Bilateral Meningioma: A Case Report and Review of the Literature
Abstract
:1. Introduction
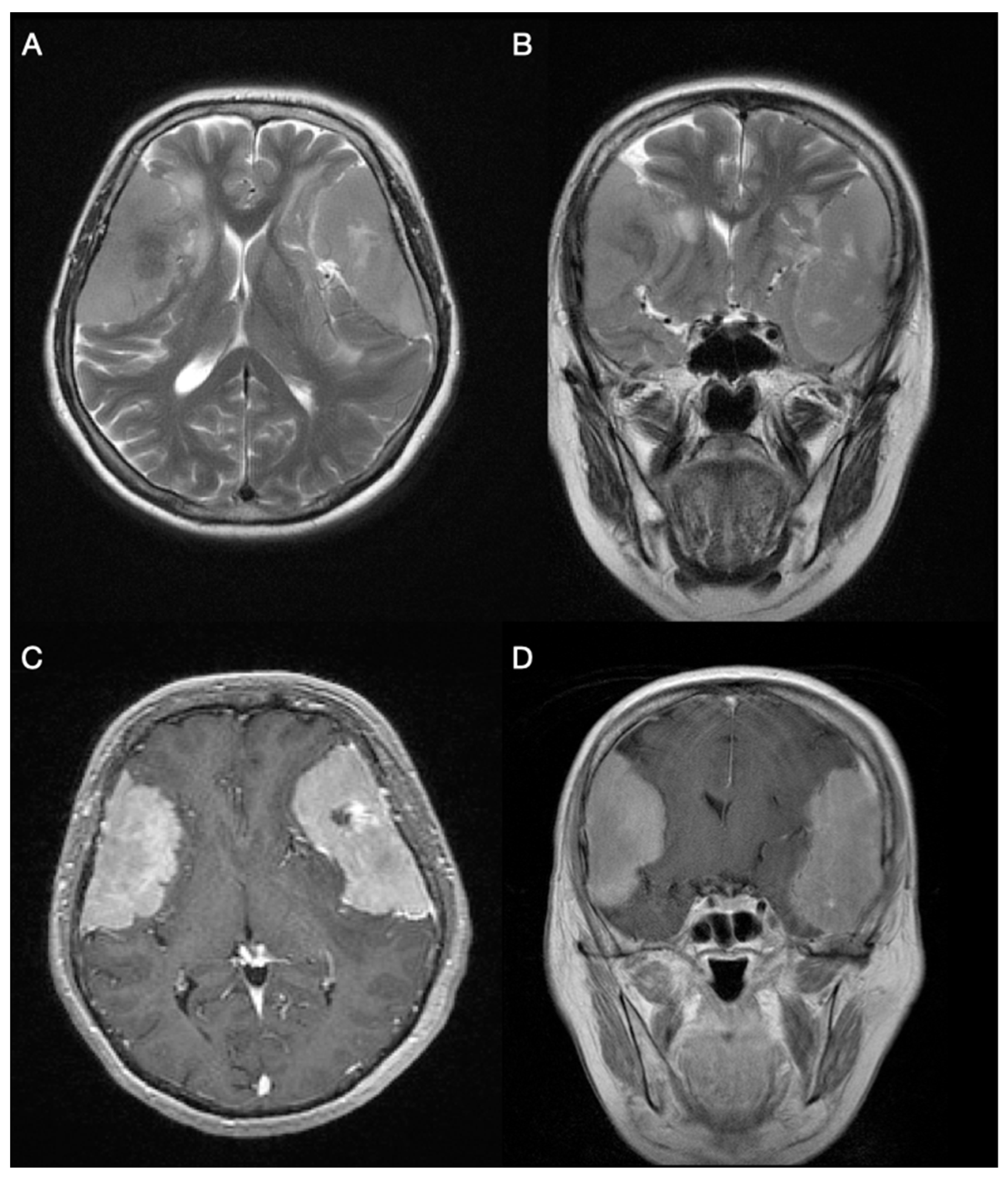
2. Case Description
3. Results
3.1. Sequence Analysis and Deletion/Duplication Testing of NF2 and SMARCB1 Genes
3.2. Levels of E- and N-Cadherins Expression
3.3. Levels of TWIST1, SNAIL and SLUG Expression
3.4. Levels of Beta-Catenin and GSK3beta Expression
3.5. Levels of DVL1 Expression
4. Discussion
5. Methods
5.1. DNA Extraction and Genetic Testing
5.2. Immunohistochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, H.; Croul, S.E. Pathology and genetics of meningiomas. Semin. Diagn. Pathol. 2011, 28, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Pećina-Šlaus, N.; Kafka, A.; Lechpammer, M. Molecular Genetics of Intracranial Meningiomas with Emphasis on Canonical Wnt Signalling. Cancers 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behling, F.; Hempel, J.-M.; Schittenhelm, J. Brain invasion in Meningioma—A prognostic potential worth exploring. Cancers 2021, 13, 3259. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Wong, A.; Vick, N.; Farhat, H. Natural history of multiple meningiomas. Surg. Neurol. Int. 2013, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Moussalem, C.; Massaad, E.; Minassian, G.B.; Ftouni, L.; Bsat, S.; Houshiemy, M.N.E.; Alomari, S.; Sarieddine, R.; Kobeissy, F.; Omeis, I. Meningioma genomics: A therapeutic challenge for clinicians. J. Integr. Neurosci. 2021, 20, 463–469. [Google Scholar] [CrossRef]
- Turgut, M.; Palaoğlu, S.; Ozcan, O.E.; Gürçay, O.; Eryilmaz, M. Multiple meningiomas of the central nervous system without the stigmata of neurofibromatosis. Clinical and therapeutic study. Neurosurg. Rev. 1997, 20, 117–123. [Google Scholar] [CrossRef]
- Domenicucci, M.; Santoro, A.; D’Osvaldo, D.H.; Delfini, R.; Cantore, G.P.; Guidetti, B. Multiple intracranial meningiomas. J. Neurosurg. 1989, 70, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Salvati, M.; Caroli, E.; Ferrante, L.; Rocchi, G.; D’Andrea, G.; Piccirilli, M.; Delfini, R. Spontaneous, multiple meningiomas. Zentralbl. Neurochir. 2004, 65, 180–184. [Google Scholar] [CrossRef]
- Leondi, A.; Valotassiou, V.; Koutsikos, J.; Tsiouris, S.; Mainta, E.; Zerva, C. Multiple meningiomas. Clin. Nucl. Med. 2005, 30, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Terrier, L.M.; François, P. Multiple meningiomas. Neurochirurgie 2016, 62, 128–135. [Google Scholar] [CrossRef]
- Pereira, B.J.A.; de Almeida, A.N.; de Aguiar, P.H.P.; Paiva, W.; Teixeira, M.J.; Marie, S. Multiple Intracranial Meningiomas: A Case Series and Review of the Literature. World Neurosurg. 2019, 122, e1536–e1541. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.; Qualmann, K.; Esquenazi, Y.; Hagan, J.; Kim, D.H. Familial syndromes involving Meningiomas provide Mechanistic insight into Sporadic Disease. Neurosurgery 2018, 83, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Ghanchi, H.; Hariri, O.R.; Takayanagi, A.; Li, G. Multiple Extradural Spinal Meningiomas in a Patient with Acquired Immunodeficiency Syndrome: Case Report and Literature Review. World Neurosurg. 2018, 117, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Koech, F.; Orege, J.; Ndiangui, F.; Macharia, B.; Mbaruku, N. Multiple intracranial meningiomas: A review of the literature and a case report. Case Rep. Surg. 2013, 2013, 131962. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; A Cree, I.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Andrioli, G.C.; Rigobello, L.; Iob, I.; Casentini, L. Multiple meningiomas. Neurochirurgia 1981, 24, 67–69. [Google Scholar] [CrossRef]
- Gelabert-González, M.; Leira-Muiño, R.; Fernández-Villa, J.M.; Iglesias-Pais, M. Multiple intracranial meningiomas. Rev. Neurol. 2003, 37, 717–722. [Google Scholar]
- Celebre, A.; Wu, M.Y.; Danielson, B.; Cohen, S.; Munoz, D.; Das, S.; Karamchandani, J. Anaplastic meningioma with extensive single-cell infiltration: A potential role for epithelial-mesenchymal transformation in the progression of a meningothelial tumour? Histopathology 2013, 62, 1111–1114. [Google Scholar] [CrossRef]
- Collord, G.; Tarpey, P.; Kurbatova, N.; Martincorena, I.; Moran, S.; Castro, M.; Nagy, T.; Bignell, G.; Maura, F.; Young, M.D.; et al. An integrated genomic analysis of anaplastic meningioma identifies prognostic molecular signatures. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogasawara, C.; Philbrick, B.D.; Adamson, D.C. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines 2021, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, M.; Foshay, K.; Abedalthagafi, M. Recent Advances in Meningioma Immunogenetics. Front. Oncol. 2020, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, P.; Brenner, A.V.; Neta, G.; Pfeiffer, R.; Wang, S.S.; Yeager, M.; Thomas, G.; Fine, H.A.; Linet, M.S.; Rothman, N.; et al. Risk of Meningioma and Common Variation in Genes Related to Innate Immunity. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1356–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallesch, M.; Pachow, D.; Blücher, C.; Firsching, R.; Warnke, J.P.; Braunsdorf, W.E.K.; Kirches, E.; Mawrin, C. Altered expression of E-Cadherin-related transcription factors indicates partial epithelial-mesenchymal transition in aggressive meningiomas. J. Neurol. Sci. 2017, 380, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M. Involvement of partial EMT in cancer progression. J. Biochem. 2018, 164, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Castro-Piedras, I.; Simmons, G.; Pruitt, K. Dishevelled: A masterful conductor of complex Wnt signals. Cell. Signal. 2018, 47, 52–64. [Google Scholar] [CrossRef]
- Bukovac, A.; Kafka, A.; Raguž, M.; Brlek, P.; Dragičević, K.; Müller, D.; Pećina-Šlaus, N. Are We Benign? What Can Wnt Signaling Pathway and Epithelial to Mesenchymal Transition Tell Us about Intracranial Meningioma Progression. Cancers 2021, 13, 1633. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N.; Kafka, A.; Vladušić, T.; Pećina, H.I.; Hrašćan, R. AXIN1 Expression and Localization in Meningiomas and Association to Changes of APC and E-cadherin. Anticancer. Res. 2016, 36, 4583–4594. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, G.T.C.; Silva-Martins, W.C.; Magalhães, K.C.S.F.; Nunes, C.B.; Soares, A.N.; Tafuri, L.S.A.; Simões, R.T. Recurrence/Regrowth in Grade I Meningioma: How to Predict? Front. Oncol. 2021, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Maggio, I.; Franceschi, E.; Tosoni, A.; Nunno, V.D.; Gatto, L.; Lodi, R.; Brandes, A.A. Meningioma: Not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021, 10, CNS72. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S.E.; Truty, R.; Lin, C.-F.; Zook, J.M.; Paul, J.; Ramey, V.H.; Salit, M.; Rehm, H.L.; Nussbaum, R.L.; Lebo, M.S. A Rigorous Interlaboratory Examination of the Need to Confirm Next-Generation SequencingeDetected Variants with an Orthogonal Method in Clinical Genetic Testing. J. Mol. Diagn. 2019, 21, 318–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Meningoma #1 | |
|---|---|
| Type | Grade I meningothelial meningioma |
| Location | Left frontotemporoparietal region |
| Consistency and dimensions | Soft consistency with dimensions 7.5 × 6 × 3 cm |
| Mitosis | Nuclei of tumor cells were hypochromatic. Only 1 mitosis found in 10 consecutive high-power fields. |
| Histological description | Nest configurations of meningothelial cells were placed in pseudosyncytial formation. Minor centers of necrosis were observed. Connective tissue, surrounding the tumor, was partially enlarged. A few psammoma bodies were spotted. On the margins of the specimen, there was a sharp transition from tumor cells to healthy brain tissue. |
| Meningoma #2 | |
|---|---|
| Type | Grade II atypical meningioma |
| Location | Right frontotemporoparietal region; tumor attached to dura |
| Consistency and dimensions | Grey, solid consistency with dimensions 7 × 4 cm |
| Mitosis | Maximum of 5 mitosis in 10 consecutive high-power fields were found |
| Histological description | The tumor was built of meningothelial cells forming a pseudosyncytial structure. Hypercellular areas with clear margins between cytoplasmic membranes were spotted. There was no necrosis inside the tissue. Hyalinization and calcification of connective tissue were seen in some areas. A sharp transition from tumor cells to healthy brain parenchyma tissue separated by layers of connective tissue. |
| Antigen | Antibody | Type | Dilution |
|---|---|---|---|
| E-cadherin | E-cadherin clone: NCH-38 Code M3612 (Dako Santa Clara, CA, USA) | Monoclonal | 1:100 |
| N-cadherin | N-cadherin (D-4): sc-8424 (Santa Cruz Biotechnology, Inc. Dallas, TX, USA) | Monoclonal | 1:200 |
| TWIST1 | Anti-Twist antibody [10E4E6] ab175430 (Abcam Cambridge, MA, USA) | Monoclonal | 1:400 |
| SNAIL&SLUG | Anti-SNAIL + SLUG antibody ab180714 (Abcam Cambridge, MA, USA) | Polyclonal | 1:200 |
| Beta-catenin (active) | Non-phospho (Active) β-Catenin (Ser33/37/Thr41), (D131A1), (Cell Signalling Technology, Danvers, MA, USA) | Monoclonal | 1:800 |
| Beta-catenin (total) | Clone b-Catenin-1, M3539 (Dako, Santa Clara, CA, USA) | Monoclonal | 1:200 |
| GSK3beta (active) | Anti-GSK3β (phospho Y216) ab75745 (Abcam, Cambridge, MA, USA) | Polyclonal | 1:100 |
| GSK3beta (inactive) | Anti-GSK3β (phospho S9) ab131097 (Abcam, Cambridge, MA, USA) | Polyclonal | 1:100 |
| DVL1 | Anti-Dishevelled/Dvl1 antibody: ab233003 (Abcam Cambridge, MA, USA) | Polyclonal | 1:200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukovac, A.; Panić, H.; Mrgan, T.; Šlaus, N.; Kafka, A.; Njirić, N.; Pećina-Šlaus, N. Bilateral Meningioma: A Case Report and Review of the Literature. Int. J. Mol. Sci. 2022, 23, 1187. https://doi.org/10.3390/ijms23031187
Bukovac A, Panić H, Mrgan T, Šlaus N, Kafka A, Njirić N, Pećina-Šlaus N. Bilateral Meningioma: A Case Report and Review of the Literature. International Journal of Molecular Sciences. 2022; 23(3):1187. https://doi.org/10.3390/ijms23031187
Chicago/Turabian StyleBukovac, Anja, Hana Panić, Tomislava Mrgan, Nika Šlaus, Anja Kafka, Niko Njirić, and Nives Pećina-Šlaus. 2022. "Bilateral Meningioma: A Case Report and Review of the Literature" International Journal of Molecular Sciences 23, no. 3: 1187. https://doi.org/10.3390/ijms23031187






