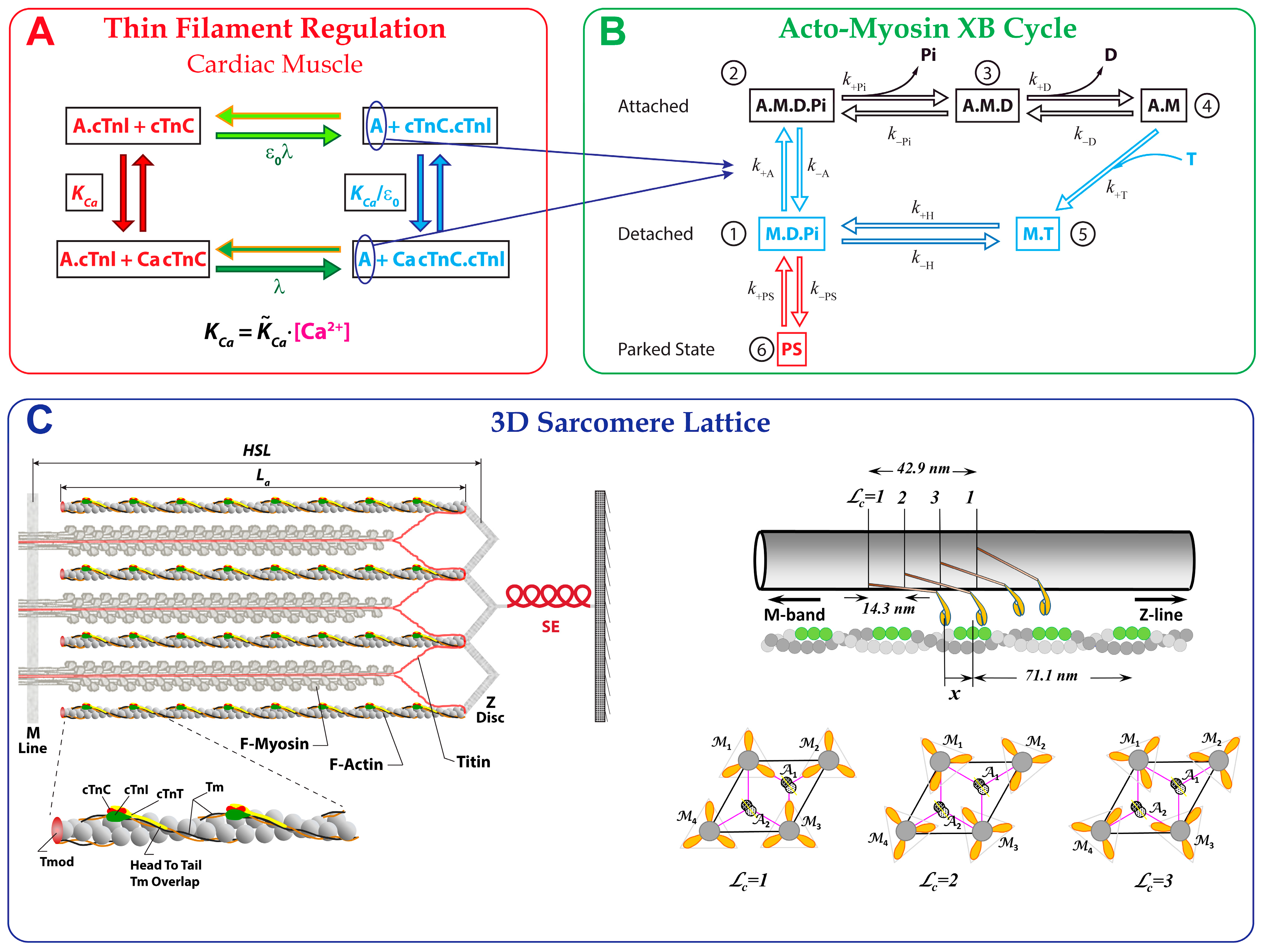
2.1. Cardiac Muscle Structure
2.2. Six-State Crossbridge Cycle
2.3. Calcium-Regulated Tension Generation
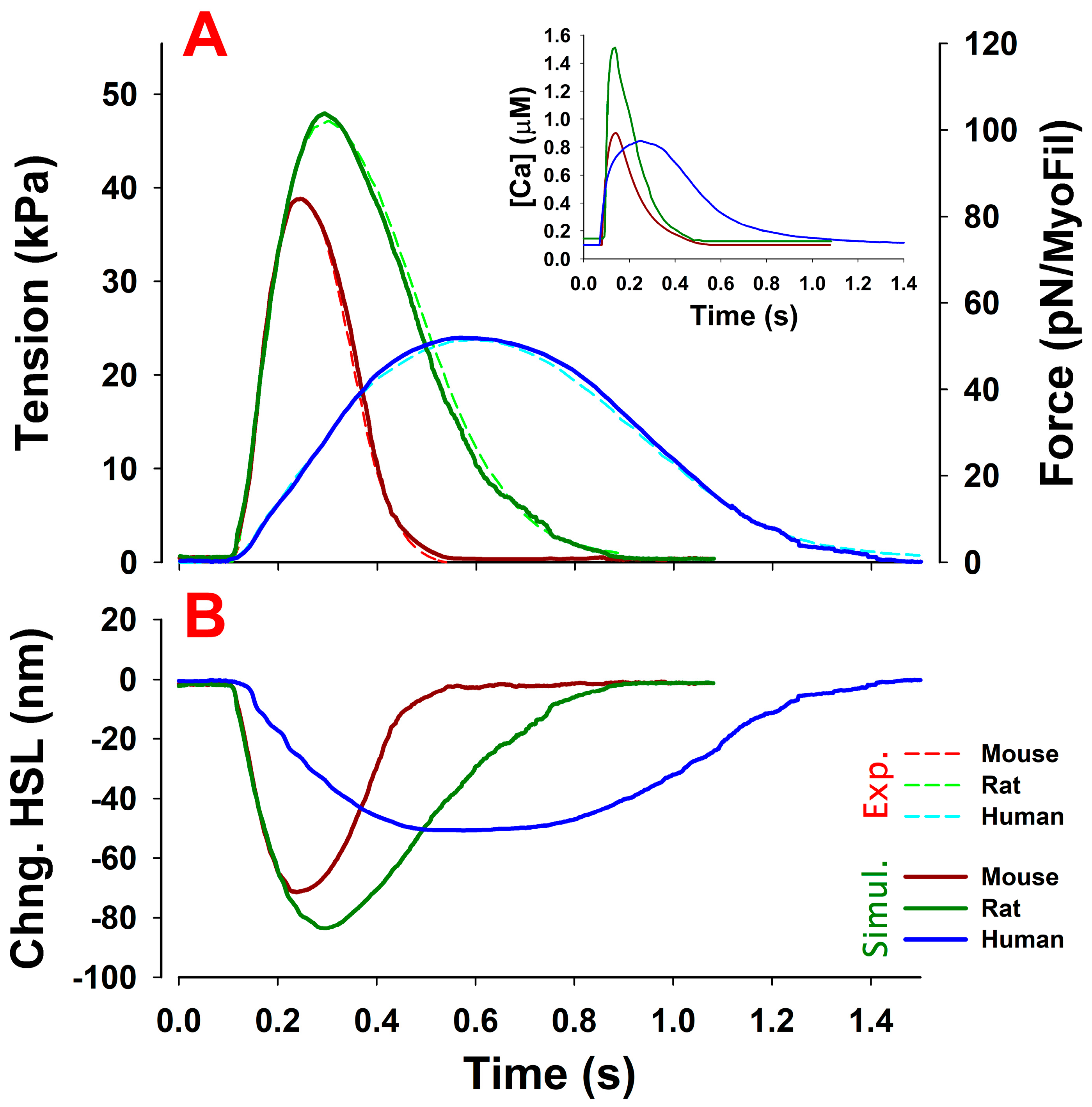
2.4. MUSICO Predictions of Twitch Transients in Intact Trabeculae from Mice, Rats and Humans
3. Results
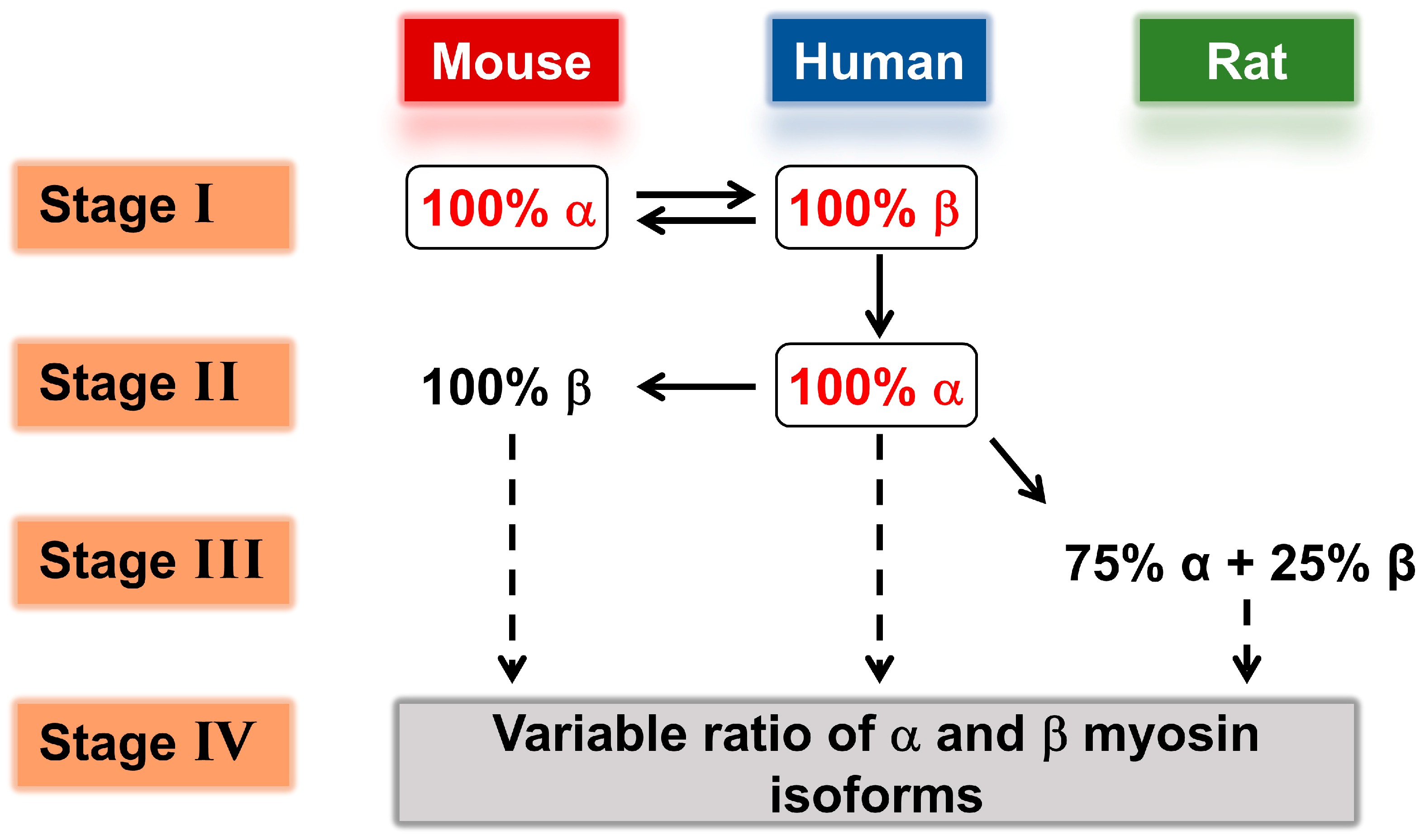
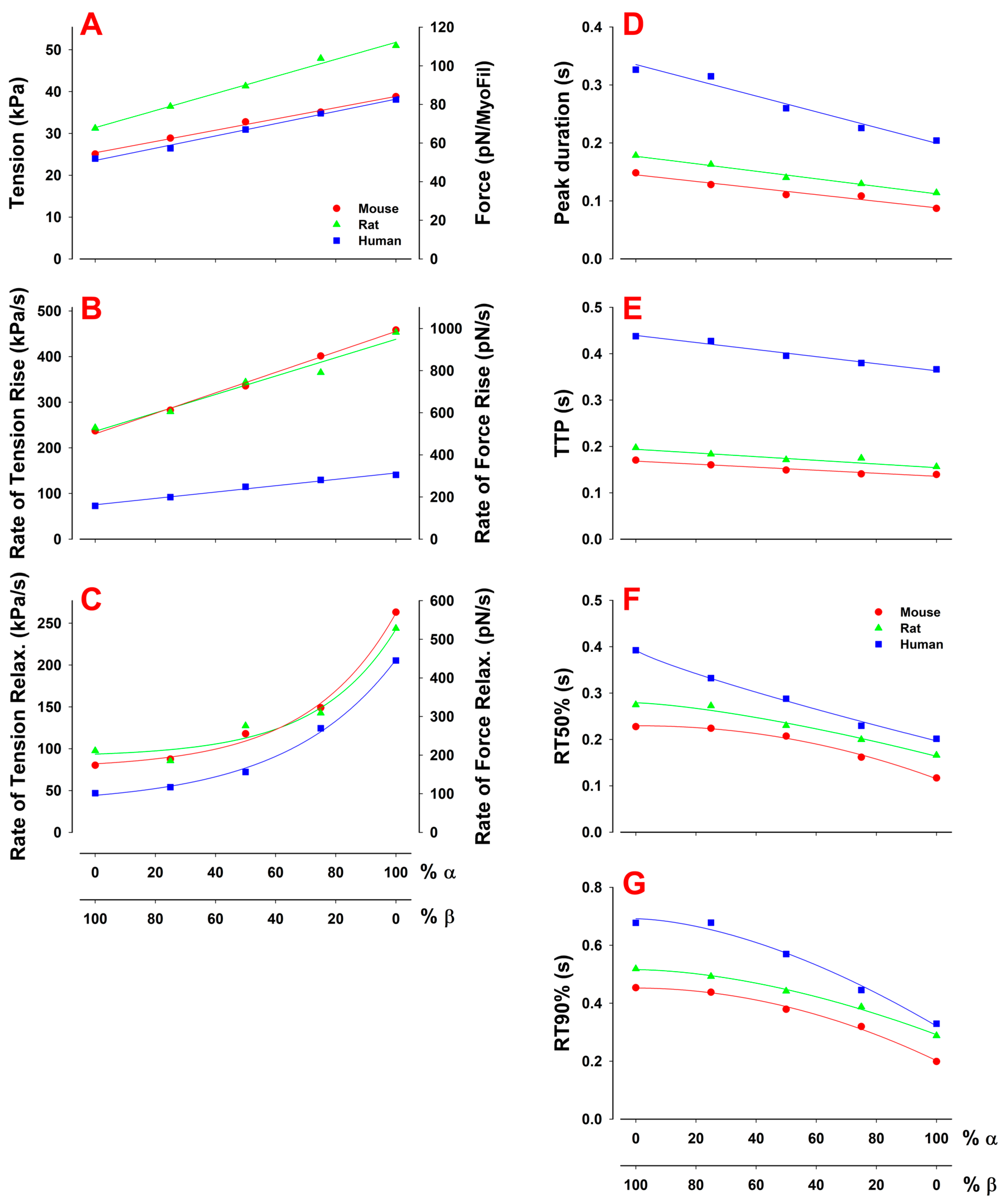
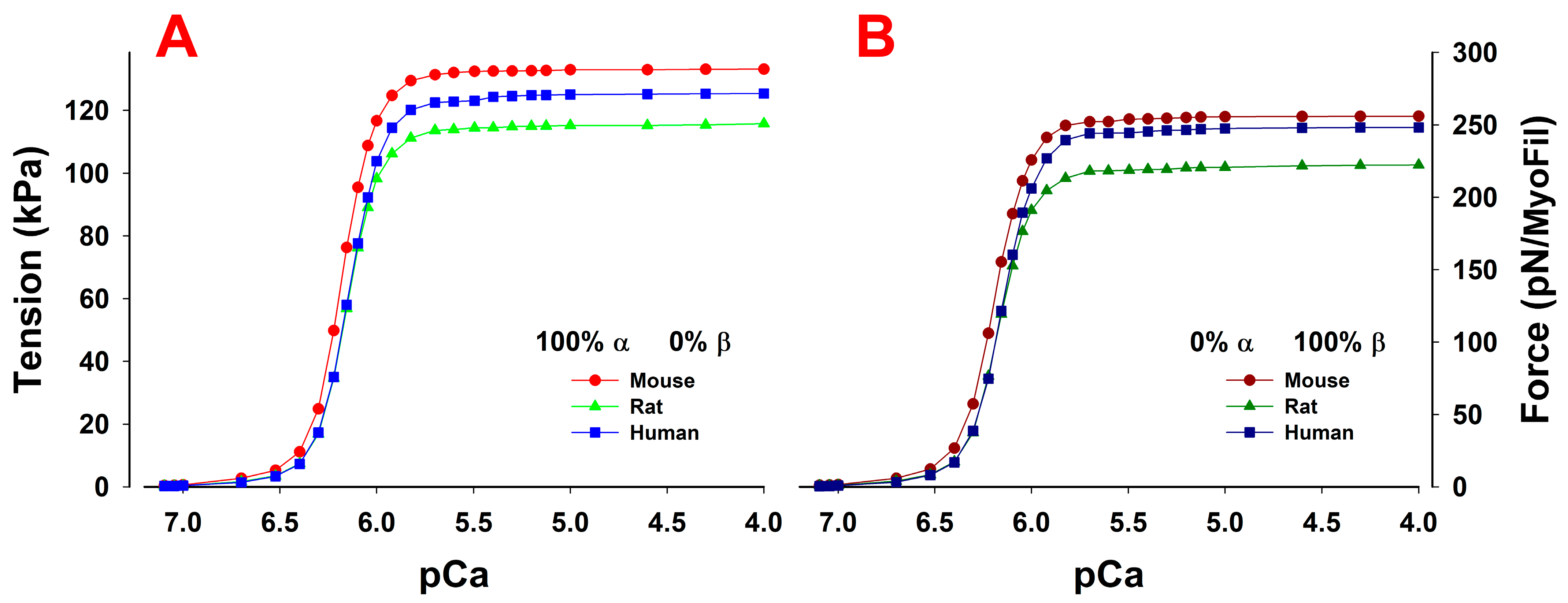
Role of Mixed Myosin α- and β-Isoforms in Cardiac Muscle Twitch Contraction
4. Discussion
Effect of Differences in Myosin Isoforms within and between Species
5. Materials and Methods
5.1. MUSICO Model Parameters
5.2. MUSICO Software Environment and Simulation Details
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mijailovich, S.M.; Prodanovic, M.; Poggesi, C.; Geeves, M.A.; Regnier, M. Multiscale modeling of twitch contractions in cardiac trabeculae. J. Gen. Physiol. 2021, 153, e202012604. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, V.; Bottinelli, R.; Poggesi, C.; Moggio, R.; Reggiani, C. Shortening velocity and myosin and myofibrillar ATPase activity related to myosin isoenzyme composition during postnatal development in rat myocardium. Circ. Res. 1989, 65, 446–457. [Google Scholar] [CrossRef]
- Chizzonite, R.A.; Zak, R. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J. Biol. Chem. 1984, 259, 12628–12632. [Google Scholar] [CrossRef]
- Miyata, S.; Minobe, W.; Bristow, M.R.; Leinwand, L.A. Myosin Heavy Chain Isoform Expression in the Failing and Nonfailing Human Heart. Circ. Res. 2000, 86, 386–390. [Google Scholar] [CrossRef]
- Reiser, P.J.; Portman, M.A.; Ning, X.-H.; Moravec, C.S. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Circ. Physiol. 2001, 280, H1814–H1820. [Google Scholar] [CrossRef]
- Belus, A.; Piroddi, N.; Ferrantini, C.; Tesi, C.; Cazorla, O.; Toniolo, L.; Drost, M.; Mearini, G.; Carrier, L.; Rossi, A.; et al. Effects of Chronic Atrial Fibrillation on Active and Passive Force Generation in Human Atrial Myofibrils. Circ. Res. 2010, 107, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Reiser, P.J.; Moravec, C.S. Sex differences in myosin heavy chain isoforms of human failing and nonfailing atria. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H265–H272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deacon, J.C.; Bloemink, M.J.; Rezavandi, H.; Geeves, M.A.; Leinwand, L.A. Identification of functional differences between recombinant human alpha and beta cardiac myosin motors. Cell. Mol. Life Sci. 2012, 69, 2261–2277. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, K.A.; Tyska, M.J.; Dupuis, D.E.; Alpert, N.R.; Warshaw, D.M. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J. Physiol. 1999, 519, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Mijailovich, S.M.; Nedic, D.; Svicevic, M.; Stojanović, B.; Regnier, M.; Geeves, M.A. Dynamic Transient Responses of Muscle Fibers with a Heterogeneous Populations of Isoforms and Mutation. Biophys. J. 2016, 110, 299a. [Google Scholar] [CrossRef]
- Ng, S.Y.; Wong, C.K.; Tsang, S.Y. Differential gene expressions in atrial and ventricular myocytes: Insights into the road of applying embryonic stem cell-derived cardiomyocytes for future therapies. Am. J. Physiol. Physiol. 2010, 299, C1234–C1249. [Google Scholar] [CrossRef]
- Walklate, J.; Ferrantini, C.; Johnson, C.A.; Tesi, C.; Poggesi, C.; Geeves, M.A. Alpha and beta myosin isoforms and human atrial and ventricular contraction. Cell. Mol. Life Sci. 2021, 78, 7309–7337. [Google Scholar] [CrossRef]
- Pellegrino, M.A.; Canepari, M.; Rossi, R.; D’Antona, G.; Reggiani, C.; Bottinelli, R. Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J. Physiol. 2003, 546, 677–689. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Prodanovic, M.; Poggesi, C.; Powers, J.D.; Davis, J.; Geeves, M.A.; Regnier, M. The effect of variable troponin C mutation thin filament incorporation on cardiac muscle twitch contractions. J. Mol. Cell. Cardiol. 2021, 155, 112–124. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Prodanovic, M.; Poggesi, C.; Regnier, M.; Geeves, M.A. Effect of Myosin Isoform on Mechanics in Intact Cardiac Trabeculae from Mice, Rats and Humans. Biophys. J. 2020, 118, 423a. [Google Scholar] [CrossRef]
- Janssen, P.M.; Biesiadecki, B.J.; Ziolo, M.T.; Davis, J.P. The Need for Speed: Mice, Men, and Myocardial Kinetic Reserve. Circ. Res. 2016, 119, 418–421. [Google Scholar] [CrossRef]
- Chase, P.B.; MacPherson, J.M.; Daniel, T.L. A Spatially Explicit Nanomechanical Model of the Half-Sarcomere: Myofilament Compliance Affects Ca2+-Activation. Ann. Biomed. Eng. 2004, 32, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Tanner, B.C.W.; Daniel, T.L.; Regnier, M. Filament Compliance Influences Cooperative Activation of Thin Filaments and the Dynamics of Force Production in Skeletal Muscle. PLoS Comput. Biol. 2012, 8, e1002506. [Google Scholar] [CrossRef] [PubMed]
- Tanner, B.C.W.; Regnier, M.; Daniel, T.L. A spatially explicit model of muscle contraction explains a relationship between activation phase, power and ATP utilization in insect flight. J. Exp. Biol. 2008, 211, 180–186. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Kayser-Herold, O.; Stojanović, B.; Nedic, D.; Irving, T.C.; Geeves, M.A. Three-dimensional stochastic model of actin–myosin binding in the sarcomere lattice. J. Gen. Physiol. 2016, 148, 459–488. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Stojanovic, B.; Nedic, D.; Svicevic, M.; Geeves, M.A.; Irving, T.C.; Granzier, H. Nebulin and Titin Modulate Crossbridge Cycling and Length Dependent Calcium Sensitivity. J. Gen. Physiol. 2019, 151, 680–704. [Google Scholar] [CrossRef]
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of Contraction in Striated Muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.R.; Granzier, H.L. Titin-based tension in the cardiac sarcomere: Molecular origin and physiological adaptations. Prog. Biophys. Mol. Biol. 2012, 110, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Mijailovich, S.M.; Kayser-Herold, O.; Li, X.; Griffiths, H.; Geeves, M.A. Cooperative regulation of myosin-S1 binding to actin filaments by a continuous flexible Tm–Tn chain. Eur. Biophys. J. 2012, 41, 1015–1032. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Prodanovic, M.; Vasovic, L.; Stojanovic, B.; Maric, M.; Prodanovic, D.; Powers, J.D.; Davis, J.; Geeves, M.A.; Regnier, M. Modulation of Calcium Sensitivity and Twitch Contractions in Cardiac Muscle with Troponin-C Mutations: Simulations and Experiments. Biophys. J. 2019, 116, 116a. [Google Scholar] [CrossRef]
- Robinson, T.F.; Winegrad, S. Variation of thin filament length in heart muscle. Nature 1977, 267, 74–75. [Google Scholar] [CrossRef]
- Caremani, M.; Pinzauti, F.; Reconditi, M.; Piazzesi, G.; Stienen, G.; Lombardi, V.; Linari, M. Size and speed of the working stroke of cardiac myosin in situ. Proc. Natl. Acad. Sci. USA 2016, 113, 3675–3680. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.M.; De Tombe, P.P. Uncontrolled sarcomere shortening increases intracellular Ca2+ transient in rat cardiac trabeculae. Am. J. Physiol. Content 1997, 272, H1892–H1897. [Google Scholar] [CrossRef]
- Geeves, M.; Griffiths, H.; Mijailovich, S.; Smith, D. Cooperative [Ca2+]-Dependent Regulation of the Rate of Myosin Binding to Actin: Solution Data and the Tropomyosin Chain Model. Biophys. J. 2011, 100, 2679–2687. [Google Scholar] [CrossRef]
- Vibert, P.; Craig, R.; Lehman, W. Steric-model for activation of muscle thin filaments 1 1 Edited by P.E. Wright. J. Mol. Biol. 1997, 266, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Popp, D.; Holmes, K. Refinement of the F-Actin Model against X-ray Fiber Diffraction Data by the Use of a Directed Mutation Algorithm. J. Mol. Biol. 1993, 234, 826–836. [Google Scholar] [CrossRef]
- Mijailovich, S.M.; Prodanovic, M.; Irving, T.C. Estimation of Forces on Actin Filaments in Living Muscle from X-ray Diffraction Patterns and Mechanical Data. Int. J. Mol. Sci. 2019, 20, 6044. [Google Scholar] [CrossRef]
- Chung, C.S.; Hoopes, C.W.; Campbell, K.S. Myocardial relaxation is accelerated by fast stretch, not reduced afterload. J. Mol. Cell. Cardiol. 2017, 103, 65–73. [Google Scholar] [CrossRef]
- Janssen, P.M.L.; Stull, L.B.; Marbán, E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am. J. Physiol. Circ. Physiol. 2002, 282, H499–H507. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Coppini, R.; Scellini, B.; Ferrara, C.; Pioner, J.M.; Mazzoni, L.; Priori, S.; Cerbai, E.; Tesi, C.; Poggesi, C. R4496C RyR2 mutation impairs atrial and ventricular contractility. J. Gen. Physiol. 2015, 147, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Pioner, J.M.; Mazzoni, L.; Gentile, F.; Tosi, B.; Rossi, A.; Belardinelli, L.; Tesi, C.; Palandri, C.; Matucci, R.; et al. Late sodium current inhibitors to treat exercise-induced obstruction in hypertrophic cardiomyopathy: An in vitro study in human myocardium. Br. J. Pharmacol. 2018, 175, 2635–2652. [Google Scholar] [CrossRef]
- Deacon, J.C.; Bloemink, M.J.; Rezavandi, H.; Geeves, M.A.; Leinwand, L.A. Erratum to: Identification of functional differences between recombinant human alpha and beta cardiac myosin motors. Cell. Mol. Life Sci. 2012, 69, 4239–4255. [Google Scholar] [CrossRef]
- Duke, T.A.J. Molecular model of muscle contraction. Proc. Natl. Acad. Sci. USA 1999, 96, 2770–2775. [Google Scholar] [CrossRef]
- Smith, D.; Geeves, M. Cooperative Regulation of Myosin-Actin Interactions by a Continuous Flexible Chain II: Actin-Tropomyosin-Troponin and Regulation by Calcium. Biophys. J. 2003, 84, 3168–3180. [Google Scholar] [CrossRef]
- Kreutziger, K.L.; Piroddi, N.; McMichael, J.T.; Tesi, C.; Poggesi, C.; Regnier, M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J. Mol. Cell. Cardiol. 2011, 50, 165–174. [Google Scholar] [CrossRef]
- Wang, D.; McCully, M.E.; Luo, Z.; McMichael, J.; Tu, A.-Y.; Daggett, V.; Regnier, M. Structural and functional consequences of cardiac troponin C L57Q and I61Q Ca2+-desensitizing variants. Arch. Biochem. Biophys. 2013, 535, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Robertson, I.M.; Li, M.X.; McCully, M.E.; Crane, M.L.; Luo, Z.; Tu, A.-Y.; Daggett, V.; Sykes, B.D.; Regnier, M. Structural and Functional Consequences of the Cardiac Troponin C L48Q Ca2+-Sensitizing Mutation. Biochemistry 2012, 51, 4473–4487. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.J.; Lorenz, M.; Evans, G.; Rosenbaum, G.; Pirani, A.; Craig, R.; Tobacman, L.S.; Lehman, W.; Holmes, K.C. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J. Struct. Biol. 2006, 155, 273–284. [Google Scholar] [CrossRef]
- Pirani, A.; Xu, C.; Hatch, V.; Craig, R.; Tobacman, L.S.; Lehman, W. Single Particle Analysis of Relaxed and Activated Muscle Thin Filaments. J. Mol. Biol. 2005, 346, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.F.; Winegrad, S. The measurement and dynamic implications of thin filament lengths in heart muscle. J. Physiol. 1979, 286, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Irving, T.; Maughan, D. In Vivo X-Ray Diffraction of Indirect Flight Muscle from Drosophila melanogaster. Biophys. J. 2000, 78, 2511–2515. [Google Scholar] [CrossRef]
- Huxley, H.; Stewart, A.; Sosa, H.; Irving, T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys. J. 1994, 67, 2411–2421. [Google Scholar] [CrossRef]
- Kojima, H.; Ishijima, A.; Yanagida, T. Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc. Natl. Acad. Sci. USA 1994, 91, 12962–12966. [Google Scholar] [CrossRef] [PubMed]
- Mijailovich, S.M.; Nedic, D.; Svicevic, M.; Stojanović, B.; Walklate, J.; Ujfalusi, Z.; Geeves, M.A. Modeling the Actin.myosin ATPase Cross-Bridge Cycle for Skeletal and Cardiac Muscle Myosin Isoforms. Biophys. J. 2017, 112, 984–996. [Google Scholar] [CrossRef]
- Johnson, C.A.; McGreig, J.E.; Vera, C.D.; Mulvihill, D.P.; Ridout, M.; Leinwand, L.A.; Wass, M.N.; Geeves, M.A. Cardiac contraction velocity has evolved to match heart rate with body size through variation in β-cardiac myosin sequence. bioRxiv 2019. [Google Scholar] [CrossRef]
- Piroddi, N.; Belus, A.; Scellini, B.; Tesi, C.; Giunti, G.; Cerbai, E.; Mugelli, A.; Poggesi, C. Tension generation and relaxation in single myofibrils from human atrial and ventricular myocardium. Pflügers Arch.-Eur. J. Physiol. 2007, 454, 63–73. [Google Scholar] [CrossRef]
- Woulfe, K.C.; Ferrara, C.; Pioner, J.M.; Mahaffey, J.H.; Coppini, R.; Scellini, B.; Ferrantini, C.; Piroddi, N.; Tesi, C.; Poggesi, C.; et al. A Novel Method of Isolating Myofibrils from Primary Cardiomyocyte Culture Suitable for Myofibril Mechanical Study. Front. Cardiovasc. Med. 2019, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; McGreig, E.J.; Jeanfavre, S.T.; Walklate, J.; Vera, C.D.; Farré, M.; Mulvihill, D.P.; Baines, A.J.; Ridout, M.; A Leinwand, L.; et al. Identification of sequence changes in myosin II that adjust muscle contraction velocity. PLoS Biol. 2021, 19, e3001248. [Google Scholar] [CrossRef] [PubMed]
- Alpert, N.R.; Brosseau, C.; Federico, A.; Krenz, M.; Robbins, J.; Warshaw, D.M. Molecular mechanics of mouse cardiac myosin isoforms. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H1446–H1454. [Google Scholar] [CrossRef]
- Davis, J.; Davis, L.C.; Correll, R.N.; Makarewich, C.A.; Schwanekamp, J.; Moussavi-Harami, F.; Wang, D.; York, A.J.; Wu, H.; Houser, S.R.; et al. A Tension-Based Model Distinguishes Hypertrophic versus Dilated Cardiomyopathy. Cell 2016, 165, 1147–1159. [Google Scholar] [CrossRef]
- Powers, J.D.; Kooiker, K.B.; Mason, A.B.; Teitgen, A.E.; Flint, G.V.; Tardiff, J.C.; Schwartz, S.D.; McCulloch, A.D.; Regnier, M.; Davis, J.; et al. Modulating the tension-time integral of the cardiac twitch prevents dilated cardiomyopathy in murine hearts. JCI Insight 2020, 5, e142446. [Google Scholar] [CrossRef]
- Johnson, C.A.; Walklate, J.; Svicevic, M.; Mijailovich, S.M.; Vera, C.; Karabina, A.; Leinwand, L.A.; Geeves, M.A. The ATPase cycle of Human Muscle Myosin II Isoforms: Adaptation of a single mechanochemical cycle for different physiological roles. bioRxiv 2019. [Google Scholar] [CrossRef]
- Lowes, B.; Gilbert, E.M.; Abraham, W.T.; Minobe, W.A.; Larrabee, P.; Ferguson, D.; Wolfel, E.E.; Lindenfeld, J.; Tsvetkova, T.; Robertson, A.D.; et al. Myocardial Gene Expression in Dilated Cardiomyopathy Treated with Beta-Blocking Agents. N. Engl. J. Med. 2002, 346, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, J.E.; Norman, H.S.; Chen, P.P.; Patel, J.R.; Moss, R.L. Transmural variation in myosin heavy chain isoform expression modulates the timing of myocardial force generation in porcine left ventricle. J. Physiol. 2008, 586, 5203–5214. [Google Scholar] [CrossRef]
- Locher, M.R.; Razumova, M.V.; Stelzer, J.E.; Norman, H.S.; Moss, R.L. Effects of low-level α-myosin heavy chain expression on contractile kinetics in porcine myocardium. Am. J. Physiol. Circ. Physiol. 2011, 300, H869–H878. [Google Scholar] [CrossRef] [PubMed]
- Luther, P.K.; Bennett, P.M.; Knupp, C.; Craig, R.; Padron, R.; Harris, S.P.; Patel, J.; Moss, R.L. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J. Mol. Biol. 2008, 384, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Sugimoto, Y.; Tanaka, H.; Ueno, Y.; Takezawa, Y.; Amemiya, Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys. J. 1994, 67, 2422–2435. [Google Scholar] [CrossRef]
- Prodanovic, M.; Irving, T.C.; Mijailovich, S.M. X-ray diffraction from nonuniformly stretched helical molecules. J. Appl. Crystallogr. 2016, 49, 784–797. [Google Scholar] [CrossRef]
- Williams, C.D.; Salcedo, M.K.; Irving, T.C.; Regnier, M.; Daniel, T.L. The length–tension curve in muscle depends on lattice spacing. Proc. R. Soc. B Boil. Sci. 2013, 280, 20130697. [Google Scholar] [CrossRef] [PubMed]

| Description | Parameter | Iso Trabeculae |
|---|---|---|
| Crossbridge Cycle | ||
| Working stroke [1,21,22,39] | 10.5 nm | |
| Second working stroke [1,21,22] | 1 nm | |
| Myosin stroke forward cap rate constant | 1000 s−1 | |
| ATP-binding and myosin detachment rate constant a | 106 s−1 | |
| Crossbridge stiffness [1,21,22,39] | 1.3 pN/nm | |
| at 25 °C | 4.116 pN·nm | |
| Parked State | ||
| Transition rate constant to “parked state” b | 200 s−1 | |
| Baseline rate constant b | 5 s−1 | |
| Amplitude b | 400 s−1 | |
| Calcium Hill function slope b | 5 | |
| Half activation point of the Hill function b | 1 μM | |
| Calcium Kinetics | ||
| Calcium-binding to cTnC equilib. rate constant [30,40] | 106 M−1 | |
| Calcium-binding rate constant to cTnC [30,40] | 7.54·107 M−1·s−1 | |
| Calcium dissociation rate constant from cTnC [41,42,43] | 75.4 s−1 | |
| cTnI-actin equilibrium rate const. at high Ca2+ | 10 | |
| cTnI-actin backward rate const. | 375 s−1 | |
| cTnI-actin-Ca cooperativity coefficient [30,40] | 0.01 | |
| CFC | ||
| Tropomyosin pinning angle [44] | −25° | |
| Myosin Tm angular displacement [44] | 10° | |
| Angular standard deviation of free CFC [25,45] | 29.7° | |
| Persistence length of Tm-cTn confined chain [25] | 50 nm | |
| Sarcomere | ||
| Length of sarcomere | 2.2 μm | |
| Reference length of actin filament [27,46] | 1.1 μm | |
| Interfilament spacing at SL = 2.2 μm [47] | 33.8 nm | |
| Thin filament elastic modulus [48,49] | 65 nN | |
| Thick filament elastic modulus [48] | 132 nN |
| Description | Parameter | Mouse | Rat | Human | |||
|---|---|---|---|---|---|---|---|
| α | β | α | β | α | β | ||
| Myosin-actin binding rate | (s−1) | 330 | 330 | 160 | 160 | 60 | 60 |
| Myosin-actin detachment rate a | (s−1) | 55 | 55 | 40 | 40 | 40 | 40 |
| Power-stroke energy change c | (kBT) | −13 | −11.3 | −13 | −11.3 | −12.5 | −11.3 |
| Myosin reverse-stroke cap rate b | (s−1) | 40 | 13 | 33 | 11 | 30 | 10 |
| ADP release rate a | (s−1) | 150 | 50 | 120 | 40 | 22 | 10 |
| Hydrolysis forward rate a | (s−1) | 150 | 70 | 150 | 63 | 150 | 30 |
| Hydrolysis backward rate a | (s−1) | 15 | 7 | 15 | 6.3 | 15 | 5 |
| α-Myosin | β-Myosin | |
|---|---|---|
| Mouse | 133.1 | 118.1 |
| Rat | 115.8 | 102.6 |
| Human | 125.4 | 114.5 |
| Hill Coefficient | pCa50 | ATPase (s−1) | ||||
|---|---|---|---|---|---|---|
| 100% α | 100% β | 100% α | 100% β | 100% α | 100% β | |
| Mouse | 4.91 | 4.70 | 6.18 | 6.19 | 2.25 | 0.90 |
| Rat | 5.00 | 4.87 | 6.15 | 6.17 | 1.56 | 0.64 |
| Human | 4.86 | 4.78 | 6.14 | 6.15 | 0.43 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prodanovic, M.; Geeves, M.A.; Poggesi, C.; Regnier, M.; Mijailovich, S.M. Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans. Int. J. Mol. Sci. 2022, 23, 1135. https://doi.org/10.3390/ijms23031135
Prodanovic M, Geeves MA, Poggesi C, Regnier M, Mijailovich SM. Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans. International Journal of Molecular Sciences. 2022; 23(3):1135. https://doi.org/10.3390/ijms23031135
Chicago/Turabian StyleProdanovic, Momcilo, Michael A. Geeves, Corrado Poggesi, Michael Regnier, and Srboljub M. Mijailovich. 2022. "Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans" International Journal of Molecular Sciences 23, no. 3: 1135. https://doi.org/10.3390/ijms23031135
APA StyleProdanovic, M., Geeves, M. A., Poggesi, C., Regnier, M., & Mijailovich, S. M. (2022). Effect of Myosin Isoforms on Cardiac Muscle Twitch of Mice, Rats and Humans. International Journal of Molecular Sciences, 23(3), 1135. https://doi.org/10.3390/ijms23031135






