Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review
Abstract
:1. Introduction
2. Activity Targeting Cholinergic Neurotransmission
3. BACE-1 Inhibitory Activity
4. α-Synuclein Inhibition
5. MAO Inhibition
6. Anti NFTs Accumulation
7. Neuroinflammation
- Microglia: the resident phagocytes of central nervous system. In the case of AD, the structure binds to soluble Aβ oligomers and Aβ fibrils via the following receptors: SCARA1, CD36, CD14, α6β1 integrin, CD47, and Toll-like receptors. Binding of Aβ with CD36, TLR4, and TLR6 leads to activation of microglia and the production of proinflammatory cytokines and chemokines [114,115].
- Astroglia: accumulates around senile plaques. The structures release cytokines, interleukins, nitric oxide, and other potentially cytotoxic molecules. ApoE is needed for astrocyte-mediated clearance of Aβ, and astrocyte-dependent lipidation of ApoE increases the capability of microglia to clear Aβ [114].
8. The Influence of Iron Ions on Neurodegeneration Process
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| AChE | acetylcholinesterase |
| AME | alternariol monomethyl ether |
| Apo E | apolipoprotein E |
| APP | amyloid precursor protein |
| BACE | β-secretase |
| BDNF | brain-derived neurotrophic factor |
| BuChE | butyrylcholinesterase |
| ChAT | choline O-acetyltransferase |
| CDK5 | cyclin-dependent kinase 5 |
| COX | cyclooxygenase |
| CRP | C-reactive protein |
| EGCG | epigallocatechin-3-gallate |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSK | glycogen synthase kinase |
| iNOS | inducible nitric oxide synthase |
| LDH | lactate dehydrogenase |
| LXR | liver X receptor |
| MAO | monoamine oxidase |
| MDA | malondialdehyde |
| MCP-1 | monocyte chemoattractant protein-1 |
| MMP | mitochondrial membrane potential |
| NF-κB | nuclear factor kappa-B |
| NGF | nerve growth factor |
| NO | nitric oxide |
| PI3K | phosphatidylinositol 3-kinase |
| PKB | protein kinase B |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| PP2A | protein phosphatase 2A |
| ROS | reactive oxygen species |
| r.t. | room temperature |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid reactive substances |
References
- Kumar, A.; Singh, A.; Ekavali, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 1–23. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Waksmundzka-Hajnos, M. An attempt to elucidate the role of iron and zinc ions in development of Alzheimer’s and Parkinson’s diseases. Biomed. Pharmacother. 2019, 111, 1277–1289. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16, e1900434. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tao, H.; Wang, S.; Xiao, J.; Wang, Y.; Su, H. Dietary intervention with edible medicinal plants and derived products for prevention of Alzheimer’s disease: A compendium of time-tested strategy. J. Funct. Foods 2021, 81, 104463. [Google Scholar] [CrossRef]
- Tiraboschi, P.; Hansen, L.A.; Alford, M.; Masliah, E.; Thal, L.J.; Corey-Bloom, J. The decline in synapses and cholinergic activity is asynchronous in Alzheimer’s disease. Neurology 2000, 55, 1278–1283. [Google Scholar] [CrossRef]
- Mesulam, M.-M. Butyrylcholinesterase in Alzheimer’s Disease. In Alzheimer Disease: Therapeutic Strategies; Giacobini, E., Becker, R.E., Eds.; Birkhäuser: Boston, MA, USA, 1994; pp. 79–83. [Google Scholar]
- Inestrosa, N.C.; Sagal, J.P.; Colombres, M. Acetylcholinesterase Interaction with Alzheimer Amyloid β. In Alzheimer’s Disease. Subcellular Biochemistry; Harris, J.R., Fahrenholz, F., Eds.; Springer: Boston, MA, USA, 2005; Volume 38, pp. 299–317. [Google Scholar]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Kaufmann, D.; Kaur Dogra, A.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules 2016, 21, 1161. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Zhang, M.-Q.; Wang, W.; Liu, L.-T.; Zhou, L.-M.; Miao, S.-K.; Wan, L.-H. Compound danshen tablet ameliorated aβ25-35-induced spatial memory impairment in mice via rescuing imbalance between cytokines and neurotrophins. BMC Complement. Altern. Med. 2014, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Song, H.; Chen, Y.; Cheng, S.; Fang, S.; Zhang, J.; Wang, Q. Effects of Bushen-Yizhi formula on age-related inflammation and oxidative stress in senescence-accelerated mice. Mol. Med. Rep. 2018, 17, 6947–6960. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. Corrigendum to “6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia” [BBRC 449 (2014) 8-13]. Biochem. Biophys. Res. Commun. 2019, 521, 545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Xu, P.; Song, S.; Liu, P.; Chi, T.; Ji, X.; Jin, G.; Qiu, S.; Hou, Y.; et al. Xanthoceras sorbifolia extracts ameliorate dendritic spine deficiency and cognitive decline via upregulation of BDNF expression in a rat model of Alzheimer’s disease. Neurosci. Lett. 2016, 629, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Kim, J.Y.; Lee, Y.; Chun, H.J.; Choi, Y.W.; Shin, H.K.; Choi, B.T.; Kim, C.M.; Lee, J. PMC-12, a traditional herbal medicine, enhances learning memory and hippocampal neurogenesis in mice. Neurosci. Lett. 2016, 617, 254–263. [Google Scholar] [CrossRef]
- Ohba, T.; Yoshino, Y.; Ishisaka, M.; Abe, N.; Tsuruma, K.; Shimazawa, M.; Oyama, M.; Tabira, T.; Hara, H. Japanese Huperzia serrata extract and the constituent, huperzine A, ameliorate the scopolamine-induced cognitive impairment in mice. Biosci. Biotechnol. Biochem. 2015, 79, 1838–1844. [Google Scholar] [CrossRef]
- Geromichalos, G.D.; Lamari, F.; Papandreou, M.A.; Trafalis, D.T.; Margarity, M.; Papageorgiou, A.; Sinakos, Z. Saffron as a Source of Novel Acetylcholinesterase Inhibitors: Molecular Docking and in Vitro Enzymatic Studies. J. Agric. Food Chem. 2012, 60, 6131–6138. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Salem, A.M.; Sabry, G.M.; Husein, A.A.; Kotob, S.E. Possible Therapeutic Uses of Salvia triloba and Piper nigrum in Alzheimer’s Disease–Induced Rats. J. Med. Food 2013, 16, 437–446. [Google Scholar] [CrossRef]
- Orhan, I.; Aslan, M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J. Ethnopharmacol. 2009, 122, 327–332. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Cholinergic Basis of Memory-Strengthening Effect of Foeniculum vulgare Linn. J. Med. Food 2006, 9, 413–417. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Mani, V.; Dundapa, T.A.; Watanabe, K.; Konishi, T. Ocimum sanctum Linn. Leaf Extracts Inhibit Acetylcholinesterase and Improve Cognition in Rats with Experimentally Induced Dementia. J. Med. Food 2011, 14, 912–919. [Google Scholar] [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Liu, X. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016, 193, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Choi, J.M.; Lee, J.; Lee, M.H.; Lee, S.; Cho, E.J. Effects of Vegetable Oils with Different Fatty Acid Compositions on Cognition and Memory Ability in Aβ25–35-Induced Alzheimer’s Disease Mouse Model. J. Med. Food 2016, 19, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Okello, E.J.; Savelev, S.U.; Perry, E.K. In vitro anti-β-secretase and dual anti-cholinesterase activities of Camellia sinensis L. (tea) relevant to treatment of dementia. Phytother. Res. 2004, 18, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G. Inhibition of Acetylcholinesterase Activity and Fe2+-Induced Lipid Peroxidation in Rat Brain In Vitro by Some Citrus Fruit Juices. J. Med. Food 2012, 15, 428–434. [Google Scholar] [CrossRef]
- Qosa, H.; Mohamed, L.A.; Batarseh, Y.S.; Alqahtani, S.; Ibrahim, B.; LeVine, H.; Keller, J.; Kaddoumi, A. Extra-virgin olive oil attenuates amyloid-β and tau pathologies in the brains of TgSwDI mice. J. Nutr. Biochem. 2015, 26, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- Okello, E.J.; Leylabi, R.; McDougall, G.J. Inhibition of acetylcholinesterase by green and white tea and their simulated intestinal metabolites. Food Funct. 2012, 3, 651–661. [Google Scholar] [CrossRef]
- Jelenković, A.; Jovanović, M.D.; Stevanović, I.; Petronijević, N.; Bokonjić, D.; Živković, J.; Igić, R. Influence of the Green Tea Leaf Extract on Neurotoxicity of Aluminium Chloride in Rats. Phytother. Res. 2013, 28, 82–87. [Google Scholar] [CrossRef]
- Mathiyazahan, D.B.; Thenmozhi, A.J.; Manivasagam, T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods 2015, 16, 423–435. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Albuquerque, H.M.; Cardoso, S.M.; Silva, A.M.; Silva, V.L. Dual-target compounds for Alzheimer’s disease: Natural and synthetic AChE and BACE-1 dual-inhibitors and their structure-activity relationship (SAR). Eur. J. Med. Chem. 2021, 221, 113492. [Google Scholar] [CrossRef]
- Bursavich, M.G.; Harrison, B.A.; Blain, J.-F. Gamma Secretase Modulators: New Alzheimer’s Drugs on the Horizon? J. Med. Chem. 2016, 59, 7389–7409. [Google Scholar] [CrossRef]
- Maia, M.A.; Sousa, M.E. BACE-1 and γ-Secretase as Therapeutic Targets for Alzheimer’s Disease. Pharmaceuticals 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pont, C.; Ginex, T.; Griñán-Ferré, C.; Scheiner, M.; Mattellone, A.; Martínez, N.; Arce, E.M.; Soriano-Fernández, Y.; Naldi, M.; De Simone, A.; et al. From virtual screening hits targeting a cryptic pocket in BACE-1 to a nontoxic brain permeable multitarget anti-Alzheimer lead with disease-modifying and cognition-enhancing effects. Eur. J. Med. Chem. 2021, 225, 113779. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, O.; Juárez-Jiménez, J.; Muñoz-Torrero, D.; Laughton, C.A.; Luque, F.J. Unveiling a novel transient druggable pocket in BACE-1 through molecular simulations: Conformational analysis and binding mode of multisite inhibitors. PLoS ONE 2017, 12, e0177683. [Google Scholar] [CrossRef]
- Mirsafian, H.; Ripen, A.M.; Merican, A.; Bin Mohamad, S. Amino Acid Sequence and Structural Comparison of BACE1 and BACE2 Using Evolutionary Trace Method. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Shimizu, H.; Tosaki, A.; Kaneko, K.; Hisano, T.; Sakurai, T.; Nukina, N. Crystal Structure of an Active Form of BACE1, an Enzyme Responsible for Amyloid β Protein Production. Mol. Cell. Biol. 2008, 28, 3663–3671. [Google Scholar] [CrossRef] [Green Version]
- McDade, E.; Voytyuk, I.; Aisen, P.; Bateman, R.J.; Carrillo, M.C.; De Strooper, B.; Haass, C.; Reiman, E.M.; Sperling, R.; Tariot, P.N.; et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat. Rev. Neurol. 2021, 17, 703–714. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Evans, C.D.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Cai, J.; Qi, X.; Kociok, N.; Skosyrski, S.; Emilio, A.; Ruan, Q.; Grant, M.B.; Saftig, P.; Serneels, L.; Golde, T.; et al. β-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol. Med. 2012, 4, 980–991. [Google Scholar] [CrossRef]
- Egan, M.F.; Mukai, Y.; Voss, T.; Kost, J.; Stone, J.; Furtek, C.; Mahoney, E.; Cummings, J.L.; Tariot, P.N.; Aisen, P.S.; et al. Further analyses of the safety of verubecestat in the phase 3 EPOCH trial of mild-to-moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 1–12. [Google Scholar] [CrossRef]
- Lopez, C.L.; Tariot, P.N.; Caputo, A.; Langbaum, J.B.; Liu, F.; Riviere, M.; Langlois, C.; Rouzade-Dominguez, M.; Zalesak, M.; Hendrix, S.; et al. The Alzheimer’s Prevention Initiative Generation Program: Study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2019, 5, 216–227. [Google Scholar] [CrossRef]
- Tüshaus, J.; Müller, A.S.; Kataka, E.S.; Zaucha, J.; Monasor, L.S.; Su, M.; Güner, G.; Jocher, G.; Tahirovic, S.; Frishman, D.; et al. An optimized quantitative proteomics method establishes the cell type-resolved mouse brain secretome. EMBO J. 2020, 39, e105693. [Google Scholar] [CrossRef] [PubMed]
- Barão, S.; Gärtner, A.; Leyva-Díaz, E.; Demyanenko, G.; Munck, S.; Vanhoutvin, T.; Zhou, L.; Schachner, M.; López-Bendito, G.; Maness, P.F.; et al. Antagonistic Effects of BACE1 and APH1B-γ-Secretase Control Axonal Guidance by Regulating Growth Cone Collapse. Cell Rep. 2015, 12, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.T.; To, D.C.; Tran, M.H.; Oh, S.H.; Kim, J.A.; Ali, Y.; Woo, M.-H.; Choi, J.S.; Min, B.S.; Nguyen, V.T.; et al. Isolation of cholinesterase and β-secretase 1 inhibiting compounds from Lycopodiella cernua. Bioorg. Med. Chem. 2015, 23, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Nuthakki, V.; Sharma, A.; Kumar, A.; Bharate, S.B. Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev. Res. 2019, 80, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Paudel, P.; Seong, S.H.; Zhou, Y.; Ha, M.T.; Min, B.S.; Jung, H.A.; Choi, J.S. Arylbenzofurans from the Root Bark of Morus alba as Triple Inhibitors of Cholinesterase, β-Site Amyloid Precursor Protein Cleaving Enzyme 1, and Glycogen Synthase Kinase-3β: Relevance to Alzheimer’s Disease. ACS Omega 2019, 4, 6283–6294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jannat, S.; Balupuri, A.; Ali, Y.; Hong, S.S.; Choi, C.W.; Choi, Y.-H.; Ku, J.-M.; Kim, W.J.; Leem, J.Y.; Kim, J.E.; et al. Inhibition of β-site amyloid precursor protein cleaving enzyme 1 and cholinesterases by pterosins via a specific structure−activity relationship with a strong BBB permeability. Exp. Mol. Med. 2019, 51, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Xu, Q.-X.; Hu, Y.; Li, G.-Y.; Xu, W.; Zhang, Y.-T.; Yang, X.-W. Multi-Target Anti-Alzheimer Activities of Four Prenylated Compounds from Psoralea fructus. Molecules 2018, 23, 614. [Google Scholar] [CrossRef] [Green Version]
- Videira, R.; Castanheira, P.; Grãos, M.; Salgueiro, L.; Faro, C.; Cavaleiro, C. A necrodane monoterpenoid fromLavandula luisieriessential oil as a cell-permeable inhibitor of BACE-1, theβ-secretase in Alzheimer’s disease. Flavour Fragr. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Kashyap, P.; Kalaiselvan, V.; Kumar, R.; Kumar, S. Ajmalicine and Reserpine: Indole Alkaloids as Multi-Target Directed Ligands Towards Factors Implicated in Alzheimer’s Disease. Molecules 2020, 25, 1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.; Zhao, J.; Zhao, Y.; Cui, Y.; Fang, W. BACE inhibitory flavanones from Balanophora involucrata Hook. Fitoterapia 2012, 83, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, R.; Nuthakki, V.K.; Singh, L.; Singh, B.; Bharate, S.S.; Bhatti, R.; Bharate, S.B. Identification of plant-based multitargeted leads for Alzheimer’s disease: In-vitro and in-vivo validation of Woodfordia fruticosa (L.) Kurz. Phytomedicine 2021, 91, 153659. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.; Chandra, S.; Diwan, A.D. Multi-Target β-Protease Inhibitors from Andrographis paniculata: In Silico and In Vitro Studies. Plants 2019, 8, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.A.; Min, B.-S.; Yokozawa, T.; Lee, J.-H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and Antioxidant Activities of Coptidis Rhizoma Alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, N.; Mahmoudian, M.; Mortazavi, P.; Hashjin, G.S. Experimental research Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Arch. Med. Sci. 2013, 1, 146–150. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, C.; Wang, X.; Yang, Z.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. 2,2′,4′-Trihydroxychalcone from Glycyrrhiza glabra as a new specific BACE1 inhibitor efficiently ameliorates memory impairment in mice. J. Neurochem. 2010, 114, 374–385. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Paris, D.; Beaulieu-Abdelahad, D.; Bachmeier, C.; Reed, J.; Ait-Ghezala, G.; Bishop, A.; Chao, J.; Mathura, V.; Crawford, F.; Mullan, M. Anatabine lowers Alzheimer’s Aβ production in vitro and in vivo. Eur. J. Pharmacol. 2011, 670, 384–391. [Google Scholar] [CrossRef]
- Rahman, H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Kim, C.-S.; Lee, K.-J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Wales, P.; Pinho, R.; Lázaro, D.F.; Outeiro, T.F. Limelight on Alpha-Synuclein: Pathological and Mechanistic Implications in Neurodegeneration. J. Parkinsons Dis. 2013, 3, 415–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brás, I.C.; Dominguez-Meijide, A.; Gerhardt, E.; Koss, D.; Lázaro, D.F.; Santos, P.I.; Vasili, E.; Xylaki, M.; Outeiro, T.F. Synucleinopathies: Where we are and where we need to go. J. Neurochem. 2020, 153, 433–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The Synucleinopathies: Twenty Years On. J. Parkinsons Dis. 2017, 7, S51–S69. [Google Scholar] [CrossRef] [Green Version]
- Outeiro, T.F. Emerging concepts in synucleinopathies. Acta Neuropathol. 2021, 141, 469–470. [Google Scholar] [CrossRef]
- Gonçalves, P.; Sodero, A.; Cordeiro, Y. Green Tea Epigallocatechin-3-gallate (EGCG) Targeting Protein Misfolding in Drug Discovery for Neurodegenerative Diseases. Biomolecules 2021, 11, 767. [Google Scholar] [CrossRef]
- Teil, M.; Arotcarena, M.-L.; Faggiani, E.; Laferriere, F.; Bezard, E.; Dehay, B. Targeting alpha-Synuclein for PD Therapeutics: A Pursuit on All Fronts. Biomolecules 2020, 10, 391. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef] [Green Version]
- Ehrnhoefer, E.D.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.; Garcia-Parrilla, M.C. Protocatechuic Acid: Inhibition of Fibril Formation, Destabilization of Preformed Fibrils of Amyloid-β and α-Synuclein, and Neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef]
- Manzoor, S.; Hoda, N. A comprehensive review of monoamine oxidase inhibitors as Anti-Alzheimer’s disease agents: A review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.; Toma, M.; Bungau, S.; Bumbu, A. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R. Monoamine Oxidases: The Biochemistry of the Proteins as Targets in Medicinal Chemistry and Drug Discovery. Curr. Top. Med. Chem. 2012, 12, 2189–2209. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Upadhyay, S.; Paliwal, S.; Saraf, S.K. Privileged scaffolds as MAO inhibitors: Retrospect and prospects. Eur. J. Med. Chem. 2018, 145, 445–497. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Hirata, M.; Kagawa, S.; Magata, Y.; Ohmomo, Y.; Temma, T. Synthesis and characterization of novel radiofluorinated probes for positron emission tomography imaging of monoamine oxidase B. J. Label. Compd. Radiopharm. 2019, 62, 580–587. [Google Scholar] [CrossRef]
- Kristal, B.S. Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic. Biol. Med. 2001, 30, 924–931. [Google Scholar] [CrossRef]
- Sparks, D.L.; Woeltz, V.M.; Markesbery, W.R. Alterations in Brain Monoamine Oxidase Activity in Aging, Alzheimer’s Disease, and Pick’s Disease. Arch. Neurol. 1991, 48, 718–721. [Google Scholar] [CrossRef]
- Barnett, J.H.; Xu, K.; Heron, J.; Goldman, D.; Jones, P. Cognitive effects of genetic variation in monoamine neurotransmitter systems: A population-based study of COMT, MAOA, and 5HTTLPR. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2010, 156, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Schneier, F.R. Pharmacotherapy of social anxiety disorder. Expert Opin. Pharmacother. 2011, 12, 615–625. [Google Scholar] [CrossRef]
- Dhull, D.K.; Jindal, A.; Dhull, R.K.; Aggarwal, S.; Bhateja, D.; Padi, S.S.V. Neuroprotective Effect of Cyclooxygenase Inhibitors in ICV-STZ Induced Sporadic Alzheimer’s Disease in Rats. J. Mol. Neurosci. 2011, 46, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, Y.; Van Dam, D.; Aerts, T.; Engelborghs, S.; Martin, J.-J.; De Deyn, P.P. The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, S.F.; Schöll, M.; Almkvist, O.; Wall, A.; Engler, H.; Långström, B.; Nordberg, A. Evidence for Astrocytosis in Prodromal Alzheimer Disease Provided by 11C-Deuterium-L-Deprenyl: A Multitracer PET Paradigm Combining 11C-Pittsburgh Compound B and 18F-FDG. J. Nucl. Med. 2012, 53, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larit, F.; Elokely, K.M.; Chaurasiya, N.D.; Benyahia, S.; Nael, M.A.; Leon, F.; Abu-Darwish, M.S.; Efferth, T.; Wang, Y.-H.; Belouahem-Abed, D.; et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine 2017, 40, 27–36. [Google Scholar] [CrossRef]
- Baek, S.C.; Choi, B.; Nam, S.-J.; Kim, H. Inhibition of monoamine oxidase A and B by demethoxycurcumin and bisdemethoxycurcumin. J. Appl. Biol. Chem. 2018, 61, 187–190. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.J.; Nam, S.-J.; Kim, H. Potent Selective Inhibition of Monoamine Oxidase A by Alternariol Monomethyl Ether Isolated from Alternaria brassicae. J. Microbiol. Biotechnol. 2017, 27, 316–320. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Zhao, J.; Pandey, P.; Doerksen, R.J.; Muhammad, I.; Tekwani, B.L. Selective Inhibition of Human Monoamine Oxidase B by Acacetin 7-Methyl Ether Isolated from Turnera diffusa (Damiana). Molecules 2019, 24, 810. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.M.; Chaurasiya, N.D.; Mohamed, N.M.; Bayoumi, S.A.L.; Tekwani, B.L.; Ross, S.A. Promising selective MAO-B inhibition by sesamin, a lignan from Zanthoxylum flavum stems. Saudi Pharm. J. 2020, 28, 409–413. [Google Scholar] [CrossRef]
- Rauhamäki, S.; Postila, P.A.; Niinivehmas, S.; Kortet, S.; Schildt, E.; Pasanen, M.; Manivannan, E.; Ahinko, M.; Koskimies, P.; Nyberg, N.; et al. Structure-Activity Relationship Analysis of 3-Phenylcoumarin-Based Monoamine Oxidase B Inhibitors. Front. Chem. 2018, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, P.; Hu, Y.; Liu, T.; Sun, J.; Wang, X. Synthesis and biological evaluation of 3-arylcoumarins as potential anti-Alzheimer’s disease agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 651–656. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yang, K.; Yu, S.; Su, J.; Yuan, S.; Han, J.; Chen, Y.; Gu, J.; Zhou, T.; Bai, R.; et al. Design, synthesis and biological evaluation of hydroxypyridinone-coumarin hybrids as multimodal monoamine oxidase B inhibitors and iron chelates against Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 367–382. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Lan, J.-S.; Ding, J.; Sun, Y.; Fang, Y.; Jiang, N.; Yang, Z.; Sun, L.; Jin, Y.; et al. Coumarin-dithiocarbamate hybrids as novel multitarget AChE and MAO-B inhibitors against Alzheimer’s disease: Design, synthesis and biological evaluation. Bioorg. Chem. 2018, 81, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Repsold, B.P.; Malan, S.F.; Joubert, J.; Oliver, D.W. Multi-targeted directed ligands for Alzheimer’s disease: Design of novel lead coumarin conjugates. SAR QSAR Environ. Res. 2018, 29, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Farber, N.B.; Rubin, E.H.; Newcomer, J.W.; Kinscherf, D.A.; Miller, J.P.; Morris, J.C.; Olney, J.W.; McKeel, D.W. Increased Neocortical Neurofibrillary Tangle Density in Subjects With Alzheimer Disease and Psychosis. Arch. Gen. Psychiatry 2000, 57, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojas, C.; Cabezas-Opazo, F.; Deaton, C.A.; Vergara, E.H.; Johnson, G.V.W.; Quintanilla, R.A. It’s all about tau. Prog. Neurobiol. 2019, 175, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Praticò, D. Alzheimer’s disease: Phenotypic approaches using disease models and the targeting of tau protein. Expert Opin. Ther. Targets 2020, 24, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef]
- Karakani, A.M.; Riazi, G.; Ghaffari, S.M.; Ahmadian, S.; Mokhtari, F.; Firuzi, M.J.; Bathaie, S.Z. Inhibitory effect of corcin on aggregation of 1N/4R human tau protein in vitro. Iran. J. Basic Med. Sci. 2015, 18, 485–492. [Google Scholar] [CrossRef]
- Feng, J.-H.; Cai, B.-C.; Guo, W.-F.; Wang, M.-Y.; Ma, Y.; Lu, Q.-X. Neuroprotective effects of Tongmai Yizhi Decoction (通脉益智汤) against Alzheimer’s disease through attenuating cyclin-dependent kinase-5 expression. Chin. J. Integr. Med. 2016, 23, 132–137. [Google Scholar] [CrossRef]
- Shi, H.; Dong, C.; Wang, M.; Liu, R.; Wang, Y.; Kan, Z.; Wang, L.; Si, G. Exploring the mechanism of Yizhi Tongmai decoction in the treatment of vascular dementia through network pharmacology and molecular docking. Ann. Transl. Med. 2021, 9, 164. [Google Scholar] [CrossRef]
- Ma, Q.; Ruan, Y.-Y.; Xu, H.; Shi, X.-M.; Wang, Z.-X.; Hu, Y.-L. Safflower yellow reduces lipid peroxidation, neuropathology, tau phosphorylation and ameliorates amyloid β-induced impairment of learning and memory in rats. Biomed. Pharmacother. 2015, 76, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, M.; Ye, R.; Wang, W.; Liu, X.; Zhang, G.; Han, J.; Zhang, Y.; Wang, B.; Zhao, J.; et al. Ginsenoside Rd Attenuates Tau Protein Phosphorylation Via the PI3K/AKT/GSK-3β Pathway After Transient Forebrain Ischemia. Neurochem. Res. 2014, 39, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Chidambaram, H.; Boral, D.; Gorantla, N.V.; Balmik, A.A.; Dangi, A.; Ramasamy, S.; Marelli, U.K.; Chinnathambi, S. EGCG impedes human Tau aggregation and interacts with Tau. Sci. Rep. 2020, 10, 12579. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Gao, W.; Wang, Q.; Zhang, L.; Li, Y.; Li, L.; Zhang, L. Cornel Iridoid Glycoside Inhibits Tau Hyperphosphorylation via Regulating Cross-Talk Between GSK-3β and PP2A Signaling. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Uversky, V.N.; Chinnathambi, S. Baicalein inhibits heparin-induced Tau aggregation by initializing non-toxic Tau oligomer formation. Cell Commun. Signal. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimer’s Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef]
- Yu, K.C.; Kwan, P.; Cheung, S.K.; Ho, A.; Baum, L. Effects of resveratrol and morin on insoluble tau in tau transgenic mice. Transl. Neurosci. 2018, 9, 54–60. [Google Scholar] [CrossRef]
- Ghasemzadeh, S.; Riazi, G.H. Inhibition of Tau amyloid fibril formation by folic acid: In-vitro and theoretical studies. Int. J. Biol. Macromol. 2019, 154, 1505–1516. [Google Scholar] [CrossRef]
- Viswanathan, G.K.; Shwartz, D.; Losev, Y.; Arad, E.; Shemesh, C.; Pichinuk, E.; Engel, H.; Raveh, A.; Jelinek, R.; Cooper, I.; et al. Purpurin modulates Tau-derived VQIVYK fibrillization and ameliorates Alzheimer’s disease-like symptoms in animal model. Cell. Mol. Life Sci. 2019, 77, 2795–2813. [Google Scholar] [CrossRef]
- Alberti, G.; Paladino, L.; Vitale, A.; Caruso Bavisotto, C.; Conway de Macario, E.; Campanella, C.; Macario, A.; Marino Gammazza, A. Functions and Therapeutic Potential of Extracellular Hsp60, Hsp70, and Hsp90 in Neuroinflammatory Disorders. Appl. Sci. 2021, 11, 736. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Kummer, M.P.; Heneka, M.T. Truncated and modified amyloid-beta species. Alzheimer’s Res. Ther. 2014, 6, 28–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2003, 101, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine. Netw. 2006, 17, 4–12. [Google Scholar]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef]
- Kalinin, S.; Gavrilyuk, V.; Polak, P.E.; Vasser, R.; Zhao, J.; Heneka, M.T.; Feinstein, D.L. Noradrenaline deficiency in brain increases β-amyloid plaque burden in an animal model of Alzheimer’s disease. Neurobiol. Aging 2007, 28, 1206–1214. [Google Scholar] [CrossRef]
- Siraj, A.; Shilpi, J.A.; Hossain, G.; Uddin, S.J.; Islam, K.; Jahan, I.A.; Hossain, H. Anti-Inflammatory and Antioxidant Activity of Acalypha hispida Leaf and Analysis of its Major Bioactive Polyphenols by HPLC. Adv. Pharm. Bull. 2016, 6, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Gainok, C.J.; Daniels, C.R.; Golembiowski, C.D.; Strickland, C.R.; Garrett, N. Investigation of the Anti-Inflammatory, Antinociceptive Effect of Ellagic Acid as Measured by Digital Paw Pressure via the Randall-Selitto Meter in Male Sprague- Dawley Rats. AANA J. 2011, 79, S28–S34. [Google Scholar]
- Xu, Z.; Liu, C.; Wang, R.; Gao, X.; Hao, C.; Liu, C. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J. Nutr. Biochem. 2020, 88, 108558. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, Z.; Wang, Y.; Xu, J.; He, X. Anti-neuroinflammatory triterpenoids from the seeds of Quercus serrata Thunb. Fitoterapia 2020, 142, 104523. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.Y.; Subedi, L.; Shin, D.; Kim, C.S.; Lee, K.R.; Kim, S.Y. A New Neolignan Derivative, Balanophonin Isolated from Firmiana simplex Delays the Progress of Neuronal Cell Death by Inhibiting Microglial Activation. Biomol. Ther. 2017, 25, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Lignan Glycosides from the Twigs of Chaenomeles sinensis and Their Biological Activities. J. Nat. Prod. 2015, 78, 1174–1178. [Google Scholar] [CrossRef]
- Suh, W.S.; Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Iridoid Glycosides from the Twigs of Sambucus williamsii var. coreana and Their Biological Activities. J. Nat. Prod. 2017, 80, 2502–2508. [Google Scholar] [CrossRef]
- Sun, J.; Gu, Y.-F.; Su, X.-Q.; Li, M.-M.; Huo, H.-X.; Zhang, J.; Zeng, K.-W.; Zhang, Q.; Zhao, Y.-F.; Li, J.; et al. Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 2014, 98, 110–116. [Google Scholar] [CrossRef]
- Zeng, Z.; Tian, R.; Feng, J.; Yang, N.-A.; Yuan, L. A systematic review on traditional medicine Toddalia asiatica (L.) Lam.: Chemistry and medicinal potential. Saudi Pharm. J. 2021, 29, 781–798. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef]
- Uranga, R.M.; Salvador, G.A. Unraveling the Burden of Iron in Neurodegeneration: Intersections with Amyloid Beta Peptide Pathology. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Ha, C.; Ryu, J.; Park, C.B. Metal Ions Differentially Influence the Aggregation and Deposition of Alzheimer’s β-Amyloid on a Solid Template. Biochemistry 2007, 46, 6118–6125. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.; Pagani, A.; Camaschella, C. Furin-mediated release of soluble hemojuvelin: A new link between hypoxia and iron homeostasis. Blood 2008, 111, 924–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, S.; Ebralidze, I.I.; She, Z.; Kraatz, H.-B. Electrochemical studies of tau protein-iron interactions—Potential implications for Alzheimer’s Disease. Electrochim. Acta 2017, 236, 384–393. [Google Scholar] [CrossRef]
- Nair, N.G.; Perry, G.; Smith, M.A.; Reddy, V.P. NMR Studies of Zinc, Copper, and Iron Binding to Histidine, the Principal Metal Ion Complexing Site of Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 20, 57–66. [Google Scholar] [CrossRef]
- Nkhili, E.; Loonis, M.; Mihai, S.; El Hajji, H.; Dangles, O. Reactivity of food phenols with iron and copper ions: Binding, dioxygen activation and oxidation mechanisms. Food Funct. 2014, 5, 1186–1202. [Google Scholar] [CrossRef]
- Engelmann, M.D.; Hutcheson, A.R.; Cheng, I.F. Stability of Ferric Complexes with 3-Hydroxyflavone (Flavonol), 5,7-Dihydroxyflavone (Chrysin), and 3‘,4‘-Dihydroxyflavone. J. Agric. Food Chem. 2005, 53, 2953–2960. [Google Scholar] [CrossRef]
- Guo, M.; Perez, C.; Wei, Y.; Rapoza, E.; Su, G.; Bou-Abdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects. Dalton Trans. 2007, 4951–4961. [Google Scholar] [CrossRef] [Green Version]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K. Polyphenols as Potential Metal Chelation Compounds Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Yang, R.; Tian, J.; Liu, Y.; Zhu, L.; Sun, J.; Meng, D.; Wang, Z.; Wang, C.; Zhou, Z.; Chen, L. Interaction mechanism of ferritin protein with chlorogenic acid and iron ion: The structure, iron redox, and polymerization evaluation. Food Chem. 2021, 349, 129144. [Google Scholar] [CrossRef]
- Chatoui, K.; Harhar, H.; El Kamli, T.; Tabyaoui, M. Chemical Composition and Antioxidant Capacity of Lepidium sativum Seeds from Four Regions of Morocco. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S.; Sharp, P.A. Quercetin Inhibits Intestinal Iron Absorption and Ferroportin Transporter Expression In Vivo and In Vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, T.; Panja, S.; Shendge, A.; Das, A.; Mandal, N. A natural antioxidant, tannic acid mitigates iron-overload induced hepatotoxicity in Swiss albino mice through ROS regulation. Environ. Toxicol. 2018, 33, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Kim, E.-Y.; Han, O. Bioactive Dietary Polyphenols Decrease Heme Iron Absorption by Decreasing Basolateral Iron Release in Human Intestinal Caco-2 Cells. J. Nutr. 2010, 140, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
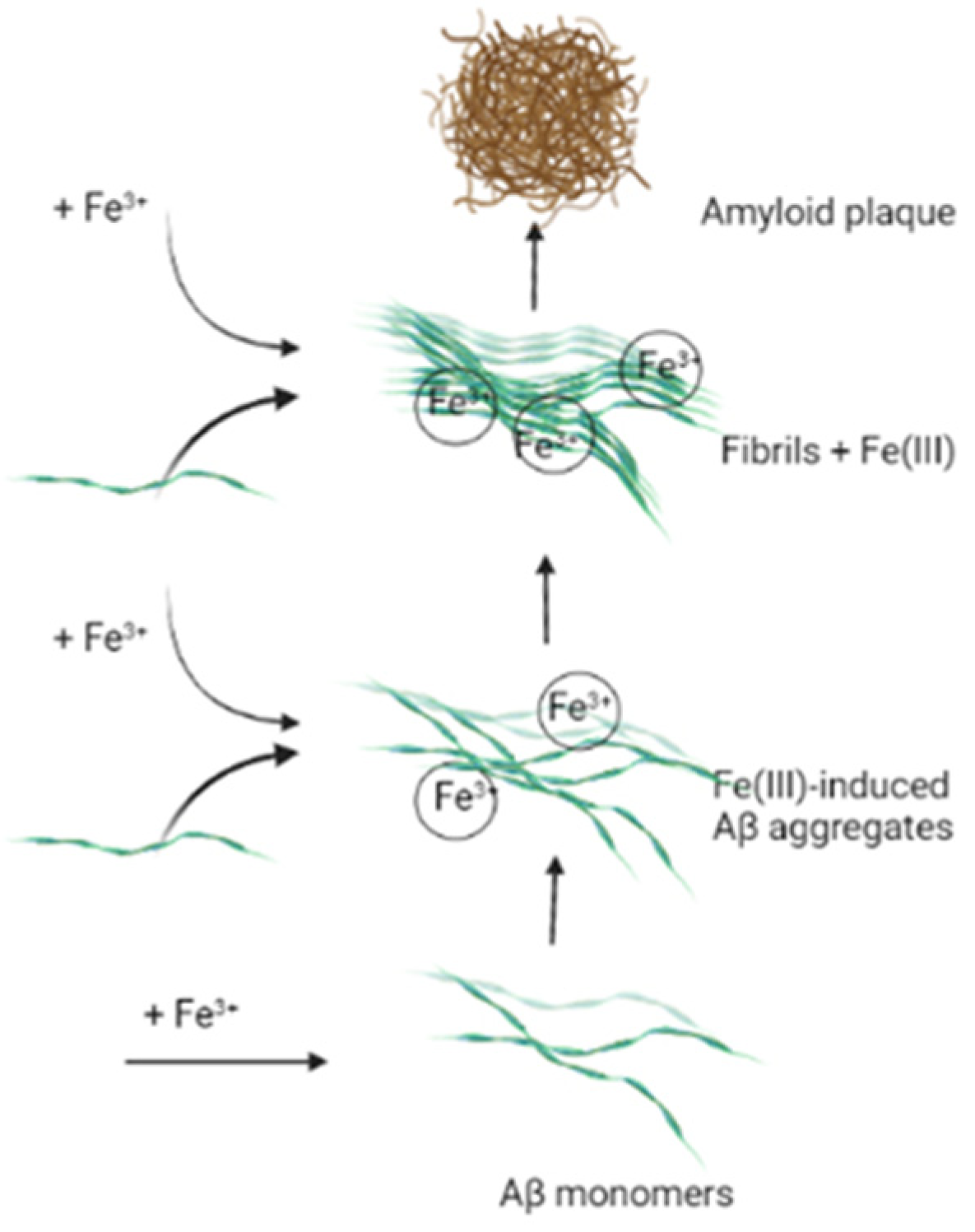
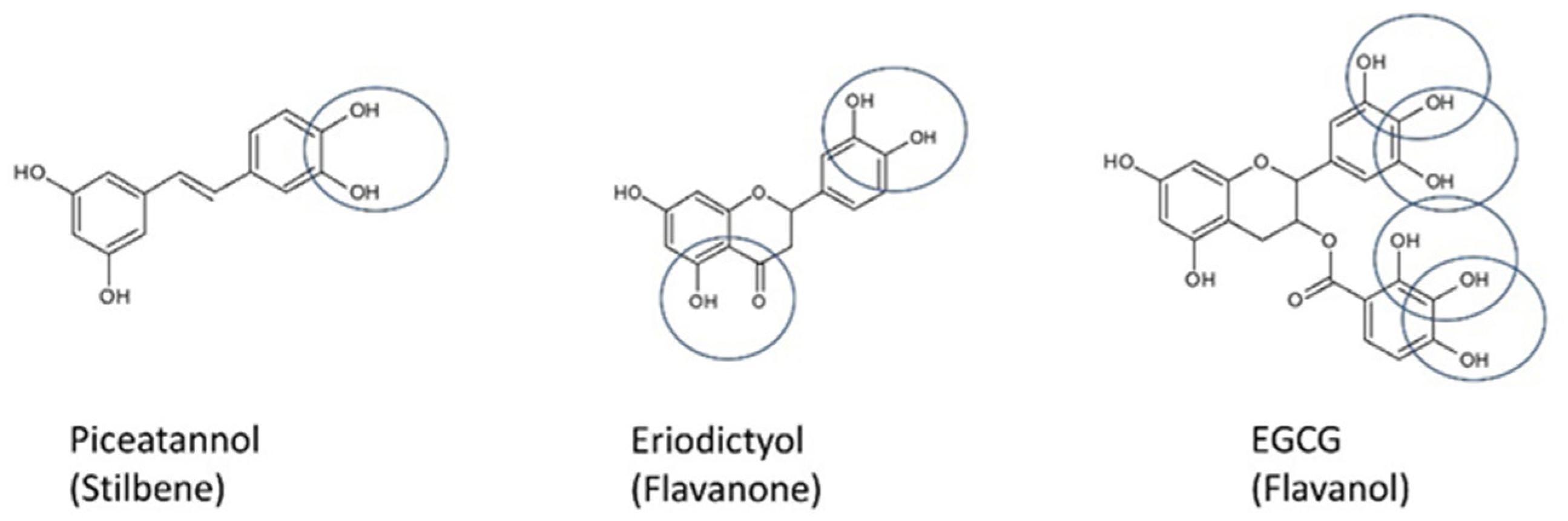
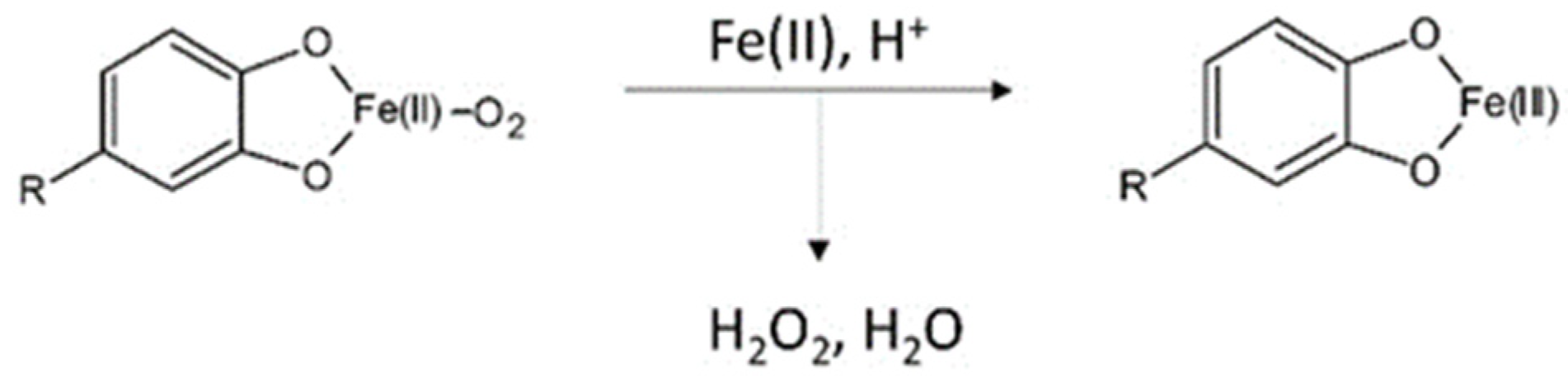
| Plant | Extract | Model and Assay | Target | Results | Ref. |
|---|---|---|---|---|---|
| Salvia triloba L. | aerial parts macerated in 70% methanol | AD rats, male (administration of AlCl3) | AChE, CRP, NF-κB, MCP-1 | ↓AChE activities in brain and serum, ↓CRP, ↓NF-κB, ↓MCP-1, ↑ACh | [19] |
| Salvia triloba | samples extracted with 75% ethanol at r.t. | in vitro enzymatic assay (AChE), Swiss albino mice, male scopolamine-induced amnesia | AChE | AChE inhibition, IC50: 0.71 mg/mL, memory-enhancing effect: 57.1 and 71.4% at 200 and 400 mg/kg, respectively | [20] |
| Melissa officinalis | samples extracted with 75% ethanol at r.t. | in vitro enzymatic assay (AChE), Swiss albino mice, male scopolamine-induced amnesia | AChE | ↓AChE activities in brain, memory-enhancing effect: no significance | [20] |
| Teucrium polium | samples extracted with 75% ethanol at r.t. | in vitro enzymatic assay (AChE), Swiss albino mice, male scopolamine-induced amnesia | AChE, | AChE inhibition, IC50: 0.55 mg/mL, memory-enhancing effect: 55.4 and 61.6% at 200 and 400 mg/kg, respectively | [20] |
| Piper nigrum | seeds extracted with 70% methanol at r.t. | SD rats, male (administration of AlCl3) | AChE, CRP, NF-κB, MCP-1 | ↓AChE activities in brain and serum, ↓CRP, ↓NF-κB, ↓MCP-1, ↑ACh | [19] |
| Foeniculum vulgare | fruit extracted with 90% methanol at r.t. | Swiss mice, scopolamine- and aging-induced amnesia | AChE | amnesia behavioral improvement, ↓AChE activities↓ in brain | [21] |
| Ocimum sanctum | water extract, refluxed at 75–80 °C | Wistar rats; male; maximal electroshock-, atropine-, and cyclosporine-induced dementia | AChE | cognitive behavioral performance improvement | [22] |
| Ocimum sanctum Linn | leaf extracted with 95% ethanol extract using Soxhlet | Wistar rats; male; maximal electroshock-, atropine-, and cyclosporine-induced dementia | AChE | cognitive behavioral performance improvement ↓AChE activities↓ in cortex, cerebellum, medulla oblongata, and midbrain region: 21%, 21%, 25%, and 30% at 500 mg/kg | [22] |
| Lavandula angustifolia Mill. | essential oils obtained from steam distillation | C57BL/6J mice, male, scopolamine-induced amnesia; H2O2-induced PC12 (1.5–50 μg/mL LO for 24 h) | AChE, ROS, MMP | cognitive behavioral performance improvement, ↓AChE activities, ↓MDA, ↑SOD activities↑, ↑GPX activities, PC12 cells model: ↓LDH, ↓NO, ↓ROS, ↑MMP | [23] |
| Olive oil (fruit oil of Olea europaea) | olive oil (rich in oleic acid) | ICR mice, male, intracerebroventricular injection of Aβ into the mice brain | MDA, NO, COX-2 | ↓MDA, ↓NO, ↓COX-2 | [24] |
| Corn oil (Zea mays) | corn oil (rich in linoleic acid) | ICR mice, male, intracerebroventricular injection of Aβ into the mouse brain | AChE, MDA, NO, iNOS, COX-2 | ↓AChE, ↓MDA, ↓NO, ↓COX-2, ↓iNOS | [24] |
| Perilla oil (Perilla frutescens) | perilla oil (rich in α-linolenic acid) | ICR mice; male, intracerebroventricular injection of Aβ into the mouse brain | AChE, MDA, NO, iNOS, COX-2, BDNF | ↓AChE, ↓MDA, ↓NO, ↓COX-2, ↓iNOS↓, ↑BDNF | [24] |
| Coffee | boiled water extraction | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 0.41 ± 0.004 mg/mL | [25] |
| Shaddock (Citrus maxima) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of 60.39% at 66.68 mL/L and of ≈ 28% at 16.67 mL/L | [26] |
| Grapefruit (Citrus paradisii) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 52% at 66.68 mL/L; AChE inhibitory rate of ≈ 29% at 16.67 mL/L | [26] |
| Lemon (Citrus limoni) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 48% at 66.68 mL/L and of ≈ 22% at 16.67 mL/L | [26] |
| Orange (Citrus sinensis) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 50% at 66.68 mL/L and of ≈ 30.89% at 16.67 mL/L | [26] |
| Tangerine (Citrus reticulata) | citrus fruit juices | Fe2+-induced malondialdehyde production in rat brain homogenate in vitro | AChE | AChE inhibitory rate of ≈ 57% at 66.68 mL/L and of ≈ 20% at 16.67 mL/L | [26] |
| Extra-virgin olive oil (Olea europaea) | extra-virgin olive oil | TgSwDI model | Aβ, tau, ApoE, PPARγ, and LXRs | cognitive behavioral performance improvement, ↓Aβ, ↓tau, ↓phosphorylation of tau, ↑ApoE, ↑PPARγ, ↑LXRs ↑Aβ clearance pathways | [27] |
| Green tea (Camellia sinensis) | water extract of green tea | in vitro enzymatic assay | AChE, BuChE, and BACE-1 | AChE inhibition, IC50: 7.2 μg/mL | [28] |
| White tea (Camellia sinensis, WTE) | water extract of white tea | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 8.06 μg/mL | [28] |
| Green tea (Camellia sinensis, GTE-PG) | water extract of green tea processed through simulated gastrointestinal digestion to obtain post-gastric digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 17.84 μg/mL | [28] |
| Green tea (Camellia sinensis, GTE-CA) | water extract of green tea processed through simulated gastrointestinal digestion to obtain colon-available digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 9.59 μg/mL | [28] |
| White tea (Camellia sinensis, WTE-PG) | water extract of white tea processed through simulated gastrointestinal digestion to obtain post-gastric digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 16.1 μg/mL | [28] |
| White tea (Camellia sinensis, WTE-CA) | water extract of white tea processed through simulated gastrointestinal digestion to obtain colon-available digested extract | in vitro enzymatic assay | AChE | AChE inhibition, IC50: 4.22 μg/mL | [28] |
| Black tea (Camellia sinensis) | water extract of black tea | in vitro enzymatic assay | AChE and BuChE | AChE inhibition, IC50: 0.06 ± 0.005 mg/mL; BuChE inhibition, IC50: 0.05 ± 0.007 mg/mL | [25] |
| Green tea (Camellia sinensis) | water extract of green tea | Wistar rats; male; injection with green tea extract, saline, or AlCl3 into the left-brain hemisphere cornu ammonis region 1 of the hippocampus | AChE | ↑COX and AChE activities with GTE injection, ↓AlCl3 neurotoxicity, 3-epigallocatechin gallate and epicatechin in extract improves cholinergic synaptic functions | [29] |
| Black tea (Camellia sinensis) | brewed at 85 °C | Wistar rats, male, AlCl3 (100 mg/kg, i.p. 60 days) induced AD | AChE, APP, β and γ secretases, Aβ | memory-enhancing effect ↓TBARS, ↑GSH, ↑SOD, ↑catalase, ↑GPx | [30] |
| In Vitro and In Silico Studies towards BACE-1 Inhibition | ||||
|---|---|---|---|---|
| Compound | Type of Study/Methodology | Mechanism of Action | Studies Results/Comment | References |
| Two serratene-type triterpenoids: lycernuic acid A with a ρ-hydroxycinnamate group as an ester substituent and 21β-hydroxyserrat-14-en-3,16-dione extracted from Lycopodiella cernua L. |
| Interactions with several pocket domains of the AChE, which were 5 Å from the inhibitors in the original complex. | IC50 = 0.23 μM and 0.98 μM, respectively. The compounds revealed higher inhibitory activity than quercetin, a positive control. | [31,45] |
| Embelin (3-undecyl-1,4-benzoquinone) from Embelia ribes |
| Molecular docking revealed entering of embelin into the active site gorge and interacting with Tyr71 (via hydrogen bonding). | IC50 = 2.11 μM. Lower activity than donepezil, a positive control. | [31,46] |
| Five arylbenzofurans: sanggenofuran A, mulberrofuran D, mulberrofuran H, morusalfuran B, and mulberrofuran D2 from the root bark of Morus alba |
| Molecular docking revealed the following interactions for mulberrofuran: D2 bound to the active allosteric site of BACE-1 through hydrogen bonds with Asn37, Ser36, and Tyr198, as well as hydrophobic interactions with Val69, Tyr71, Trp76, Phe108, Tyr198, and Ile126 | Sanggenofuran A revealed lower activity (IC50 = 5.64 μM) than mulberrofuran D (IC50 = 3.74 μM), and both compounds were less active than quercetin (IC50= 3.38 μM). The remaining compounds revealed higher activity in comparison to quercetin: mulberrofuran D2, mulberrofuran H, and morusalfuran B, for which IC50 was equal to: 0.73 μM, 1.04 μM, and 2.03 μM, respectively. | [31,47] |
| Fifteen ptesorin derivatives from Pteridium aquilinum |
| (2R)-Pteroside D was able to bind (hydrogen bonds) with Asn37, Trp76, and Ile126; (2R,3R)-pteroside C was able to create hydrogen bonds with Ser36, Asn37, Asp228, and Thr231, as well as hydrophobic interactions with Ala39, Trp76, Val69, Ile118, and Arg129; (3S)-pteroside D was able to create hydrogen bonds with Ser36, Asn37, Ile126, and Asp228, as well as hydrophobic interactions with Val69, Tyr71, Trp76, and Arg128. | The most active compounds were the following: (2R)-pteroside D, (2S,3R)-pteroside C, (2R,3R)-pteroside C, and (3S)-pteroside D (IC50 = 2.55, 9.17, 3.77, and 27.4 μM, respectively). (2R)-Pteroside D, (2R,3R)-pteroside C, and (3S)-pteroside D revealed higher inhibitory activity than quercetin. The compounds revealed the ability to bind with crucial amino acid residues, creating BACE-1 binding sites. | [48] |
| Three phlorotannins: eckol, dieckol, and 8,8′-bieckol isolated from Ecklonia cava |
| Dieckol revealed the ability to interact with Trp76, Thr232, and Lys321 through hydrogen bonds. 8,8′-Bieckol interacted with the BACE-1 active site by hydrogen bonding interactions with Lys107, Gly230, Thr231, and Ser325. | Dieckol and 8,8′-bieckol revealed higher inhibitory activity than reseveratrol (positive control) with IC50 = 2.34 and 1.62 μM, respectively. | [49] |
| Flavonoids and non-flavonoids: caffeic acid, hydroxytyrosol, oleuropein, verbascoside, quercetin, rutin, and luteloin isolated from Olea europaea L. |
| The compound structure analysis suggests that the 3,4-dihydroxy group and double bond in olive biophenols can interfere with hydrogen bonds of the NH2 group and NH hydrogens in the core structure of the BACE-1 enzyme. The higher activity of flavonoid olive biophenols in comparison to non-flavonoid olive biophenols results from their chemistry-a 15-carbon skeleton consisting of two benzene rings linked via the heterocyclic pyrene ring-C. | Caffeic acid, hydroxytyrosol, oleuropein, verbascoside, quercetin, rutin, and luteloin revealed higher inhibitory activity than positive control epigallocatechin gallate, with the following IC50 values: 16.67, 0.035, 2.76, 0.0063, 0.55, 0.0038, and 0.52 μM, respectively. | [50] |
| Flavonoids: bavachin, bavachinin, bavachalcone, and iso-bavalchacone isolated from Psoralea fructus |
| Structure analysis of studied compounds revealed that the chalcone backbone of bavachalcone and isobavachalcone was more flexible, which allowed them to fit more easily to the conformations of Aβ42 and enabled more hydrogen bonds than the flavanone of bavachin and bavachinin. Bavachalcone and isobavachalcone may stabilize Aβ42 monomers through their strong bindings, whereas bavachinin might induce intricate conformational changes of Aβ42 through binding, which leads to the off-pathway aggregation. | BACE-1 inhibition: 14% (bavachin at concentration 100 μM), 20% (bavachinin at concentration 100 μM), 68% (bavalchacone at concentration 100 μM), and 34% (iso-bavalchacone at concentration of 100 μM). | [51] |
| Linalool and 2,3,4,4-tetramethyl-5-methylene-cyclopent-2-enone isolated from Lavandula luisieri |
| Lack of mechanisms analysis. | Inhibitory activity for linalool was equal to 4.7, whereas 2,3,4,4-tetramethyl-5-methylene-cyclopent-2-enone was 31.8% at a concentration of 45 μg/mL. | [52] |
| Ajmalicine and reserpine |
| Strong binding of the compounds to the catalytic site of BACE-1. Reserpine interacted with Thr72, Asp32, and Asp217 by five hydrogen bonds, whereas ajmalicine was able to create hydrophobic interactions with Asp32 and Asp228. Thanks to the reserpine indole ring, the compound acted as a hydrogen bond donor capable of creating double hydrogen bonds with the catalytic site of the enzyme, whereas ajmalicine bound more strongly to the enzyme by hydrophobic interactions. | AJM showed the maximum inhibition of BACE-1 activity to be 69% at 50 μM concentration, whereas RES imparted 47% inhibition at the same concentration. | [53] |
| (S)-5,7,3′,5′-Tetrahydroxy-flavanone-7-O-(6″-galloyl)-β-D-glucopyranose (1); flavanone: (S)-5,7,3′,5′- tetrahydroxy-flavanone-7-O-β-D-glucopyranose (2), and dihydrochalcones: 4,2′,6′-trihydroxy-dihydrochalcone-4′-O-(6″-galloyl)-β-D-glucopyranose (3); 3,4,2′,6′-tetrahydroxydihydroflavone- 4′-O-β-D-glucopyranose (4); 3,4,2′,6′-tetrahydroxy- dihydrochalcone-4′-O-(6″-galloyl)-β-D-glucopyranose (5); and phloretin 4′-O-[4′, 6′-O-(S)-HHDP]-β-D-glucoside (6) isolated from Balanophora involucrata Hook. |
| Lack of mechanism analysis. | In vitro studies revealed the activity of the compounds to inhibit BACE-1; nevertheless, only compounds 1, 2, 4, and 5 turned out to be a little more active than the positive control. None of the substances achieved an inhibition capacity of 50% at 10 μM concentration. | [54] |
| The chemical components of W. fruticosa viz. botulin, betulinic acid, ursolic acid, ellagic acid, quercetin, kaempferol, oenothein C, and cyanidn-3,5-diglucoside. |
| The high activity of ellagic acid resulted from hydrogen bonding with Thr231, Asp228, Gly34, and Trp76 amino acid residues. Additionally, hydrophobic interactions were observed between aromatic rings of the acid and Trp115 and Tyr71 residues. | Among the compounds, ellagic acid and quercetin revealed the highest activity (70% BACE-1 inhibition at 100 μM). The most active was ellagic acid (IC50 = 16.2 μM). | [55] |
| 3,4-di-o-Caffeylquinic acid, apigenin, and 7-o-methylwoonin isolated from A. paniculata |
| The 3,4-di-o-caffeylquinic acid was able to bind with Trp71, Phe108, Gly34, Arg128 (first pose) and Ile126, Trp76, and Tyr198 (second pose) by hydrogen bonds. Hydrophobic interactions were also observed. | BACE-1 inhibition assay indicates that 3,4-di-o-caffeylquinic acid is the most promising inhibitor (activity slightly higher than quercetin), whereas the activity of 7-o-methylwogonin was similar to quercetin and the activity of apigenin was slightly weaker than quercetin. In accordance with molecular docking, 3,4-di-o-caffeylquinic acid showed the highest ability to bind with the BACE-1 active site. Hydrophobic interactions and hydrogen bonds allow achieving selective BACE-1 inhibition by the compound. | [56] |
| Proroberberine alkaloids: berberine, palmatine, jateorrhizine, epiberberine, coptisine, groenlandicine, and eporphine alkaloid-magnoflorine from Coptidis Rhizoma |
| The activity of epiberberine and groenlandicine is closely related with the presence of the methylenedioxy group in the D ring that is responsible for the BACE-1 inhibitory activity of protoberberine alkaloids. | Among the compounds, only epiberberine and groenlandicine revealed good, non-competitive BACE-1 inhibitory activities, with IC50 = 8.55 and 19.68 μM, respectively. | [57] |
| In Vivo and Ex Vivo Studies towards BACE-1 Inhibition | ||||
| Compound | Animal Models/Type of Study/Methodology | Mechanism of Action | Studies Results/Comment | References |
| Berberine (isoquinoline alkaloid) | New Zealand white rabbits. Lesion (pro-Alzheimer’s disease) was induced by aluminum-maltol injection into intraventricular fissure. Berberine chloride (50 mg/kg) was administered intragastrically once daily for 14 days. Histopatological examinations (brain tissue) were performed. BACE-1 activity was detectable by RP-HPLC. | The mechanism of CNS cell damage prevention by berberine was based on BACE-1 inhibition, as well as its antioxidant, anti-inflammatory, and AChE inhibitory activities. | Results indicated that berberine chloride has a preventative effect on the degeneration of the hippocampus, along with the ability to decrease the activity of BACE-1. Berberine prevented the increase in enzyme activity in 40% of all cases, as compared with the control group. | [58] |
| 2,2′,4′-Trihydroxychalcone (TDC) from Glycyrrhiza glabra | APP-PS1 double transgenic mice model (B6C3-Tg (APPswe, PS1dE9)). The studied substance was administered i.p. by 100 days to two groups with different doses (9 mg/kg/day and 3 mg/kg/day). The mice were applied to the MWM spatial memory test. Additionally, Western blot analysis for BACE-1 was conducted. | This is a specific non-competitive BACE-1 inhibitor. Taking into account the low molecular weight of TDC, it is highly probable that the compound is able to cross the blood–brain barrier in vivo. | Administration of TDC (9 mg/kg/day) caused significant decreasing of Aβ production and senile plaque formation. The activity resulted in memory improvement, as observed in the Morris water maze test. It was also determined that the level of BACE-1 in TDC-treated Tg mice was almost kept unchanged, as compared with those in the vehicle-treated Tg mice. | [59] |
| Gallic acid | Male B6.Cg-Tg(APPswe, PSEN1dE9) 85Dbo/Mmjax mice (bearing ‘Swedish’ APPK595N/M596L and PS1 exon 9-deleted mutant human transgenes) on a congenic C57BL/6J background (designated APP/PS1 mice). GA was administered with 20 mg/kg/day for 6 months. Two behavioral tests were conducted: Y-maze and RAWM. | The activity of gallic acid towards BACE-1 inhibition led to nonamyloidogenic APP metabolic effects. GA is able to inhibit the enzyme activity post-translationally. | Gallic acid demonstrated the ability to mitigate impaired learning and memory and reduce cerebral amyloidosis. A 6 month oral therapy based on GA completely remediated behavioral deficits, ameliorated cerebral amyloidosis, and reduced amyloid abundance. | [60] |
| Anatabine | Measurement of BACE-1 expression by RT-qPCR according to SHSY-5Y cells. Pharmacokinetic studies of anatabine were performed using 43-week-old B6/SJL F1 mice. The studied substance was administered i.p. at dosages of 0.5 and 2.0 mg/kg/day over 4 days. | Mechanism of Aβ reduction was based on the impact of anatabine on BACE-1 transcription. The compound was able to reduce BACE-1 protein levels in human neuronal-like SHSY-5Y cells. | Reduction was indicated of two forms of amyloid (soluble-40% reduction and insoluble-30% inhibition) after 4 days of drug administration at a dosage of 2 mg/kg. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtunik-Kulesza, K.; Oniszczuk, T.; Mołdoch, J.; Kowalska, I.; Szponar, J.; Oniszczuk, A. Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review. Int. J. Mol. Sci. 2022, 23, 1212. https://doi.org/10.3390/ijms23031212
Wojtunik-Kulesza K, Oniszczuk T, Mołdoch J, Kowalska I, Szponar J, Oniszczuk A. Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review. International Journal of Molecular Sciences. 2022; 23(3):1212. https://doi.org/10.3390/ijms23031212
Chicago/Turabian StyleWojtunik-Kulesza, Karolina, Tomasz Oniszczuk, Jarosław Mołdoch, Iwona Kowalska, Jarosław Szponar, and Anna Oniszczuk. 2022. "Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review" International Journal of Molecular Sciences 23, no. 3: 1212. https://doi.org/10.3390/ijms23031212
APA StyleWojtunik-Kulesza, K., Oniszczuk, T., Mołdoch, J., Kowalska, I., Szponar, J., & Oniszczuk, A. (2022). Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review. International Journal of Molecular Sciences, 23(3), 1212. https://doi.org/10.3390/ijms23031212








