Toward Xeno-Free Differentiation of Human Induced Pluripotent Stem Cell-Derived Small Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Results
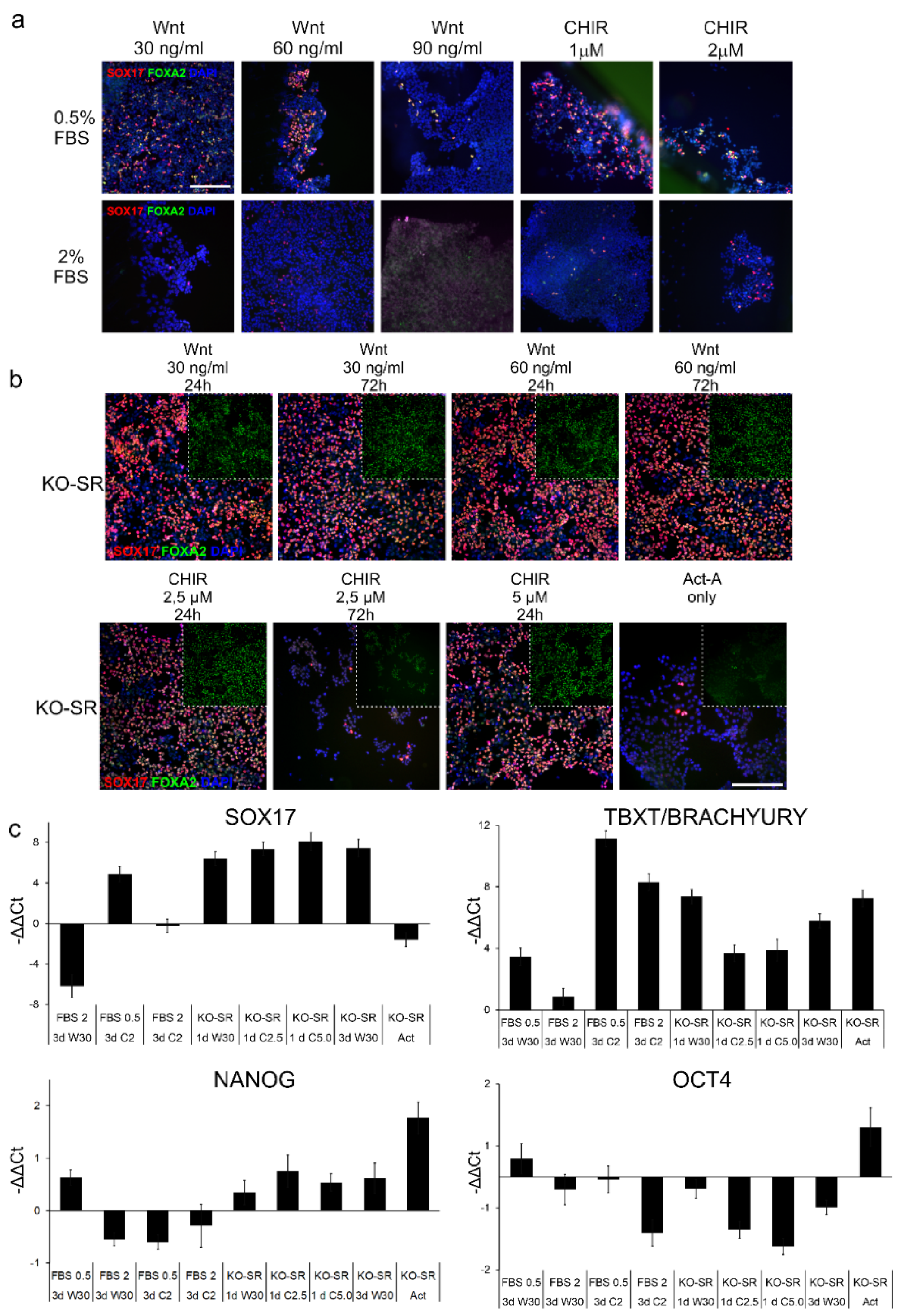
2.1. Effects of Serum Replacement and Wnt-Signaling Inducers on DE-Differentiation
2.2. Effects of Substrata on the Differentiation toward Definitive Endoderm
2.3. Induction toward an Instestinal Lineage
2.4. Induction toward Small Intestinall Epithelial Cells
2.5. Functional Characterisation of Produced Small Intestinall Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. IPSC Culture
4.2. In Vitro Differentiation to Definitive Endoderm
4.3. In Vitro Differentiation to Posterior Definitive Endoderm
4.4. In Vitro Differentiation to Small Intestinal Epithelial Cells
4.5. Caco-2 Culture
4.6. Immunofluorescence Staining and Imaging
4.7. qRT-PCR
4.8. Functionality Assays
4.8.1. Dipeptide Uptake Assay
4.8.2. Calcein AM Extrusion Assay
4.9. Statistical Significance
4.10. Ethical Issues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.-G.; Chen, M.; Zhao, S.; Wang, X. Intestinal Models for Personalized Medicine: From Conventional Models to Microfluidic Primary Intestine-on-a-chip. Stem Cell Rev. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.H.; Araújo, F.; Martínez, E.; Barrias, C.; Sarmento, B. iPSC-Derived Enterocyte-like Cells for Drug Absorption and Metabolism Studies. Trends Mol. Med. 2018, 24, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Coia, H.G.; Karl, J.P.; Pantoja-Feliciano, I.G.; Zachos, N.C.; Racicot, K. Intestinal in vitro and ex vivo Models to Study Host-Microbiome Interactions and Acute Stressors. Front. Physiol. 2018, 9, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Lennernas, H.; Welage, L.S.; Barnett, J.L.; Landowski, C.P.; Foster, D.; Fleisher, D.; Lee, K.; Amidon, G.L. Comparison of Human Duodenum and Caco-2 Gene Expression Profiles for 12,000 Gene Sequences Tags and Correlation with Permeability of 26 Drugs. Pharm. Res. 2002, 19, 1400–1416. [Google Scholar] [CrossRef] [PubMed]
- Aldhous, M.C.; Shmakov, A.N.; Bode, J.; Ghosh, S. Characterization of conditions for the primary culture of human small intestinal epithelial cells. Clin. Exp. Immunol. 2001, 125, 32–40. [Google Scholar] [CrossRef]
- Grossmann, J.; Walther, K.; Artinger, M.; Kiessling, S.; Steinkamp, M.; Schmautz, W.K.; Stadler, F.; Bataille, F.; Schultz, M.; Schsolmerich, J.; et al. Progress on isolation and short-term ex-vivo culture of highly purified non-apoptotic human intestinal epithelial cells (IEC). Eur. J. Cell Biol. 2003, 82, 262–270. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef]
- Spence, J.R.; Mayhew, C.N.; Rankin, S.A.; Kuhar, M.F.; Vallance, J.E.; Tolle, K.; Hoskins, E.E.; Kalinichenko, V.V.; Wells, S.I.; Zorn, A.M.; et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 2011, 470, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Kitano, K.; Schwartz, D.M.; Zhou, H.; Gilpin, S.E.; Wojtkiewicz, G.R.; Ren, X.; Sommer, C.A.; Capilla, A.V.; Mathisen, D.J.; Goldstein, A.M.; et al. Bioengineering of functional human induced pluripotent stem cell-derived intestinal grafts. Nat. Commun. 2017, 8, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.B.; Lee, H.; Son, Y.S.; Lee, J.H.; Cho, H.S.; Lee, M.O.; Oh, J.H.; Lee, J.; Kim, S.; Jung, C.R.; et al. In vitro and in vivo imaging and tracking of intestinal organoids from human induced pluripotent stem cells. FASEB J. 2018, 32, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahmani, S.; Breyner, N.M.; Su, H.-M.; Verdu, E.F.; Didar, T.F. Intestinal organoids: A new paradigm for engineering intestinal epithelium in vitro. Biomaterials 2019, 194, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Braverman, J.; Yilmaz, Ö.H. From 3D Organoids back to 2D Enteroids. Dev. Cell 2018, 44, 533–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorne, C.A.; Chen, I.W.; Sanman, L.E.; Cobb, M.H.; Wu, L.F.; Altschuler, S.J. Enteroid Monolayers Reveal an Autonomous WNT and BMP Circuit Controlling Intestinal Epithelial Growth and Organization. Dev. Cell 2018, 44, 624–633.e4. [Google Scholar] [CrossRef] [Green Version]
- Roodsant, T.; Navis, M.; Aknouch, I.; Renes, I.B.; van Elburg, R.M.; Pajkrt, D.; Wolthers, K.C.; Schultsz, C.; van der Ark, K.C.H.; Sridhar, A.; et al. A Human 2D Primary Organoid-Derived Epithelial Monolayer Model to Study Host-Pathogen Interaction in the Small Intestine. Front. Cell. Infect. Microbiol. 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, S.; Morooka, M.; Otera, K.; Kume, S. A cost-effective system for differentiation of intestinal epithelium from human induced pluripotent stem cells. Sci. Rep. 2015, 5, 17297. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, A.L.; Gyurdieva, A.V.; Mabus, J.R.; Ferguson, C.; Yan, Z.; Hornby, P.J. Alternative functional in vitro models of human intestinal epithelia. Front. Pharmacol. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negoro, R.; Takayama, K.; Kawai, K.; Harada, K.; Sakurai, F.; Hirata, K.; Mizuguchi, H. Efficient Generation of Small Intestinal Epithelial-like Cells from Human iPSCs for Drug Absorption and Metabolism Studies. Stem Cell Rep. 2018, 11, 1539–1550. [Google Scholar] [CrossRef] [Green Version]
- Kauffman, A.L.; Ekert, J.E.; Gyurdieva, A.V.; Rycyzyn, M.A.; Hornby, P.J. Directed differentiation protocols for successful human intestinal organoids derived from multiple induced pluripotent stem cell lines. Stem Cell Biol. Res. 2015, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; Mizuno, S.; Hashita, T.; Iwao, T.; Matsunaga, T. Using human iPS cell-derived enterocytes as novel in vitro model for the evaluation of human intestinal mucosal damage. Inflamm. Res. 2018, 67, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Mizuno, S.; Hashita, T.; Iwao, T.; Matsunaga, T. Establishment of a novel culture method for maintaining intestinal stem cells derived from human induced pluripotent stem cells. Biol. Open 2020, 9, bio049064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negoro, R.; Takayama, K.; Nagamoto, Y.; Sakurai, F.; Tachibana, M.; Mizuguchi, H. Modeling of drug-mediated CYP3A4 induction by using human iPS cell-derived enterocyte-like cells. Biochem. Biophys. Res. Commun. 2016, 472, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, K.; Negoro, R.; Ichikawa, M.; Yamashita, T.; Deguchi, S.; Harada, K.; Hirata, K.; Takayama, K.; Mizuguchi, H. Establishment of SLC15A1/PEPT1-Knockout Human-Induced Pluripotent Stem Cell Line for Intestinal Drug Absorption Studies. Mol. Ther.-Methods Clin. Dev. 2020, 17, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani-Dalini, S.; Azarpira, N.; Sangtarash, M.H.; Soleimanpour-Lichaei, H.R.; Yaghobi, R.; Lorzadeh, S.; Sabet, A.; Sarshar, M.; Al-Abdullah, I.H. Optimization of activin-A: A breakthrough in differentiation of human induced pluripotent stem cell into definitive endoderm. 3 Biotech 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.-L.; Karlsson, J. Expression of Thirty-six Drug Transporter Genes in Human Intestine, Liver, Kidney, and Organotypic Cell Lines. Drug Metab. Dispos. 2007, 35, 1333–1340. [Google Scholar] [CrossRef] [Green Version]
- Eneroth, A.; Åström, E.; Hoogstraate, J.; Schrenk, D.; Conrad, S.; Kauffmann, H.-M.; Gjellan, K. Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction. Eur. J. Pharm. Sci. 2001, 12, 205–214. [Google Scholar] [CrossRef]
- Legrand, O.; Simonin, G.; Perrot, J.-Y.; Zittoun, R.; Marie, J.-P. Pgp and MRP Activities Using Calcein-AM Are Prognostic Factors in Adult Acute Myeloid Leukemia Patients. Blood 1998, 91, 4480–4488. [Google Scholar] [CrossRef]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.K.K.; Valdez, I.A.; Dirice, E.; Kulkarni, R.N. Comparable generation of activin-induced definitive endoderm via additive Wnt or BMP signaling in absence of serum. Stem Cell Rep. 2014, 3, 5–14. [Google Scholar] [CrossRef] [Green Version]
- McLean, A.B.; D’Amour, K.A.; Jones, K.L.; Krishnamoorthy, M.; Kulik, M.J.; Reynolds, D.M.; Sheppard, A.M.; Liu, H.; Xu, Y.; Baetge, E.E.; et al. Activin A efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 2007, 25, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamminen, K.; Balboa, D.; Toivonen, S.; Pakarinen, M.P.; Wiener, Z.; Alitalo, K.; Otonkoski, T. Intestinal commitment and maturation of human pluripotent stem cells is independent of exogenous FGF4 and rspondin1. PLoS ONE 2015, 10, e0134551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brafman, D.A.; Phung, C.; Kumar, N.; Willert, K. Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions. Cell Death Differ. 2013, 20, 369–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor-Weiner, H.; Schwarzbauer, J.E.; Engler, A.J. Defined extracellular matrix components are necessary for definitive endoderm induction. Stem Cells Dayt. Ohio 2013, 31, 2084–2094. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, C.H.; Petersen, D.R.; Moeller, J.B.; Hansson, M.; Dufva, M. Collagen Type I Improves the Differentiation of Human Embryonic Stem Cells toward Definitive Endoderm. PLoS ONE 2015, 10, e0145389. [Google Scholar] [CrossRef] [Green Version]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Aumailley, M.; Brucknertuderman, L.; Carter, W.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J. A simplified laminin nomenclature. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef]
- Beaulieu, J.-F. Extracellular Matrix Components and Integrins in Relationship to Human Intestinal Epithelial Cell Differentiation. Prog. Histochem. Cytochem. 1997, 31, III. [Google Scholar] [CrossRef]
- Teller, I.C.; Auclair, J.; Herring, E.; Gauthier, R.; Ménard, D.; Beaulieu, J.-F. Laminins in the developing and adult human small intestine: Relation with the functional absorptive unit. Dev. Dyn. 2007, 236, 1980–1990. [Google Scholar] [CrossRef]
- Kodama, N.; Iwao, T.; Kabeya, T.; Horikawa, T.; Niwa, T.; Kondo, Y.; Nakamura, K.; Matsunaga, T. Inhibition of mitogen-activated protein kinase kinase, DNA methyltransferase, and transforming growth factor-β promotes differentiation of human induced pluripotent stem cells into enterocytes. Drug Metab. Pharmacokinet. 2016, 31, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Kodama, N.; Iwao, T.; Katano, T.; Ohta, K.; Yuasa, H.; Matsunaga, T. Characteristic Analysis of Intestinal Transport in Enterocyte-Like Cells Differentiated from Human Induced Pluripotent Stem Cells. Drug Metab. Dispos. 2016, 44, 1662–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, K.; Negoro, R.; Yamashita, T.; Kawai, K.; Ichikawa, M.; Mori, T.; Nakatsu, N.; Harada, K.; Ito, S.; Yamada, H.; et al. Generation of Human iPSC–Derived Intestinal Epithelial Cell Monolayers by CDX2 Transduction. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 513–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiamehr, M.; Klettner, A.; Richert, E.; Koskela, A.; Koistinen, A.; Skottman, H.; Kaarniranta, K.; Aalto-Setälä, K.; Juuti-Uusitalo, K. Compromised barrier function in human induced pluripotent stem-cell-derived retinal pigment epithelial cells from type 2 diabetic patients. Int. J. Mol. Sci. 2019, 20, 3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojala, M.; Prajapati, C.; Pölönen, R.P.; Rajala, K.; Pekkanen-Mattila, M.; Rasku, J.; Larsson, K.; Aalto-Setälä, K. Mutation-specific phenotypes in hiPSC-derived cardiomyocytes carrying either myosin-binding protein C or α-tropomyosin mutation for hypertrophic cardiomyopathy. Stem Cells Int. 2016, 2016, 530–545. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego Calif.) 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kabeya, T.; Qiu, S.; Hibino, M.; Nagasaki, M.; Kodama, N.; Iwao, T.; Matsunaga, T. Cyclic AMP signaling promotes the differentiation of human induced pluripotent stem cells into intestinal epithelial cells S. Drug Metab. Dispos. 2018, 46, 1411–1419. [Google Scholar] [CrossRef] [Green Version]
- Omkvist, D.H.; Brodin, B.; Nielsen, C.U. Ibuprofen is a non-competitive inhibitor of the peptide transporter hPEPT1 (SLC15A1): Possible interactions between hPEPT1 substrates and ibuprofen: Ibuprofen is an inhibitor of SLC15A1. Br. J. Pharmacol. 2010, 161, 1793–1805. [Google Scholar] [CrossRef] [Green Version]
- Iwao, T.; Kodama, N.; Kondo, Y.; Kabeya, T.; Nakamura, K.; Horikawa, T.; Niwa, T.; Kurose, K.; Matsunaga, T. Generation of enterocyte-like cells with pharmacokinetic functions from human induced pluripotent stem cells using small-molecule compounds. Drug Metab. Dispos. 2015, 43, 603–610. [Google Scholar] [CrossRef] [Green Version]





| Primary Antibodies | |||||
| Target | Target marker for | Manufacturer | Catalog | Host | Dilution |
| Oct4 | pluripotency | R&D | AF1759 | goat | 1:300 |
| Nanog | pluripotency | R&D | AF1997 | goat | 1:300 |
| Sox17 | endoderm | R&D | AF1924 | goat | 1:300 |
| Cdx2 | posterior definitive endoderm | Abcam | ab76541 | rabbit | 1:300 |
| Foxa2 | endoderm | EMD Millipore | 07-633 | rabbit | 1:300 |
| Villin | brush border | Abcam | ab130751 | rabbit | 1:200 |
| Pept1 | enterocytes | Novus Biologicals | NBPI-92005 | rabbit | 1:100 |
| Chromogranin A | enteroendocrine cells | Abcam | ab15160 | rabbit | 1:100 |
| Secondary Antibodies | |||||
| Target | Manufacturer | Wavelength | Cat | Host | dilution |
| Mouse IgG | Life Technologies | 568 | A11031 | Goat | 1:500 |
| Goat IgG | Invitrogen | 568 | A11057 | Donkey | 1:1000 |
| Rabbit IgG | Invitrogen | 488 | A21206 | Donkey | 1:500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saari, J.; Siddique, F.; Korpela, S.; Mäntylä, E.; Ihalainen, T.O.; Kaukinen, K.; Aalto-Setälä, K.; Lindfors, K.; Juuti-Uusitalo, K. Toward Xeno-Free Differentiation of Human Induced Pluripotent Stem Cell-Derived Small Intestinal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 1312. https://doi.org/10.3390/ijms23031312
Saari J, Siddique F, Korpela S, Mäntylä E, Ihalainen TO, Kaukinen K, Aalto-Setälä K, Lindfors K, Juuti-Uusitalo K. Toward Xeno-Free Differentiation of Human Induced Pluripotent Stem Cell-Derived Small Intestinal Epithelial Cells. International Journal of Molecular Sciences. 2022; 23(3):1312. https://doi.org/10.3390/ijms23031312
Chicago/Turabian StyleSaari, Jaakko, Fatima Siddique, Sanna Korpela, Elina Mäntylä, Teemu O. Ihalainen, Katri Kaukinen, Katriina Aalto-Setälä, Katri Lindfors, and Kati Juuti-Uusitalo. 2022. "Toward Xeno-Free Differentiation of Human Induced Pluripotent Stem Cell-Derived Small Intestinal Epithelial Cells" International Journal of Molecular Sciences 23, no. 3: 1312. https://doi.org/10.3390/ijms23031312







