Foliate-Targeting Quantum Dots-β-Cyclodextrin Nanocarrier for Efficient Delivery of Unsymmetrical Bisacridines to Lung and Prostate Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
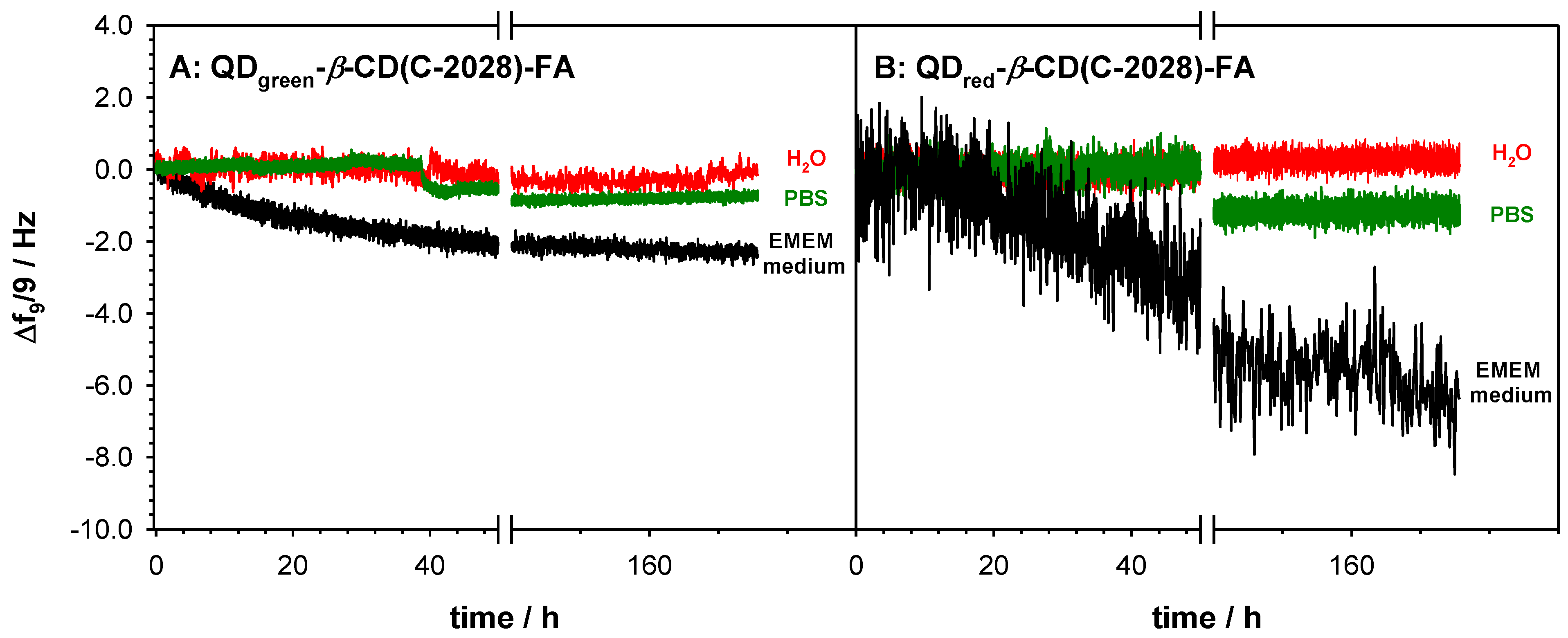
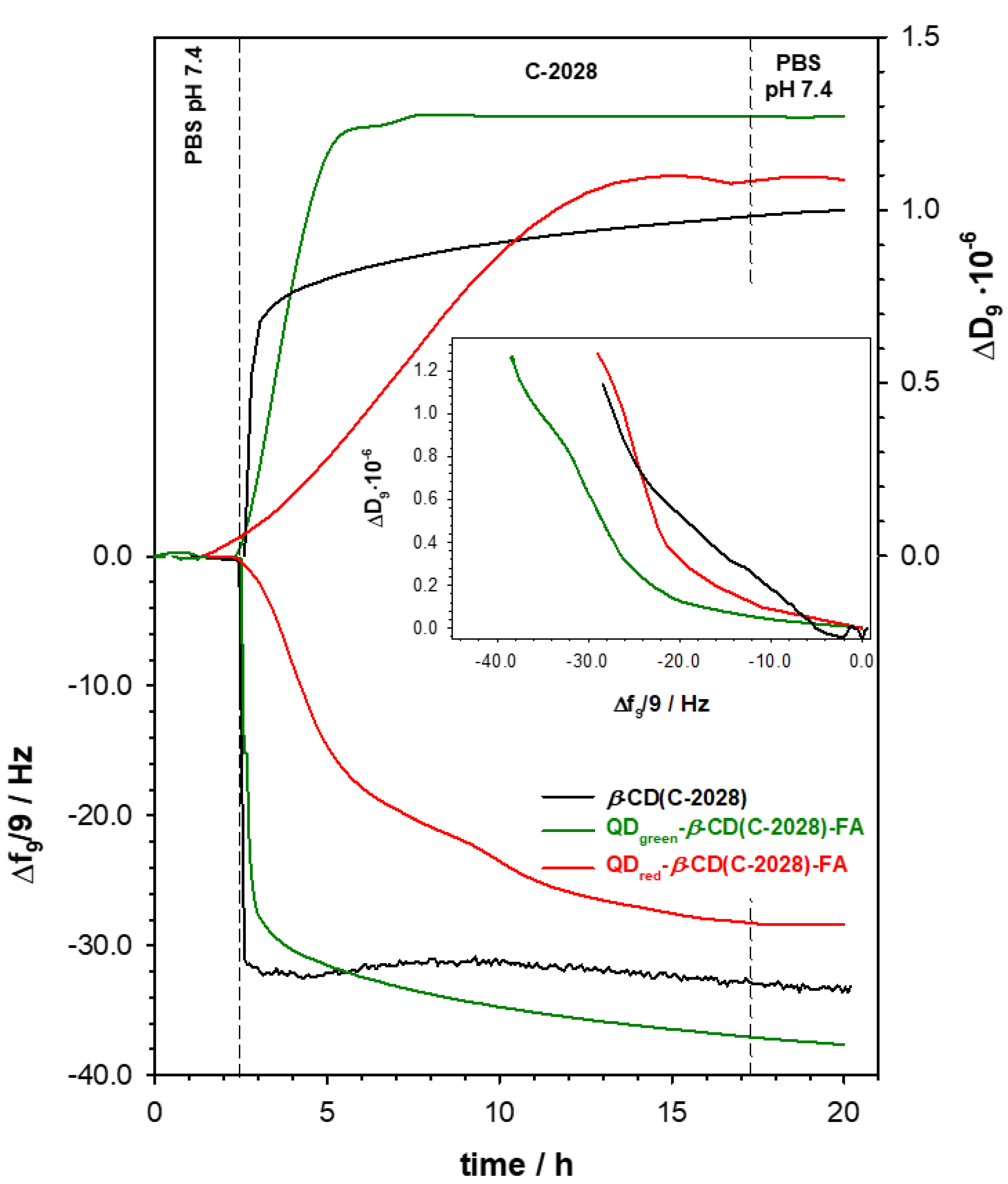
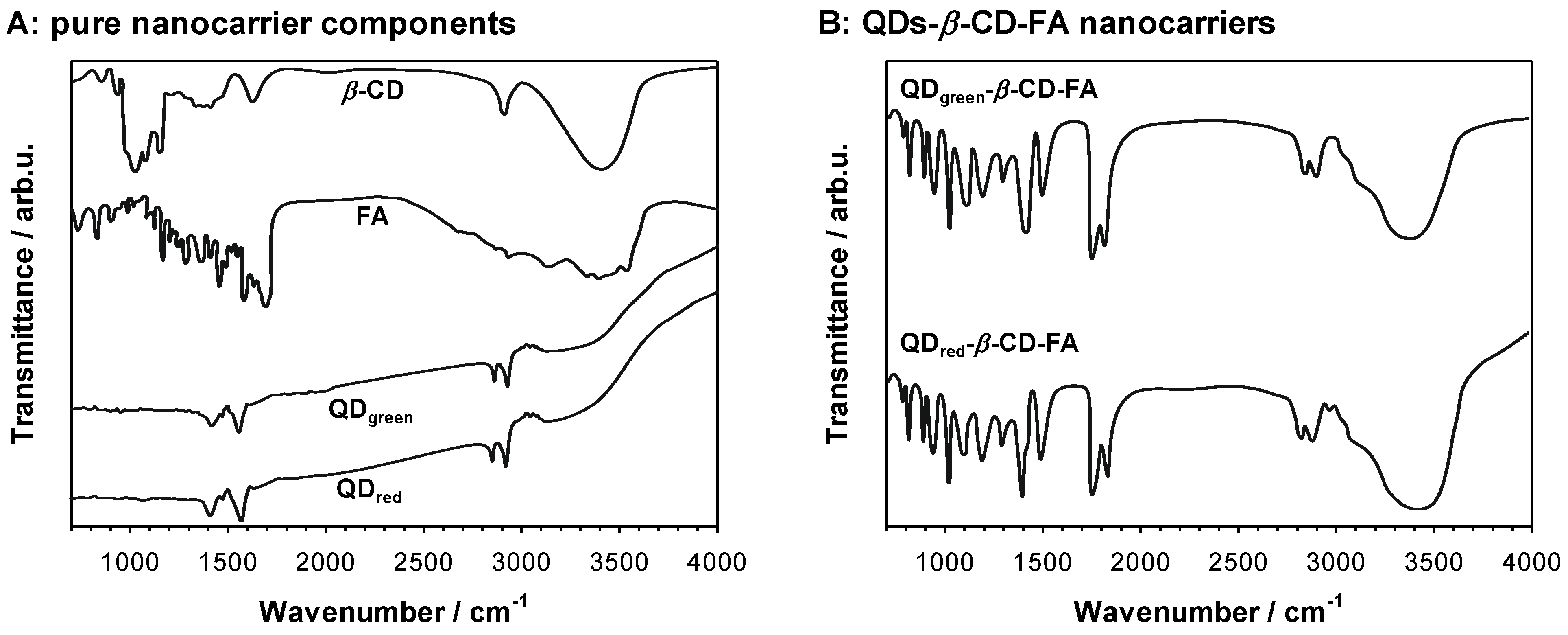
2.1. Size and Stability of QDs-β-CD-FA-C-2028 Nanoconjugates
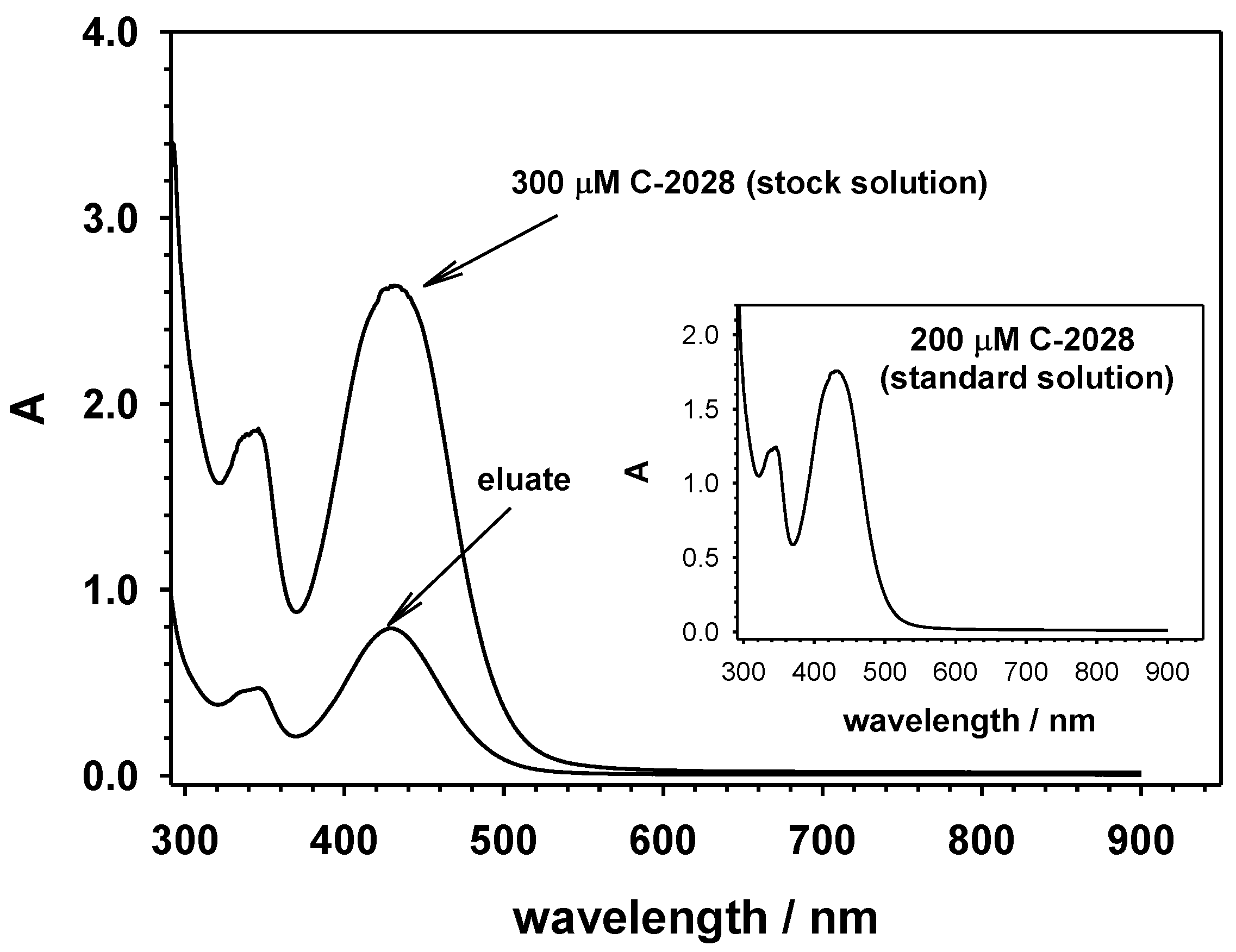
2.2. The Amount of C-2028 Accumulated in QDs-β-CD-FA Nanocarriers
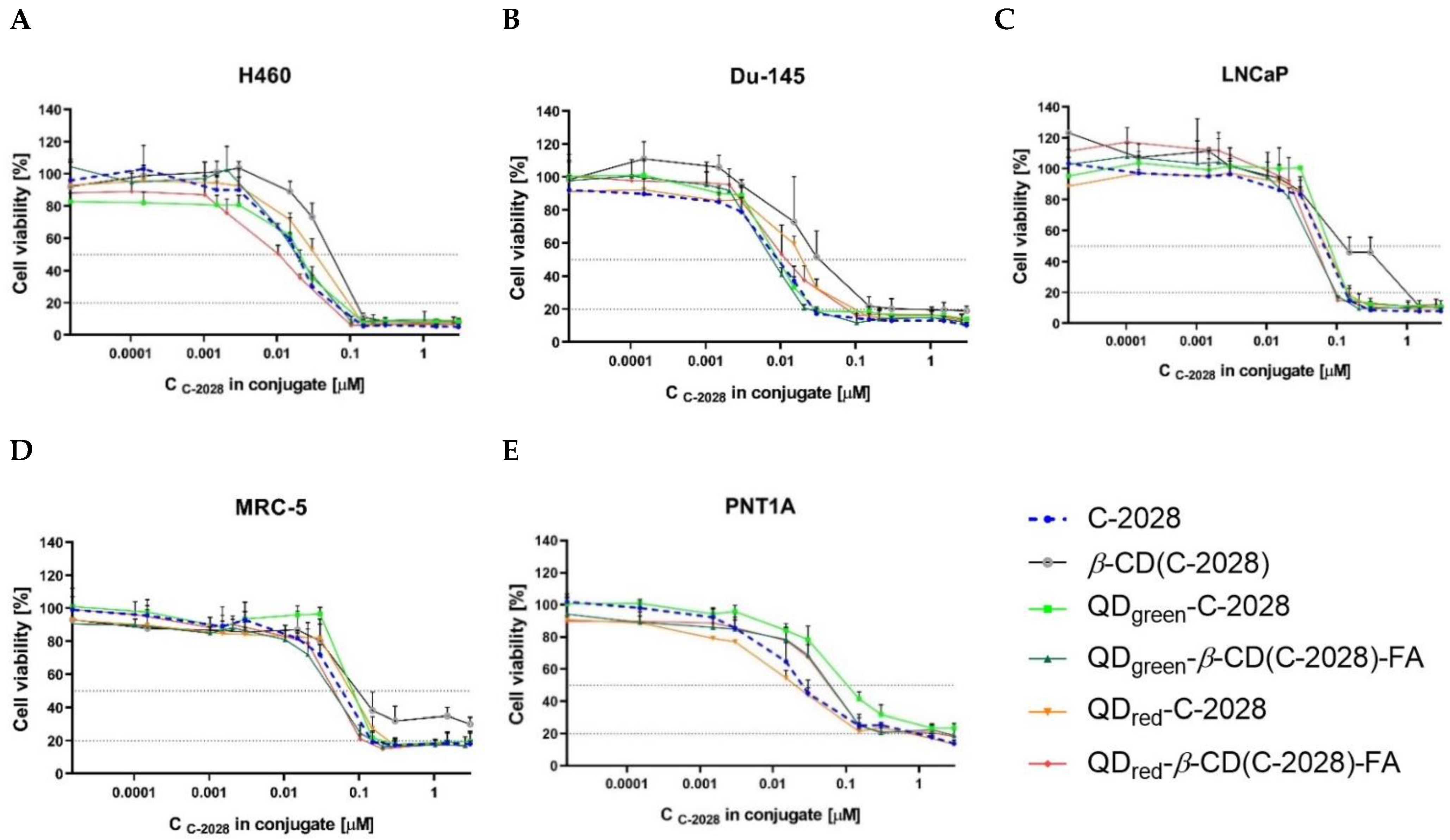
2.3. Cytotoxicity
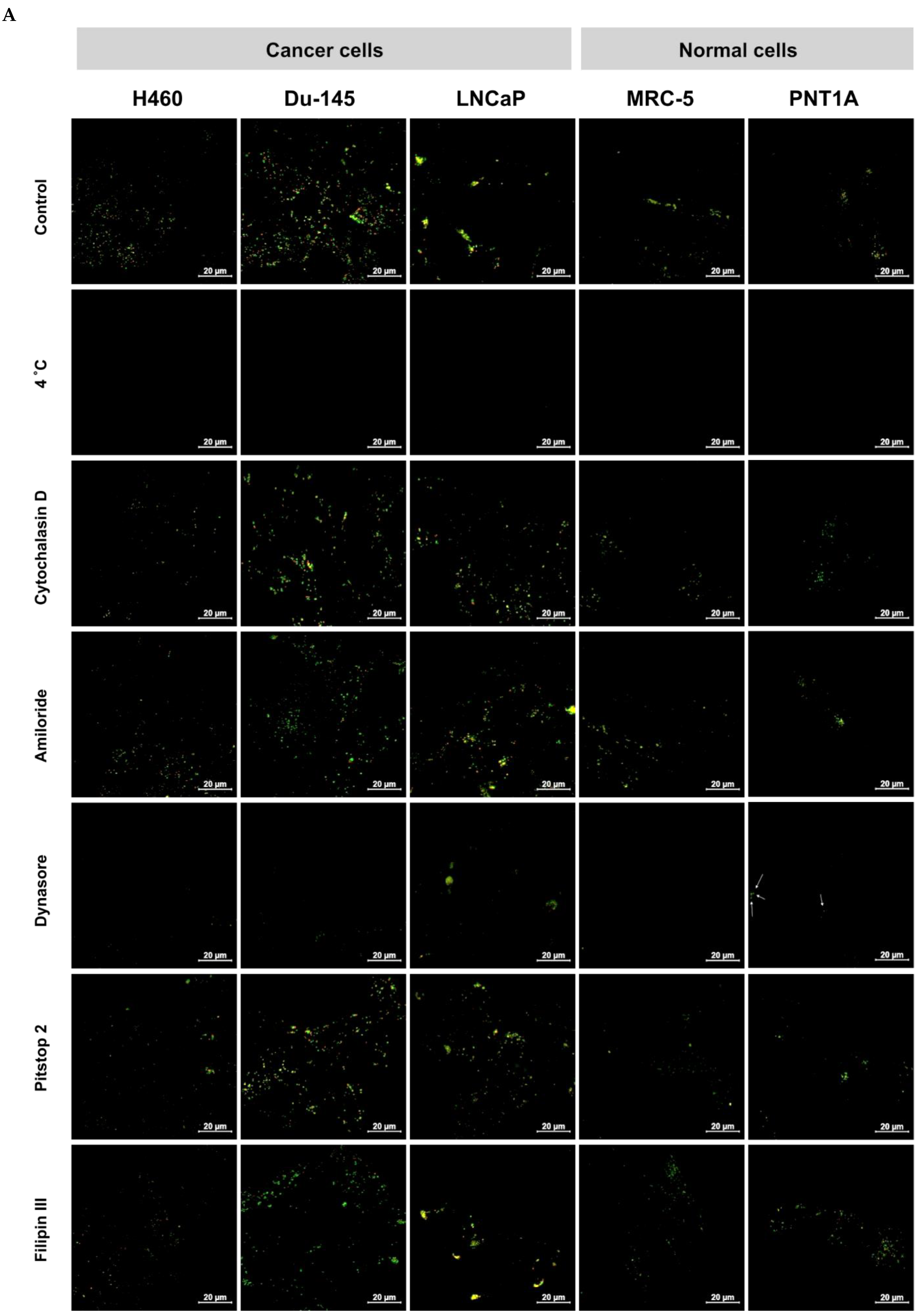
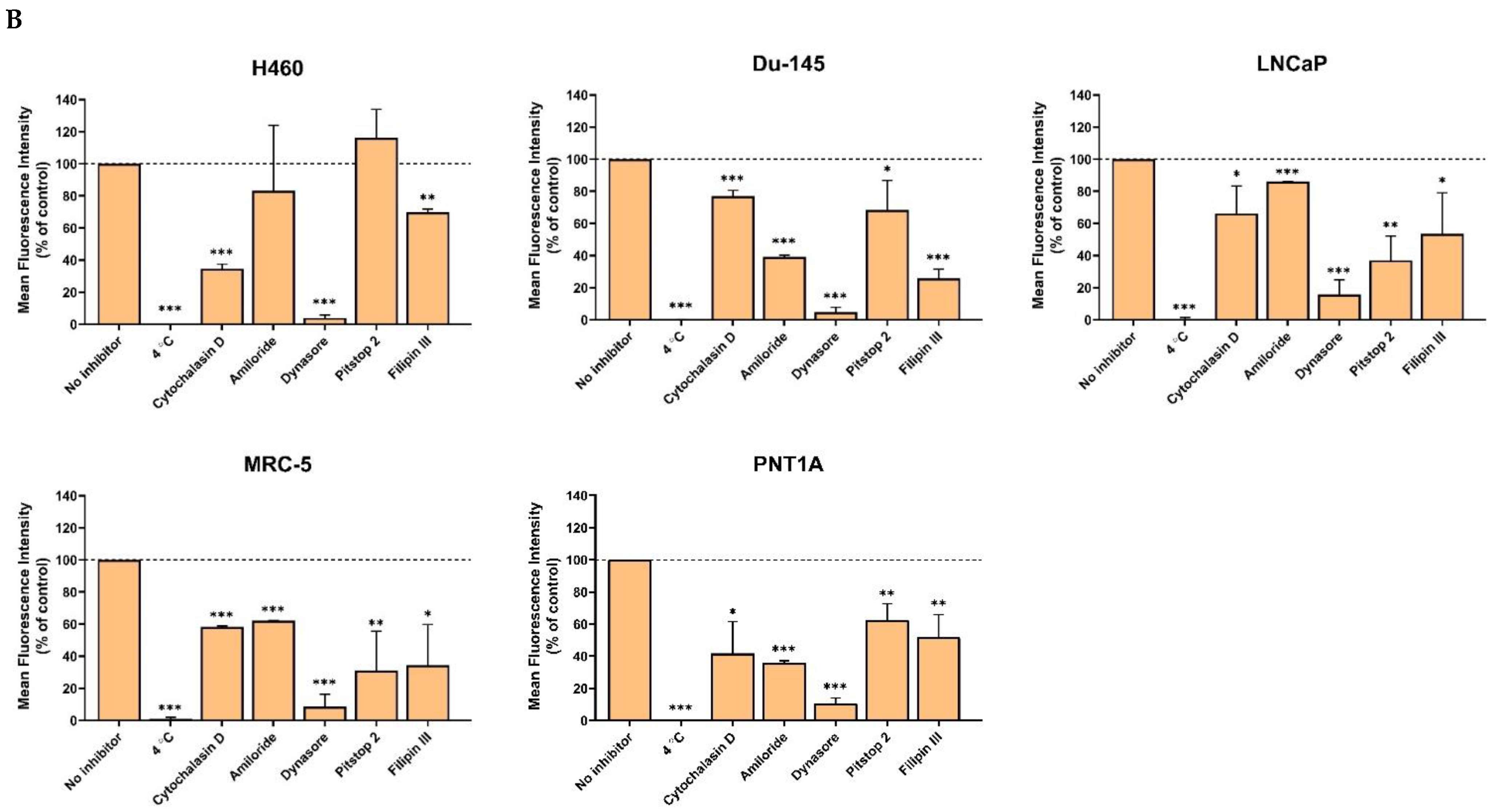
2.4. Cellular Uptake
2.5. Internalization Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Applied Techniques
3.2.2. Synthesis of β-Cyclodextrin Functionalized Quantum Dots
3.2.3. Synthesis of Folic Acid Functionalized β-Cyclodextrin Containing Quantum Dots
3.2.4. Selective Loading of C-2028 into QD-β-CD-FA Nanoconjugates
3.2.5. Cell Culture
3.2.6. MTT Assay
3.2.7. Confocal Microscopy Imaging
3.2.8. Stability Analysis
3.2.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Huang, F.; Ren, C.; Yang, L.; Liu, J.; Cheng, Z.; Chu, L.; Liu, J. Targeted Chemo-Photodynamic Combination Platform Based on the DOX Prodrug Nanoparticles for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 13016–13028. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Karim, K.; Chowdhury, E.H. Nanoparticles Targeting Receptors on Breast Cancer for Efficient Delivery of Chemotherapeutics. Biomedicine 2021, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Pashayan, N.; Antoniou, A.C.; Ivanus, U.; Esserman, L.J.; Easton, D.F.; French, D.; Sroczynsk, G.; Hall, P.; Cuzick, J.; Evans, D.G.; et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 2020, 17, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Howard, C.M.; Tilley, A.M.; Subramaniyan, B.; Tiwari, A.K.; Ruch, R.J.; Raman, D. Novel and Alternative Targets Against Breast Cancer Stemness to Combat Chemoresistance. Front. Oncol. 2019, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Babu, R.J. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Shim, M.K.; Um, W.; Yoon, H.Y.; Ryu, J.H.; Lim, D.-K.; Kim, K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics 2019, 9, 7906–7923. [Google Scholar] [CrossRef]
- Jia, Y.; Omri, A.; Krishnan, L.; McCluskie, M.J. Potential applications of nanoparticles in cancer immunotherapy. Hum. Vaccines Immunother. 2017, 13, 63–74. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.G. Nanoparticles: Synthesis and applications. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Kaul, S.C.; Wadhwa, R.; Miyako, E. Folic Acid Receptor-Mediated Targeting Enhances the Cytotoxicity, Efficacy, and Selectivity of Withania somnifera Leaf Extract: In vitro and in vivo Evidence. Front. Oncol. 2019, 9, 602. [Google Scholar] [CrossRef]
- Liao, Y.; Guo, Z.; Xia, X.; Liu, Y.; Huang, C.; Jiang, L.; Wang, X.; Liu, L.; Huang, H. Inhibition of EGFR signaling with Spautin-1 represents a novel therapeutics for prostate cancer. J. Exp. Clin. Cancer Res. 2019, 38, 157. [Google Scholar] [CrossRef]
- Jeong, S.M.; Hwang, S.; Seong, R.H. Transferrin receptor regulates pancreatic cancer growth by modulating mitochondrial respiration and ROS generation. Biochem. Biophys 2016, 471, 373–379. [Google Scholar] [CrossRef]
- Kübler, E.; Albrecht, H. Large set data mining reveals overexpressed GPCRs in prostate and breast cancer: Potential for active targeting with engineered anti-cancer nanomedicines. Oncotarget 2018, 9, 24882–24897. [Google Scholar] [CrossRef]
- Kampen, K.R. Membrane proteins: The key players of a cancer cell. J. Membr. Biol. 2011, 242, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar] [PubMed]
- Song, E.-Q.; Zhang, Z.-L.; Luo, Q.-Y.; Lu, W.; Shi, Y.-B.; Pang, D.-W. Tumor Cell Targeting Using Folate-Conjugated Fluorescent Quantum Dots and Receptor-Mediated Endocytosis. Clin. Chem. 2009, 55, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, H.; Lee, R.J. Targeted drug delivery via folate receptors. Expert Opin. Drug Deliv. 2008, 5, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Wibowoa, A.S.; Singha, M.; Reedera, K.M.; Cartera, J.J.; Kovacha, A.R.; Menga, W.; Ratnamb, M.; Zhanga, M.; Dann III, C.M. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. Proc. Natl. Acad. Sci. USA 2013, 110, 15180–15188. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updates 2014, 17, 89–95. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Maity, A.R.; Nandi, S.; Stepensky, D.; Jelinek, R. Imaging Cancer Cells Expressing the Folate Receptor with Carbon Dots Produced from Folic Acid. ChemBioChem 2016, 17, 614–619. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, T.; Wang, C.; Li, Y.; Lin, Z.; Zhao, M.; Zhu, B. Novel functionalized nanoparticles for tumor-targeting co-delivery of doxorubicin and siRNA to enhance cancer therapy. Int. J. Nanomed. 2018, 13, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J. Advances of cyclodextrin polymers for the delivery of biotech drugs. JB&B 2016, 1, 7–17. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Pilch, J.; Matysiak-Brynda, E.; Kowalczyk, A.; Bujak, P.; Mazerska, Z.; Nowicka, A.M.; Augustin, E. New Unsymmetrical Bisacridine Derivatives Noncovalently Attached to Quaternary Quantum Dots Improve Cancer Therapy by Enhancing Cytotoxicity toward Cancer Cells and Protecting Normal Cells. ACS Appl. Mater. Interfaces 2020, 12, 1727–17289. [Google Scholar] [CrossRef]
- Paluszkiewicz, E.; Horowska, B.; Borowa-Mazgaj, B.; Peszyńska-Sularz, G.; Paradziej-Łukowicz, J.; Augustin, E.; Konopa, J.; Mazerska, Z. Design, synthesis and high antitumor potential of new unsymmetrical bisacridine derivatives towards human solid tumors, specifically pancreatic cancers and their unique ability to stabilize DNA G quadruplexes. Eur. J. Med. Chem. 2020, 204, 112599. [Google Scholar] [CrossRef]
- Pilch, J.; Kowalik, P.; Bujak, P.; Nowicka, A.M.; Augustin, E. Quantum Dots as a Good Carriers of Unsymmetrical Bisacridines for Modulating Cellular Uptake and the Biological Response in Lung and Colon Cancer Cells. Nanomaterials 2021, 11, 462. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Coleman, A.W.; Nicolis, I.; Keller, N.; Dalbiez, J.P. Aggregation of cyclodextrins: An explanation of the abnormal solubility of β-cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 13, 139–143. [Google Scholar] [CrossRef]
- Bonini, M.; Rossi, S.; Karlsson, G.; Almgren, M.; Lo Nostro, P.; Baglioni, P. Self-assembly of ‚I-cyclodextrin in water. part 1: Cryo-TEM and dynamic and static light scattering. Langmuir 2006, 22, 1478–1484. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Mishra, D.K.; Deshpande, A.; Pethe, A.M. Levels of drug targeting. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: London, UK, 2019; pp. 269–305. [Google Scholar] [CrossRef]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.; Swiderska-Środa, A.; Farghali, A.A.; Wojnarowicz, J.; Stefanek, A.; Gierlotka, S.; Opalinska, A.; Allayeh, A.K.; Ciach, T.; Lojkowski, W. Folic acid–conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget 2018, 9, 26466–26490. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Shmeeda, H.; Amitay, Y.; Ohana, P.; Kumar, S.; Gabizon, A. Targeting of folate-conjugated liposomes with co-entrapped drugs to prostate cancer cells via prostate-specific membrane antigen (PSMA). Nanomedicine 2018, 14, 1407–1416. [Google Scholar] [CrossRef]
- Rudzinski, J.K.; Govindasamy, N.P.; Lewis, J.D.; Jurasz, P. The role of the androgen receptor in prostate cancer-induced platelet aggregation and platelet-induced invasion. J. Thromb. Haemost. 2020, 18, 2976–2986. [Google Scholar] [CrossRef]
- Coll-Bastus, N.; Mao, X.; Young, B.D.; Sheer, D.; Lu, Y.-J. DNA replication-dependent induction of gene proximity by androgen. Hum. Mol. Genet. 2015, 24, 963–971. [Google Scholar] [CrossRef][Green Version]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Johannes, L.; Parton, R.G.; Bassereau, P.; Mayor, S. Building endocytic pits without clathrin. Nat. Rev. Mol. Cell Biol. 2015, 16, 311–321. [Google Scholar] [CrossRef]
- Dalal, C.; Saha, A.; Jana, N.R. Nanoparticle Multivalency Directed Shifting of Cellular Uptake Mechanism. J. Phys. Chem. C 2016, 120, 6778–6786. [Google Scholar] [CrossRef]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of Clathrin- and Caveolae-Mediated Endocytosis in Gene Transfer Mediated by Lipo- and Polyplexes. Mol. Ther. 2005, 12, 468–474. [Google Scholar] [CrossRef]
- Bittleman, K.R.; Dong, S.; Roman, M.; Lee, Y.W. Folic Acid-Conjugated Cellulose Nanocrystals Show High Folate-Receptor Binding Affinity and Uptake by KB and Breast Cancer Cells. ACS Omega 2018, 3, 13952–13959. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, S.; Modrejewski, J.; Walter, J.G.; Livney, Y.D.; Assaraf, Y.G. Cancer cell-selective, clathrin-mediated endocytosis of aptamer decorated nanoparticles. Oncotarget 2018, 9, 20993–21006. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Huang, J.; Feng, Q.; Chen, X.; Liu, X.; Li, X.; Zhang, T.; Xiao, S.; Li, H.; Zhong, Z.; et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int. J. Nanomed. 2019, 14, 6957–6970. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed. Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]




| Type of Measurement | Water | PBS | EMEM Medium | |||
|---|---|---|---|---|---|---|
| QDgreen-β-CD (C-2028)-FA | QDred-β-CD (C-2028)-FA | QDgreen-β-CD (C-2028)-FA | QDred-β-CD (C-2028)-FA | QDgreen-β-CD (C-2028)-FA | QDred-β-CD (C-2028)-FA | |
| size | 1st: 145 ± 19 | 1st: 149 ± 10 | 1st: 156 ± 23 | 1st: 167 ± 27 | 1st: 189 ± 31 (96%) 856 ± 45 (4%) | 1st: 191 ± 36 (95%) 925 ± 41 (5%) |
| 2nd: 148 ± 15 | 2nd: 147 ± 12 | 2nd: 160 ± 25 | 2nd: 171 ± 30 | 2nd: 195 ± 29 (97%) 840 ± 52 (3%) | 2nd: 199 ± 38 (92%) 964 ± 33 (8%) | |
| 3rd: 151 ± 23 | 3rd: 149 ± 11 | 3rd: 162 ± 27 | 3rd: 175 ± 29 | 3rd: 197 ± 36 (92%) 920 ± 24 (8%) | 3rd: 205 ± 33 (89%) 1003 ± 45 (11%) | |
| 4th: 146 ± 21 | 4th: 153 ± 14 | 4th: 161 ± 24 | 4th: 180 ± 33 | 4th: 192 ± 24 (93%) 950 ± 65 (7%) | 4th: 204 ± 41 (87%) 1019 ± 67 (13%) | |
| 5th: 154 ± 18 | 5th: 152 ± 16 | 5th: 164 ± 25 | 5th: 186 ± 27 | 5th: 199 ± 28 (89%) 894 ± 71 (11%) | 5th: 210 ± 37 (81%) 1035 ± 73 (19%) | |
| 6th: 156 ± 17 | 6th: 154 ± 14 | 6th: 167 ± 29 | 6th: 192 ± 31 | 6th: 200 ± 32 (85%) 980 ± 29 (15%) | 6th: 212 ± 44 (79%) 1106 ± 62 (21%) | |
| 7th: 159 ± 26 | 7th: 155 ± 15 | 7th: 170 ± 31 | 7th: 195 ± 35 | 7th: 206 ± 33 (83%) 905 ± 47 (17%) | 7th: 215 ± 42 (90%) 1134 ± 58 (20%) | |
| PDI | 1st: 0.105 | 1st: 0.118 | 1st: 0.120 | 1st: 0.125 | 1st: 0.180 | 1st: 0.201 |
| 2nd: 0.108 | 2nd: 0.121 | 2nd: 0.119 | 2nd: 0.129 | 2nd: 0.192 | 2nd: 0.250 | |
| 3rd: 0.111 | 3rd: 0.120 | 3rd: 0.123 | 3rd: 0.135 | 3rd: 0.205 | 3rd: 0.307 | |
| 4th: 0.104 | 4th: 0.119 | 4th: 0.127 | 4th: 0.133 | 4th: 0.262 | 4th: 0.355 | |
| 5th: 0.106 | 5th: 0.123 | 5th: 0.124 | 5th: 0.135 | 5th: 0.297 | 5th: 0.390 | |
| 6th: 0.109 | 6th: 0.125 | 6th: 0.129 | 6th: 0.139 | 6th: 0.315 | 6th: 0.431 | |
| 7th: 0.112 | 7th: 0.127 | 7th: 0.131 | 7th: 0.138 | 7th: 0.352 | 7th: 0.468 | |
| Compound | Cell Line | |||||
|---|---|---|---|---|---|---|
| H460 | Du-145 | LNCaP | MRC-5 | PNT1A | ||
| C-2028 | IC50 | 0.016 ± 0.001 | 0.009 ± 0.001 | 0.066 ± 0.004 | 0.018 ± 0.002 | 0.028 ± 0.012 |
| IC80 | 0.035 ± 0.003 | 0.024 ± 0.002 | 0.133 ± 0.004 | 0.138 ± 0.006 | 1.14 ± 0.32 | |
| β-CD(C-2028) | IC50 | 0.054 ± 0.007 ** | 0.033 ± 0.017 * | 0.132 ± 0.039 * | 0.109 ± 0.044 | 0.033 ± 0.010 |
| IC80 | 0.120 ± 0.003 ** | 0.370 ± 0.149 * | 0.654 ± 0.055 *** | n.d. | 2.52 ± 0.59 * | |
| QDgreen-C-2028 | IC50 | 0.018 ± 0.005 | 0.010 ± 0.001 | 0.088 ± 0.015 | 0.082 ± 0.006 ** | 0.104 ± 0.022 ** |
| IC80 | 0.083 ± 0.024 * | 0.028 ± 0.002 * | 0.155 ± 0.027 | 0.184 ± 0.060 | n.d. | |
| QDgreen-β-CD(C-2028)-FA | IC50 | 0.020 ± 0.003 | 0.008 ± 0.001 | 0.044 ± 0.003 ** | 0.057 ± 0.011 | 0.064 ± 0.014 * |
| IC80 | 0.060 ± 0.004 | 0.022 ± 0.004 | 0.096 ± 0.002 *** | 0.156 ± 0.022 | 0.265 ± 0.044 * | |
| QDred-C-2028 | IC50 | 0.037 ± 0.008 * | 0.019 ± 0.002 *** | 0.072 ± 0.013 | 0.081 ± 0.003 *** | 0.021 ± 0.006 |
| IC80 | 0.097 ± 0.008 ** | 0.101 ± 0.017 *** | 0.160 ± 0.044 | 0.250 ± 0.067 * | 0.835 ± 0.131 | |
| QDred-β-CD(C-2028)-FA | IC50 | 0.010 ± 0.003 | 0.012 ± 0.006 | 0.048 ± 0.008 | 0.041 ± 0.009 * | 0.060 ± 0.007 * |
| IC80 | 0.040 ± 0.013 | 0.084 ± 0.008 *** | 0.092 ± 0.008 ** | 0.091 ± 0.005 *** | 0.258 ± 0.016 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilch, J.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Kasprzak, A.; Paluszkiewicz, E.; Augustin, E.; Nowicka, A.M. Foliate-Targeting Quantum Dots-β-Cyclodextrin Nanocarrier for Efficient Delivery of Unsymmetrical Bisacridines to Lung and Prostate Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1261. https://doi.org/10.3390/ijms23031261
Pilch J, Kowalik P, Kowalczyk A, Bujak P, Kasprzak A, Paluszkiewicz E, Augustin E, Nowicka AM. Foliate-Targeting Quantum Dots-β-Cyclodextrin Nanocarrier for Efficient Delivery of Unsymmetrical Bisacridines to Lung and Prostate Cancer Cells. International Journal of Molecular Sciences. 2022; 23(3):1261. https://doi.org/10.3390/ijms23031261
Chicago/Turabian StylePilch, Joanna, Patrycja Kowalik, Agata Kowalczyk, Piotr Bujak, Artur Kasprzak, Ewa Paluszkiewicz, Ewa Augustin, and Anna M. Nowicka. 2022. "Foliate-Targeting Quantum Dots-β-Cyclodextrin Nanocarrier for Efficient Delivery of Unsymmetrical Bisacridines to Lung and Prostate Cancer Cells" International Journal of Molecular Sciences 23, no. 3: 1261. https://doi.org/10.3390/ijms23031261
APA StylePilch, J., Kowalik, P., Kowalczyk, A., Bujak, P., Kasprzak, A., Paluszkiewicz, E., Augustin, E., & Nowicka, A. M. (2022). Foliate-Targeting Quantum Dots-β-Cyclodextrin Nanocarrier for Efficient Delivery of Unsymmetrical Bisacridines to Lung and Prostate Cancer Cells. International Journal of Molecular Sciences, 23(3), 1261. https://doi.org/10.3390/ijms23031261







