Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology
Abstract
1. Introduction
2. Chronobiology
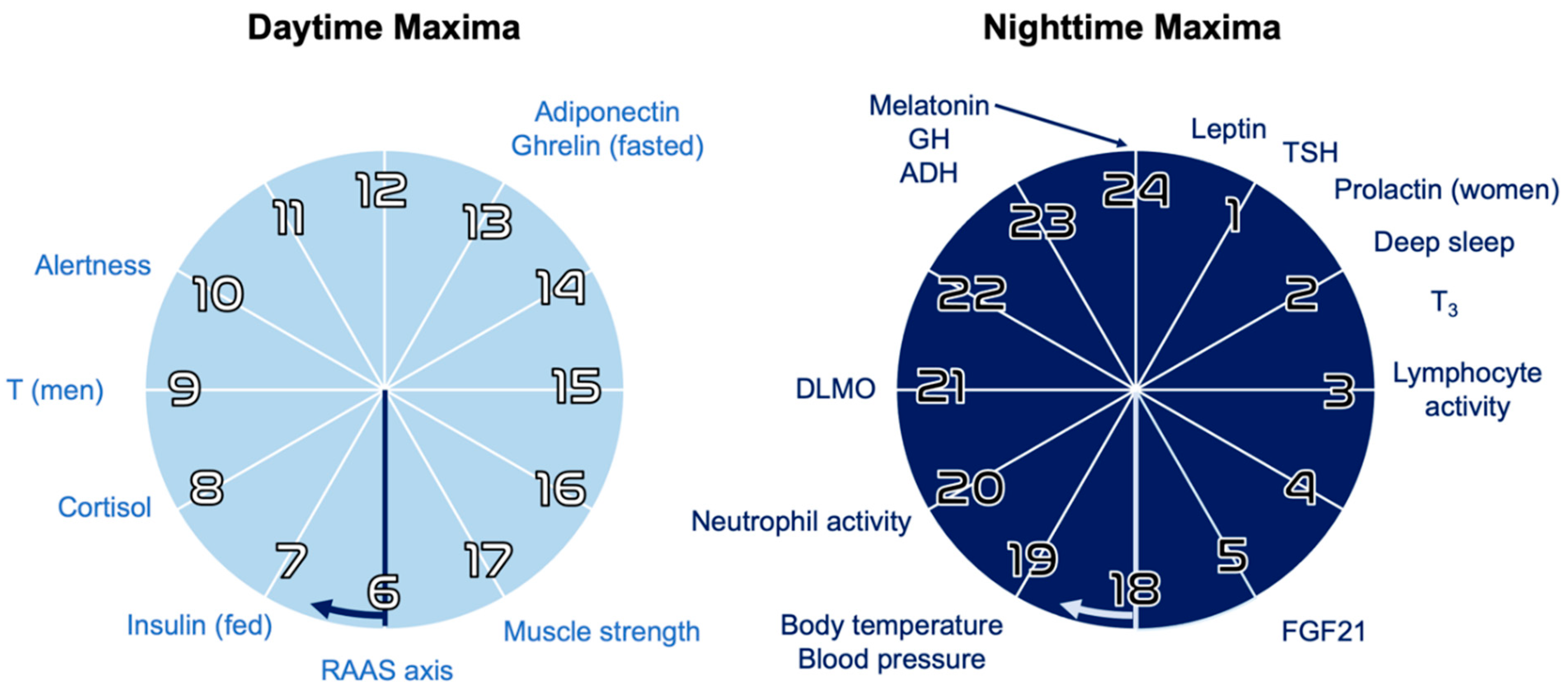
2.1. Circadian Rhythms
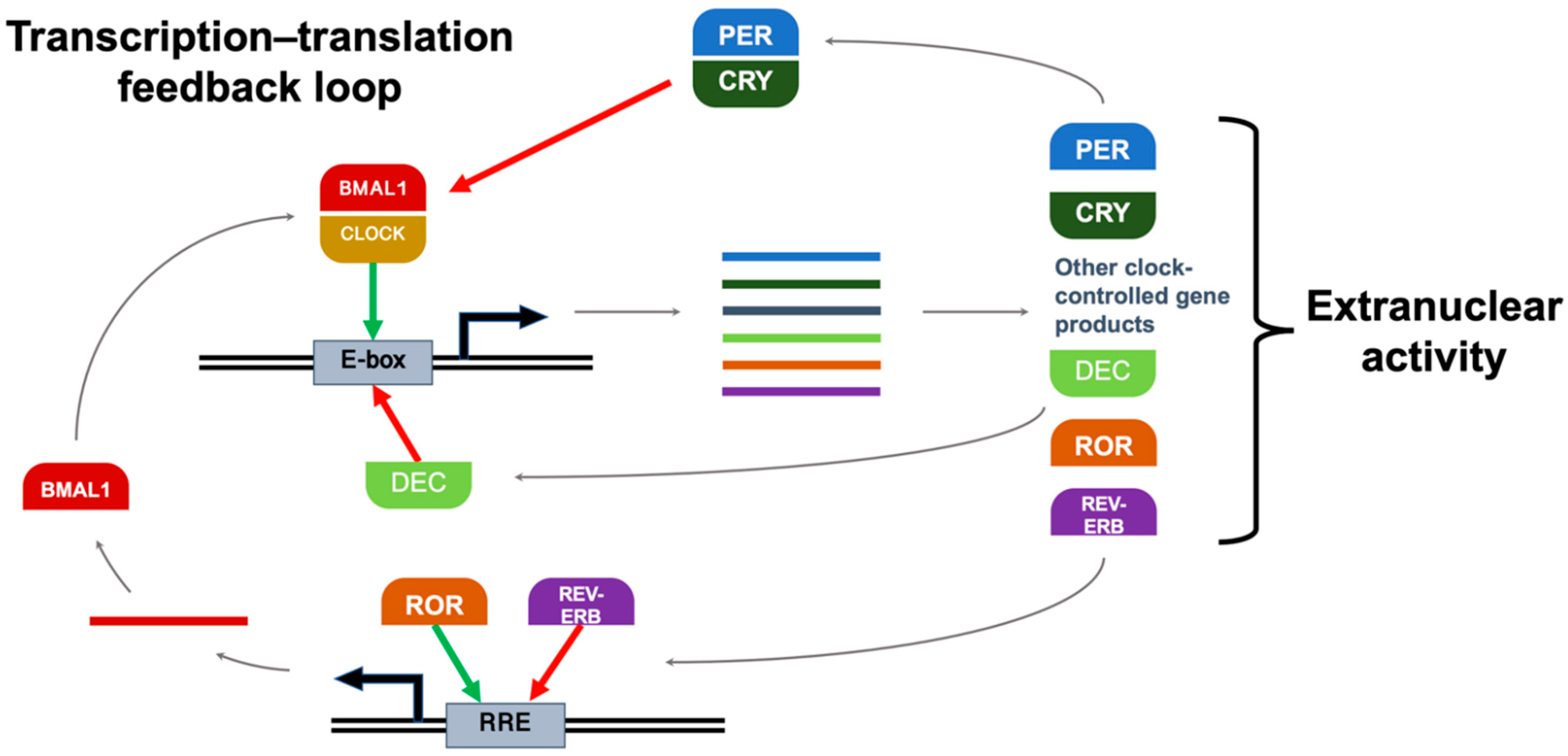
2.2. Clock Genes
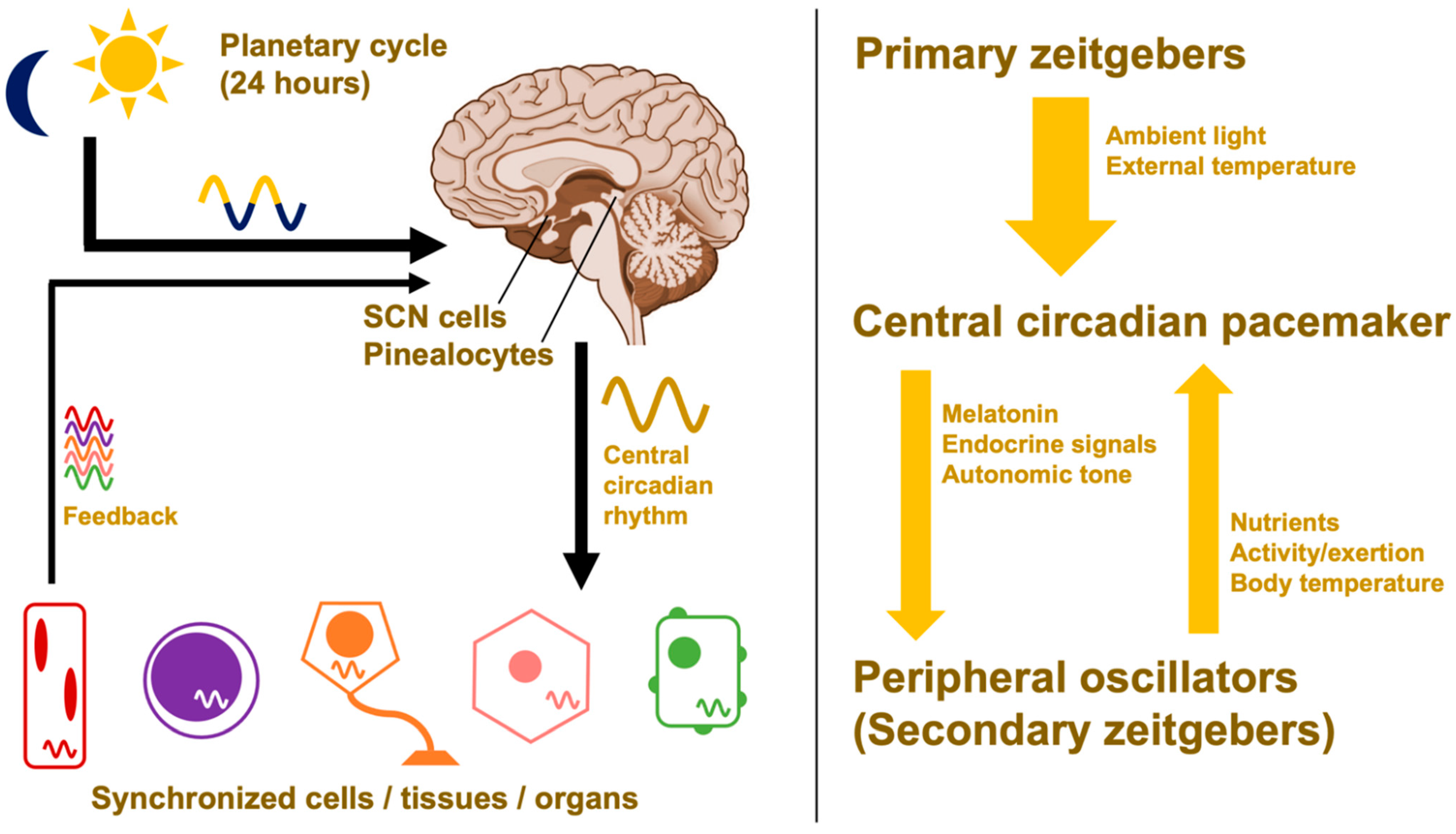
2.3. Hierarchical Organization and Zeitgebers
2.4. Circadian Amplitude
3. Circadian Disruptions and Breast Cancer
3.1. Epidemiology
3.2. Impact of Circadian Disruption on Health Disparities
3.3. Melatonin and Breast Cancer
3.4. Molecular Clock Dysfunction and Breast Cancer Risk
3.5. Breast Cancer Outcomes and Treatment Response
4. Circadian Regulation and DNA Damage Responses
4.1. Radiobiological Principles
4.2. Cell Cycle Gating
4.3. Double-Strand DNA Breaks
4.4. Hypoxia Responses and Reoxygenation
5. Metabolic Circadian Entrainment and Regulation
5.1. Epidemiologic Data Linking Metabolism and Circadian Dysregulation
5.2. Energy Sensing
5.3. Dietary Modulation
6. Leveraging Circadian Rhythms for Therapeutic Benefit
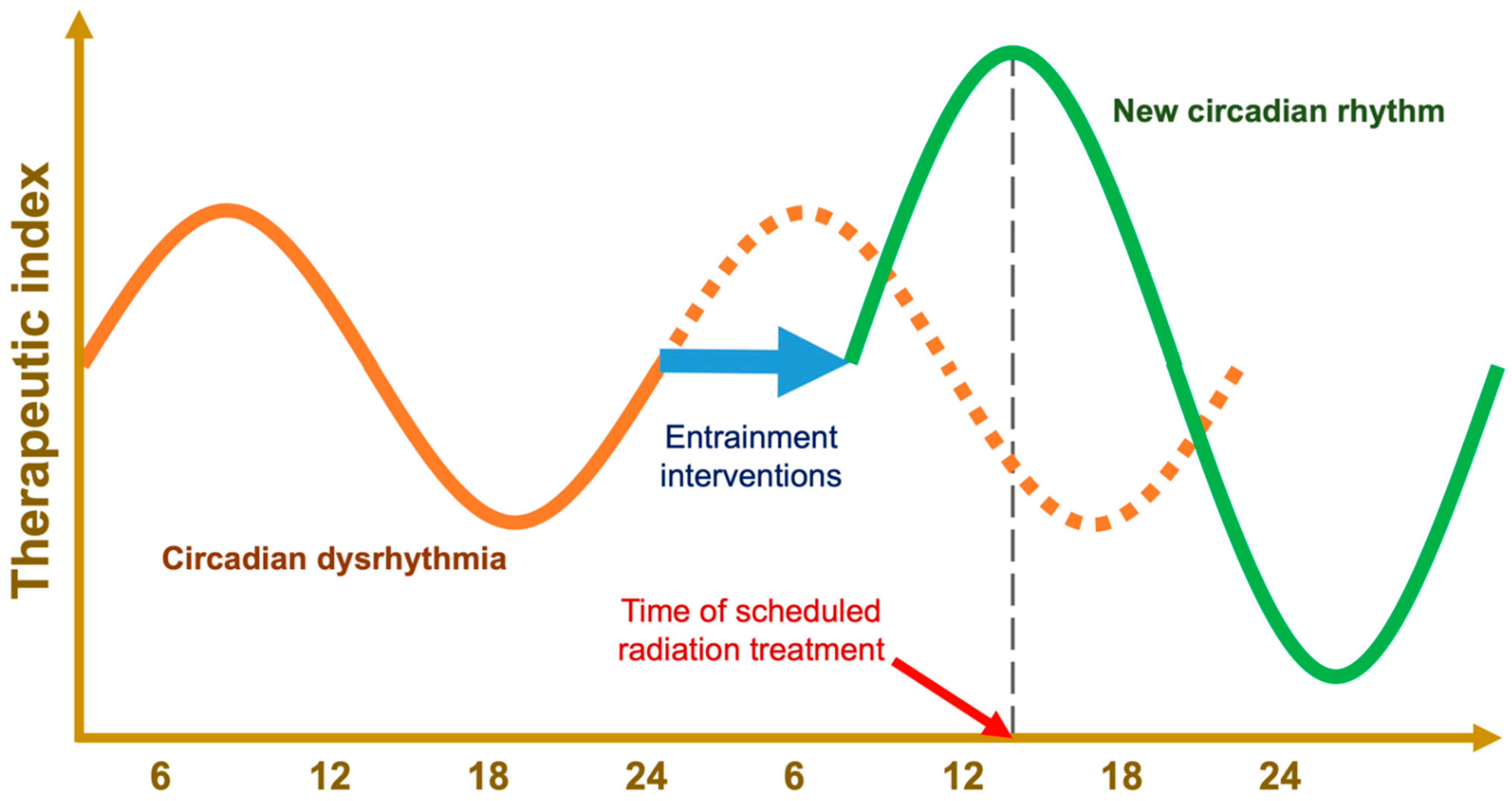
6.1. Chronoradiotherapy
6.2. Chronopharmaceuticals
7. Rational Circadian and Metabolic Interventions
7.1. Limitations of Past Research
7.2. Future Directions
7.3. Zeitgeber Diet
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.H.; Lazar, M.A. Transcriptional Control of Circadian Rhythms and Metabolism: A Matter of Time and Space. Endocr. Rev. 2020, 41, 707–732. [Google Scholar] [CrossRef] [PubMed]
- Maor, R.; Dayan, T.; Ferguson-Gow, H.; Jones, K.E. Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat. Ecol. Evol. 2017, 1, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the Fourth Dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018, 359. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, W.J.; Klerman, E.B. Circadian Neurobiology and the Physiologic Regulation of Sleep and Wakefulness. Neurol. Clin. 2019, 37, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.A.; Flynn-Evans, E.E.; Aeschbach, D.; Brainard, G.C.; Czeisler, C.A.; Lockley, S.W. Diurnal Spectral Sensitivity of the Acute Alerting Effects of Light. Sleep 2014, 37, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef]
- Bailey, S.M.; Udoh, U.S.; Young, M.E. Circadian regulation of metabolism. J. Endocrinol. 2014, 222, R75–R96. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Meléndez-Fernández, O.; Walton, J.; DeVries, A.; Nelson, R. Clocks, Rhythms, Sex, and Hearts: How Disrupted Circadian Rhythms, Time-of-Day, and Sex Influence Cardiovascular Health. Biomolecules 2021, 11, 883. [Google Scholar] [CrossRef]
- Hilton, M.; Umali, M.; Czeisler, C.; Wyatt, J.; Shea, S. Endogenous circadian control of the human autonomic nervous system. Comput. Cardiol. 2000, 27, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Arjona, A.; Silver, A.C.; Walker, W.E.; Fikrig, E. Immunity’s fourth dimension: Approaching the circadian–immune connection. Trends Immunol. 2012, 33, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Labrecque, N.; Cermakian, N. Circadian Clocks in the Immune System. J. Biol. Rhythm. 2015, 30, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Cannon, P.; Mendoza-Viveros, L.; Yuen, A.; Kærn, M.; Cheng, H.-Y.M. The Circadian Molecular Clock Regulates Adult Hippocampal Neurogenesis by Controlling the Timing of Cell-Cycle Entry and Exit. Cell Rep. 2013, 5, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Cui, M.; Xiao, H.; Luo, D.; Zhang, X.; Zhao, S.; Zheng, Q.; Li, Y.; Zhao, Y.; Dong, J.; Li, H.; et al. Circadian Rhythm Shapes the Gut Microbiota Affecting Host Radiosensitivity. Int. J. Mol. Sci. 2016, 17, 1786. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Circadian rhythms: A regulator of gastrointestinal health and dysfunction. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 411–424. [Google Scholar] [CrossRef]
- Alvarez, Y.; Glotfelty, L.G.; Blank, N.; Dohnalová, L.; Thaiss, C.A. The Microbiome as a Circadian Coordinator of Metabolism. Endocrinology 2020, 161, bqaa059. [Google Scholar] [CrossRef]
- Levine, D.; Hong, H.; Weidemann, B.J.; Ramsey, K.M.; Affinati, A.H.; Schmidt, M.S.; Cedernaes, J.; Omura, C.; Braun, R.; Lee, C.; et al. NAD+ Controls Circadian Reprogramming through PER2 Nuclear Translocation to Counter Aging. Mol. Cell 2020, 78, 835–849.e7. [Google Scholar] [CrossRef]
- Deharo, D.; Kines, K.J.; Sokolowski, M.; Dauchy, R.T.; Streva, V.A.; Hill, S.M.; Hanifin, J.P.; Brainard, G.C.; Blask, D.E.; Belancio, V.P. Regulation of L1 expression and retrotransposition by melatonin and its receptor: Implications for cancer risk associated with light exposure at night. Nucleic Acids Res. 2014, 42, 7694–7707. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Vargas, N.N.; Navarro-Espíndola, R.; Guzmán-Ruíz, M.A.; del Basualdo, M.C.; Espitia-Bautista, E.; López-Bago, A.; Lascurain, R.; Córdoba-Manilla, C.; Buijs, R.M.; Escobar, C. Circadian disruption promotes tumor growth by anabolic host metabolism; Experimental evidence in a rat model. BMC Cancer 2017, 17, 625. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; Knudsen, K.E. Cancer and the Circadian Clock. Cancer Res. 2019, 79, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Lam, M.T.Y.; Panda, S. Interplay between Circadian Clock and Cancer: New Frontiers for Cancer Treatment. Trends Cancer 2019, 5, 475–494. [Google Scholar] [CrossRef]
- Shen, H.; Cook, K.; Gee, H.E.; Hau, E. Hypoxia, metabolism, and the circadian clock: New links to overcome radiation resistance in high-grade gliomas. J. Exp. Clin. Cancer Res. 2020, 39, 129. [Google Scholar] [CrossRef]
- Dong, D.; Yang, D.; Lin, L.; Wang, S.; Wu, B. Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem. Pharmacol. 2020, 178, 114045. [Google Scholar] [CrossRef]
- Chan, S.; Rowbottom, L.; McDonald, R.; Zhang, L.; Bjarnason, G.A.; Tsao, M.; Danjoux, C.; Barnes, E.; Lam, H.; Popovic, M.; et al. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann. Palliat. Med. 2016, 5, 267–279. [Google Scholar] [CrossRef]
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a circadian time cue—Evidence from human studies. Nat. Rev. Endocrinol. 2020, 16, 213–223. [Google Scholar] [CrossRef]
- Reinke, H.; Asher, G. Crosstalk between metabolism and circadian clocks. Nat. Rev. Mol. Cell Biol. 2019, 20, 227–241. [Google Scholar] [CrossRef]
- Peek, C.B.; Levine, D.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2016, 25, 86–92. [Google Scholar] [CrossRef]
- Melkani, G.C.; Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 2017, 595, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Bhawal, U.K.; Yoshimura, T.; Muragaki, Y. DEC1 and DEC2 Crosstalk between Circadian Rhythm and Tumor Progression. J. Cancer 2016, 7, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Yoshida, A.; Chitnis, N.; Altman, B.J.; Tameire, F.; Oran, A.; Gennaro, V.; Armeson, K.E.; McMahon, S.B.; Wertheim, G.B.; et al. A PERK–miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat. Cell Biol. 2018, 20, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.-Y.; Yu, W.; Sena, L.A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C.; et al. Circadian Clock NAD+ Cycle Drives Mitochondrial Oxidative Metabolism in Mice. Science 2013, 342, 1243417. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Patel, V.R.; Eckel-Mahan, K.; Peleg, S.; Forne, I.; Ladurner, A.; Baldi, P.; Imhof, A.; Sassone-Corsi, P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 3339–3344. [Google Scholar] [CrossRef] [PubMed]
- Sides, M.B.; Johnston, S.L.; Sirek, A.; Lee, P.H.; Blue, R.S.; Antonsen, E.L.; Basner, M.; Douglas, G.L.; Epstein, A.; Flynn-Evans, E.E.; et al. Bellagio II Report: Terrestrial Applications of Space Medicine Research. Aerosp. Med. Hum. Perform. 2021, 92, 650–669. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, L.; Cao, M.; Song, F.; Zheng, H.; Zhu, X.; Wei, Q.; Zhang, W.; Chen, K. The role of polymorphisms in circadian pathway genes in breast tumorigenesis. Breast Cancer Res. Treat. 2010, 127, 531–540. [Google Scholar] [CrossRef]
- Chen, Z.; Yoo, S.-H.; Takahashi, J.S. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 231–252. [Google Scholar] [CrossRef]
- Nagoshi, E.; Saini, C.; Bauer, C.; Laroche, T.; Naef, F.; Schibler, U. Circadian gene expression in individual fibroblasts: Cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 2004, 119, 693–705. [Google Scholar] [CrossRef]
- Smith, M.R.; Revell, V.L.; Eastman, C.I. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009, 10, 287–294. [Google Scholar] [CrossRef]
- Brainard, G.C.; Barger, L.K.; Soler, R.R.; Hanifin, J.P. The development of lighting countermeasures for sleep disruption and circadian misalignment during spaceflight. Curr. Opin. Pulm. Med. 2016, 22, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Lewis, T.T.; Guo, N.; Jackson, C.L.; Sims, M.; Wilson, J.G.; Roux, A.V.D.; Williams, D.R.; Redline, S. Associations between everyday discrimination and sleep quality and duration among African-Americans over time in the Jackson Heart Study. Sleep 2021, 44, zsab162. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Leaderer, D.; Zheng, T.; Hoffman, A.E.; Stevens, R.G.; Zhu, Y. Genetic and epigenetic associations of circadian gene TIMELESS and breast cancer risk. Mol. Carcinog. 2012, 51, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Lesicka, M.; Jabłońska, E.; Wieczorek, E.; Pepłońska, B.; Gromadzińska, J.; Seroczyńska, B.; Kalinowski, L.; Skokowski, J.; Reszka, E. Circadian Gene Polymorphisms Associated with Breast Cancer Susceptibility. Int. J. Mol. Sci. 2019, 20, 5704. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J. The influence of ketogenic therapy on the 5 R’s of radiobiology. Int. J. Radiat. Biol. 2017, 95, 394–407. [Google Scholar] [CrossRef]
- Gerbes, A.L.; Arbogast, B.J. The Influence of Timeshift on Circadian Rhythm of Sensitivity to X-Irradiation in Mice. Chronobiol. Int. 1984, 1, 177–184. [Google Scholar] [CrossRef][Green Version]
- Abdollahi, H. Radiotherapy dose painting by circadian rhythm based radiomics. Med. Hypotheses 2019, 133, 109415. [Google Scholar] [CrossRef]
- Xing, W.; Busino, L.; Hinds, T.R.; Marionni, S.T.; Saifee, N.H.; Bush, M.F.; Pagano, M.; Zheng, N. SCF FBXL3 ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 2013, 496, 64–68. [Google Scholar] [CrossRef]
- Uetani, N.; Hardy, S.; Gravel, S.-P.; Kiessling, S.; Pietrobon, A.; Wong, N.N.; Chénard, V.; Cermakian, N.; St-Pierre, J.; Tremblay, M.L. PRL2 links magnesium flux and sex-dependent circadian metabolic rhythms. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Cadenas, C.; Van De Sandt, L.; Edlund, K.; Lohr, M.; Hellwig, B.; Marchan, R.; Schmidt, M.; Rahnenführer, J.; Oster, H.; Hengstler, J.G. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 2014, 13, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- Peeples, L. Medicine’s secret ingredient—i’? it’s in the timing. Nature 2018, 556, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Li, S.; Qu, X.; Wang, M.; Bai, X.; Xu, Z.; Ao, X.; Jia, Z.; Jiang, X.; Yang, Y.; et al. DEC1 regulates breast cancer cell proliferation by stabilizing cyclin E protein and delays the progression of cell cycle S phase. Cell Death Dis. 2015, 6, e1891. [Google Scholar] [CrossRef]
- Fang, W.; Li, Q.; Wang, M.; Zheng, M.; Xu, H. DEC2 Serves as Potential Tumor Suppressor in Breast Carcinoma. Dis. Markers 2020, 2020, 6053154. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Yamaguchi, S.; Mitsui, S.; Emi, A.; Shimoda, F.; Okamura, H. Control mechanism of the circadian clock for timing of cell division in vivo. Science 2003, 302, 255–259. [Google Scholar] [CrossRef]
- Bieler, J.; Cannavo, R.; Gustafson, K.; Gobet, C.; Gatfield, D.; Naef, F. Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Mol. Syst. Biol. 2014, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, J.; Montellier, E.; Sassone-Corsi, P. Molecular Cogs: Interplay between Circadian Clock and Cell Cycle. Trends Cell Biol. 2018, 28, 368–379. [Google Scholar] [CrossRef]
- Astaburuaga, R.; Basti, A.; Li, Y.; Herms, D.; Relógio, A. Circadian regulation of physiology: Relevance for space medicine. Reach 2019, 14–15, 100029. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Harper, E.; Talbot, C.J. Is it time to change radiotherapy: The dawning of chronoradiotherapy? Clin. Oncol. 2019, 31, 326–335. [Google Scholar] [CrossRef]
- Jones, S.E.; Lane, J.M.; Wood, A.R.; Van Hees, V.T.; Tyrrell, J.; Beaumont, R.N.; Jeffries, A.R.; Dashti, H.S.; Hillsdon, M.; Ruth, K.S.; et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; Schneider, L.; Cheung, J.; Bertrand, S.J.; Kariharan, T.; Pack, A.; Gehrman, P.R. Genetic Basis of Chronotype in Humans: Insights From Three Landmark GWAS. Sleep 2016, 40, zsw048. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.M.; Bruce, J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocr. 2004, 25, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.; Korf, H.; Kuffer, L.; Groß, J.V.; Erren, T.C. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: A systematic review. BMJ Open Sport Exerc. Med. 2018, 4, e000443. [Google Scholar] [CrossRef]
- West, K.E.; Jablonski, M.R.; Warfield, B.; Cecil, K.S.; James, M.; Ayers, M.A.; Maida, J.; Bowen, C.; Sliney, D.H.; Rollag, M.D.; et al. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J. Appl. Physiol. 2011, 110, 619–626. [Google Scholar] [CrossRef]
- Smith, M.R.; Eastman, C.I. Phase Delaying the Human Circadian Clock with Blue-Enriched Polychromatic Light. Chronobiol. Int. 2009, 26, 709–725. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Nelson, R.J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 2017, 7, e1017. [Google Scholar] [CrossRef]
- Maruani, J.; Geoffroy, P.A. Bright Light as a Personalized Precision Treatment of Mood Disorders. Front. Psychiatry 2019, 10, 85. [Google Scholar] [CrossRef]
- Brainard, G.C.; Coyle, W.; Ayers, M.; Kemp, J.; Warfield, B.; Maida, J.; Bowen, C.; Bernecker, C.; Lockley, S.W.; Hanifin, J.P. Solid-state lighting for the International Space Station: Tests of visual performance and melatonin regulation. Acta Astronaut. 2013, 92, 21–28. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, H.; Ji, M.; Wang, Z.; Wu, W. Low circadian clock genes expression in cancers: A meta-analysis of its association with clinicopathological features and prognosis. PLoS ONE 2020, 15, e0233508. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.K.; Harvey, S.L.; Sammons, P.J.; Anderson, A.P.; Kopalle, H.M.; Banham, A.; Partch, C.L. Cancer/Testis Antigen PASD1 Silences the Circadian Clock. Mol. Cell 2015, 58, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, M.; Song, L. PAS Domain Containing Repressor 1 (PASD1) Promotes Glioma Cell Proliferation Through Inhibiting Apoptosis In Vitro. Med. Sci. Monit. 2019, 25, 6955–6964. [Google Scholar] [CrossRef] [PubMed]
- Straif, K.; Baan, R.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007, 8, 1065–1066. [Google Scholar] [CrossRef]
- He, C.; Anand, S.T.; Ebell, M.H.; Vena, J.E.; Robb, S.W. Circadian disrupting exposures and breast cancer risk: A meta-analysis. Int. Arch. Occup. Environ. Health 2014, 88, 533–547. [Google Scholar] [CrossRef]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef]
- Salamanca-Fernández, E.; Rodríguez-Barranco, M.; Guevara, M.; Ardanaz, E.; Olry de Labry Lima, A.; Sánchez, M.J. Night-shift work and breast and prostate cancer risk: Updating the evidence from epidemiological studies. Sist Sanit Navar 2018, 41, 211–226. [Google Scholar]
- Garcia-Saenz, A.; Sánchez de Miguel, A.; Espinosa, A.; Valentin, A.; Aragonés, N.; Llorca, J.; Amiano, P.; Martín Sánchez, V.; Guevara, M.; Capelo, R.; et al. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain study). Environ. Health Perspect. 2018, 126, 047011. [Google Scholar] [CrossRef]
- Bustamante-Montes, L.P.; Flores-Meza, B.; Hernández-Valero, M.A.; Cárdenas-López, A.; Dolores-Velázquez, R.; Borja-Bustamante, P.; Borja-Aburto, V.H. Night Shift Work and Risk of Breast Cancer in Women. Arch. Med. Res. 2019, 50, 393–399. [Google Scholar] [CrossRef]
- Ritonja, J.; McIsaac, M.A.; Sanders, E.; Kyba, C.C.M.; Grundy, A.; Cordina-Duverger, E.; Spinelli, J.J.; Aronson, K.J. Outdoor light at night at residences and breast cancer risk in Canada. Eur. J. Epidemiol. 2020, 35, 579–589. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP Cancer Hazard Assessment Report on Night Shift Work and Light at Night; National Toxicology Program: Durham, NC, USA, 2021.
- Zhu, Y.; Brown, H.N.; Zhang, Y.; Stevens, R.G.; Zheng, T. Period3 structural variation: A circadian biomarker associated with breast cancer in young women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 268–270. [Google Scholar]
- Monsees, G.M.; Kraft, P.; Hankinson, S.E.; Hunter, D.J.; Schernhammer, E.S. Circadian genes and breast cancer susceptibility in rotating shift workers. Int. J. Cancer 2012, 131, 2547–2552. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Liquet, B.; Menegaux, F.; Plancoulaine, S.; Laurent-Puig, P.; Mulot, C.; Cordina-Duverger, E.; Sanchez, M.; Arveux, P.; Kerbrat, P.; et al. Breast cancer risk, nightwork, and circadian clock gene polymorphisms. Endocr.-Relat. Cancer 2014, 21, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Reszka, E.; Przybek, M.; Muurlink, O.; Pepłonska, B. Circadian gene variants and breast cancer. Cancer Lett. 2017, 390, 137–145. [Google Scholar] [CrossRef]
- Bracci, M.; Ciarapica, V.; Zabaleta, M.E.; Tartaglione, M.F.; Pirozzi, S.; Giuliani, L.; Piva, F.; Valentino, M.; Ledda, C.; Rapisarda, V.; et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers 2019, 11, 1146. [Google Scholar] [CrossRef]
- Soucise, A.; Vaughn, C.; Thompson, C.L.; Millen, A.E.; Freudenheim, J.L.; Wactawski-Wende, J.; Phipps, A.I.; Hale, L.; Qi, L.; Ochs-Balcom, H.M. Sleep quality, duration, and breast cancer aggressiveness. Breast Cancer Res. Treat. 2017, 164, 169–178. [Google Scholar] [CrossRef]
- Eastman, C.I.; Molina, T.A.; Dziepak, M.E.; Smith, M.R. Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans). Chronobiol. Int. 2012, 29, 1072–1077. [Google Scholar] [CrossRef]
- Garland, J. Energy management—A critical role in cancer induction? Crit. Rev. Oncol./Hematol. 2013, 88, 198–217. [Google Scholar] [CrossRef]
- Bartsch, H.; Buchberger, A.; Franz, H.; Bartsch, C.; Maidonis, I.; Mecke, D.; Bayer, E. Effect of melatonin and pineal extracts on human ovarian and mammary tumor cells in a chemosensitivity assay. Life Sci. 2000, 67, 2953–2960. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, P.; Dai, H.; Li, Q.; Hu, L.; Peng, J.; Jiang, S.; Xu, Y.; Wu, Z.; Nie, H.; et al. Timeless regulates sphingolipid metabolism and tumor cell growth through Sp1/ACER2/S1P axis in ER-positive breast cancer. Cell Death Dis. 2020, 11, 892. [Google Scholar] [CrossRef]
- Ashimori, A.; Nakahata, Y.; Sato, T.; Fukamizu, Y.; Matsui, T.; Yoshitane, H.; Fukada, Y.; Shinohara, K.; Bessho, Y. Attenuated SIRT1 Activity Leads to PER2 Cytoplasmic Localization and Dampens the Amplitude of Bmal1 Promoter-Driven Circadian Oscillation. Front. Neurosci. 2021, 15, 647589. [Google Scholar] [CrossRef] [PubMed]
- Lesicka, M.; Jablonska, E.; Wieczorek, E.; Seroczyńska, B.; Siekierzycka, A.; Skokowski, J.; Kalinowski, L.; Wasowicz, W.; Reszka, E. Altered circadian genes expression in breast cancer tissue according to the clinical characteristics. PLoS ONE 2018, 13, e0199622. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Stevens, R.G.; Hoffman, A.E.; Tjonneland, A.; Vogel, U.; Zheng, T.; Hansen, J. Epigenetic Impact of Long-Term Shiftwork: Pilot Evidence From Circadian Genes and Whole-Genome Methylation Analysis. Chronobiol. Int. 2011, 28, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Climent, J.; Perez-Losada, J.; Quigley, D.A.; Kim, I.-J.; Delrosario, R.; Jen, K.-Y.; Bosch, A.; Lluch, A.; Mao, J.-H.; Balmain, A. Deletion of the PER3 Gene on Chromosome 1p36 in Recurrent ER-Positive Breast Cancer. J. Clin. Oncol. 2010, 28, 3770–3778. [Google Scholar] [CrossRef]
- Winter, S.L.; Bosnoyan-Collins, L.; Pinnaduwagez, D.; Andrulis, I.L. Expression of the circadian clock genes pert, per2 in sporadic, familial breast tumors. Neoplasia 2007, 9, 797–800. [Google Scholar] [CrossRef]
- Hill, S.M.; Belancio, V.P.; Dauchy, R.T.; Xiang, S.; Brimer, S.; Mao, L.; Hauch, A.; Lundberg, P.W.; Summers, W.; Yuan, L.; et al. Melatonin: An inhibitor of breast cancer. Endocr.-Relat. Cancer 2015, 22, R183–R204. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar] [CrossRef]
- Xiang, S.; Dauchy, R.T.; Hoffman, A.E.; Pointer, D.; Frasch, T.; Blask, D.E.; Hill, S.M. Epigenetic inhibition of the tumor suppressor ARHI by light at night-induced circadian melatonin disruption mediates STAT3-driven paclitaxel resistance in breast cancer. J. Pineal Res. 2019, 67, e12586. [Google Scholar] [CrossRef]
- Dakup, P.P.; Porter, K.I.; Gajula, R.P.; Goel, P.N.; Cheng, Z.; Gaddameedhi, S. The circadian clock protects against ionizing radiation-induced cardiotoxicity. FASEB J. 2020, 34, 3347–3358. [Google Scholar] [CrossRef]
- Johnson, K.; Chang-Claude, J.; Critchley, A.-M.; Kyriacou, C.; Lavers, S.; Rattay, T.; Seibold, P.; Webb, A.; West, C.; Symonds, R.; et al. Genetic Variants Predict Optimal Timing of Radiotherapy to Reduce Side-effects in Breast Cancer Patients. Clin. Oncol. 2019, 31, 9–16. [Google Scholar] [CrossRef]
- Noh, J.M.; Choi, D.H.; Park, H.; Huh, S.J.; Park, W.; Seol, S.W.; Jeong, B.K.; Nam, S.J.; Lee, J.E.; Kil, W.-H. Comparison of acute skin reaction following morning versus late afternoon radiotherapy in patients with breast cancer who have undergone curative surgical resection. J. Radiat. Res. 2014, 55, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.; Kang, T.-H.; Reardon, J.T.; Lee, J.H.; Ozturk, N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010, 584, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Konturek, S.J.; Tomaszewska, R.; Leja-Szpak, A.; Bonior, J.; Nawrot, K.; Palonek, M.; Stachura, J.; Pawlik, W.W. The circadian rhythm of melatonin modulates the severity of caerulein-induced pancreatitis in the rat. J. Pineal Res. 2004, 37, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gaddameedhi, S.; Selby, C.P.; Kaufmann, W.K.; Smart, R.C.; Sancar, A. Control of skin cancer by the circadian rhythm. Proc. Natl. Acad. Sci. USA 2011, 108, 18790–18795. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Ren, P.; Su, Z.; Cao, H.; Zhou, J.; Zou, X.; Fu, S.; Lin, S.; Fan, J.; et al. Synergistic Effect of Combination Topotecan and Chronomodulated Radiation Therapy on Xenografted Human Nasopharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 356–362. [Google Scholar] [CrossRef]
- Borgs, L.; Beukelaers, P.; Vandenbosch, R.; Belachew, S.; Nguyen, L.; Malgrange, B. Cell “circadian” cycle: New role for mammalian core clock genes. Cell Cycle 2009, 8, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Ripperger, J.A.; Hoegger, D.C.; Bruegger, P.; Buch, T.; Birchler, T.; Mueller, A.; Albrecht, U.; Contaldo, C.; Brown, S.A. NONO couples the circadian clock to the cell cycle. Proc. Natl. Acad. Sci. USA 2013, 110, 1592–1599. [Google Scholar] [CrossRef]
- Liu, Z.; Selby, C.P.; Yang, Y.; Lindsey-Boltz, L.A.; Cao, X.; Eynullazada, K.; Sancar, A. Circadian regulation of c-MYC in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 21609–21617. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-H.; Leem, S.-H. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res. 2014, 42, 4427–4434. [Google Scholar] [CrossRef]
- Yang, X.; Wood, P.A.; Hrushesky, W.J. Mammalian TIMELESS is required for ATM-dependent CHK2 activation and G2/M checkpoint control. J. Biol. Chem. 2010, 285, 3030–3034. [Google Scholar] [CrossRef]
- Fu, L.; Pelicano, H.; Liu, J.; Huang, P.; Lee, C.C. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef]
- Wright, W.D.; Shah, S.S.; Heyer, W.-D. Homologous recombination and the repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10524–10535. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xiang, Y.; Ozguc, F.M.; Kim, Y.; Liu, C.-J.; Park, P.K.; Hu, Q.; Diao, L.; Lou, Y.; Lin, C.; et al. The Genomic Landscape and Pharmacogenomic Interactions of Clock Genes in Cancer Chronotherapy. Cell Syst. 2018, 6, 314–328.e2. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Morinibu, A.; Koyasu, S.; Goto, Y.; Hiraoka, M.; Harada, H. A circadian clock gene, PER 2, activates HIF-1 as an effector molecule for recruitment of HIF-1α to promoter regions of its downstream genes. FEBS J. 2017, 284, 3804–3816. [Google Scholar] [CrossRef]
- Bondurant, L.D.; Potthoff, M.J. Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu. Rev. Nutr. 2018, 38, 173–196. [Google Scholar] [CrossRef]
- Guo, M.; Liu, T.; Li, P.; Wang, T.; Zeng, C.; Yang, M.; Li, G.; Han, J.; Wu, W.; Zhang, R. Association Between Metabolic Syndrome and Breast Cancer Risk: An Updated Meta-Analysis of Follow-Up Studies. Front. Oncol. 2019, 9, 1290. [Google Scholar] [CrossRef]
- Maury, E. Off the Clock: From Circadian Disruption to Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef]
- Engin, A. Circadian Rhythms in Diet-Induced Obesity. Adv. Exp. Med. Biol. 2017, 960, 19–52. [Google Scholar] [CrossRef]
- Rutter, J.; Reick, M.; Wu, L.C.; McKnight, S.L. Regulation of Clock and NPAS2 DNA Binding by the Redox State of NAD Cofactors. Science 2001, 293, 510–514. [Google Scholar] [CrossRef]
- Stubblefield, J.J.; Terrien, J.; Green, C.B. Nocturnin: At the crossroads of clocks and metabolism. Trends Endocrinol. Metab. 2012, 23, 326–333. [Google Scholar] [CrossRef]
- Onder, Y.; Laothamatas, I.; Berto, S.; Sewart, K.; Kilaru, G.; Bordieanu, B.; Stubblefield, J.J.; Konopka, G.; Mishra, P.; Green, C.B. The Circadian Protein Nocturnin Regulates Metabolic Adaptation in Brown Adipose Tissue. iScience 2019, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Estrella, M.A.; Du, J.; Chen, L.; Rath, S.; Prangley, E.; Chitrakar, A.; Aoki, T.; Schedl, P.; Rabinowitz, J.; Korennykh, A. The metabolites NADP+ and NADPH are the targets of the circadian protein Nocturnin (Curled). Nat. Commun. 2019, 10, 2367. [Google Scholar] [CrossRef] [PubMed]
- Champ, C.E.; Baserga, R.; Mishra, M.V.; Jin, L.; Sotgia, F.; Lisanti, M.P.; Pestell, R.G.; Dicker, A.P.; Simone, N.L. Nutrient Restriction and Radiation Therapy for Cancer Treatment: When Less Is More. Oncologist 2013, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Simone, B.A.; Champ, C.E.; Rosenberg, A.L.; Berger, A.C.; Monti, D.A.; Dicker, A.P.; Simone, N.L. Selectively starving cancer cells through dietary manipulation: Methods and clinical implications. Future Oncol. 2013, 9, 959–976. [Google Scholar] [CrossRef]
- Saleh, A.; Simone, B.; Palazzo, J.; Savage, J.E.; Sano, Y.; Dan, T.; Jin, L.; Champ, C.; Zhao, S.; Lim, M.; et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 2013, 12, 1955–1963. [Google Scholar] [CrossRef]
- Simone, B.A.; Dan, T.; Palagani, A.; Jin, L.; Han, S.Y.; Wright, C.; Savage, J.E.; Gitman, R.; Lim, M.K.; Palazzo, J.; et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle 2016, 15, 2265–2274. [Google Scholar] [CrossRef]
- Agostini, D.; Natalucci, V.; Baldelli, G.; De Santi, M.; Zeppa, S.D.; Vallorani, L.; Annibalini, G.; Lucertini, F.; Federici, A.; Izzo, R.; et al. New Insights into the Role of Exercise in Inhibiting mTOR Signaling in Triple-Negative Breast Cancer. Oxid. Med. Cell. Longev. 2018, 2018, 5896786. [Google Scholar] [CrossRef]
- Haus, E. Chronobiology of the mammalian response to ionizing radiation potential applications in oncology. Chronobiol. Int. 2002, 19, 77–100. [Google Scholar] [CrossRef]
- Negoro, H.; Iizumi, T.; Mori, Y.; Matsumoto, Y.; Chihara, I.; Hoshi, A.; Sakurai, H.; Nishiyama, H.; Ishikawa, H. Chronoradiation Therapy for Prostate Cancer: Morning Proton Beam Therapy Ameliorates Worsening Lower Urinary Tract Symptoms. J. Clin. Med. 2020, 9, 2263. [Google Scholar] [CrossRef]
- Shuboni-Mulligan, D.D.; Breton, G.; Smart, D.; Gilbert, M.; Armstrong, T.S. Radiation chronotherapy—Clinical impact of treatment time-of-day: A systematic review. J. Neuro-Oncol. 2019, 145, 415–427. [Google Scholar] [CrossRef]
- Dallmann, R.; Okyar, A.; Lévi, F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol. Med. 2016, 22, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Phillips, C.L.; Wong, K.; McLachlan, A.J.; Saini, B. Timing of Administration: For Commonly-Prescribed Medicines in Australia. Pharmaceutics 2016, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Tamai, T.K.; Nakane, Y.; Ota, W.; Kobayashi, A.; Ishiguro, M.; Kadofusa, N.; Ikegami, K.; Yagita, K.; Shigeyoshi, Y.; Sudo, M.; et al. Identification of circadian clock modulators from existing drugs. EMBO Mol. Med. 2018, 10, e8724. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.M.; Colwell, C.S. How to fix a broken clock. Trends Pharmacol. Sci. 2013, 34, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, N.; Vandermeer, B.; Pandya, R.; Hooton, N.; Tjosvold, L.; Hartling, L.; Baker, G.; Vohra, S.; Klassen, T. Melatonin for treatment of sleep disorders. Evid. Rep. Technol. Assess. Summ. 2004, 108, 1–7. [Google Scholar]
- Carocci, A.; Catalano, A.; Sinicropi, M.S. Melatonergic drugs in development. Clin. Pharmacol. Adv. Appl. 2014, 6, 127–137. [Google Scholar] [CrossRef]
- Ben-David, M.A.; Elkayam, R.; Gelernter, I.; Pfeffer, R.M. Melatonin for Prevention of Breast Radiation Dermatitis: A Phase II, Prospective, Double-Blind Randomized Trial. Isr. Med. Assoc. J. 2016, 18, 188–192. [Google Scholar]
- Innominato, P.F.; Lim, A.S.; Palesh, O.; Clemons, M.; Trudeau, M.; Eisen, A.; Wang, C.; Kiss, A.; Pritchard, K.I.; Bjarnason, G.A. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Support. Care Cancer 2015, 24, 1097–1105. [Google Scholar] [CrossRef]
- Madsen, M.T.; Hansen, M.V.; Andersen, L.T.; Hageman, I.; Rasmussen, L.S.; Bokmand, S.; Rosenberg, J.; Gögenur, I. Effect of Melatonin on Sleep in the Perioperative Period after Breast Cancer Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Sleep Med. 2016, 12, 225–233. [Google Scholar] [CrossRef]
- Johnson, J.A.; Garland, S.N.; Carlson, L.E.; Savard, J.; Simpson, J.S.A.; Ancoli-Israel, S.; Campbell, T.S. Bright light therapy improves cancer-related fatigue in cancer survivors: A randomized controlled trial. J. Cancer Surviv. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Farhood, B.; Goradel, N.H.; Mortezaee, K.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Mahyari, H.M.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; et al. Melatonin as an adjuvant in radiotherapy for radioprotection and radiosensitization. Clin. Transl. Oncol. 2018, 21, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Campa, C.; Gonzalez, A.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E.J.; Cos, S. Melatonin enhances the inhibitory effect of aminoglutethimide on aromatase activity in MCF-7 human breast cancer cells. Breast Cancer Res. Treat. 2005, 94, 249–254. [Google Scholar] [CrossRef]
- Wilson, S.T.; Blask, D.E.; Lemus-Wilson, A.M. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J. Clin. Endocrinol. Metab. 1992, 75, 669–670. [Google Scholar] [CrossRef]
- Lissoni, P.; Ardizzoia, A.; Barni, S.; Paolorossi, F.; Tancini, G.; Meregalli, S.; Esposti, D.; Zubelewicz, B.; Braczowski, R. A randomized study of tamoxifen alone versus tamoxifen plus melatonin in estrogen receptor-negative heavily pretreated metastatic breast-cancer patients. Oncol. Rep. 1995, 2, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Marzouk, M.A.; Adhikari, S.; Wright, T.D.; Miller, B.P.; Matossian, M.D.; Elliott, S.; Wright, M.K.; Alzoubi, M.S.; Collins-Burow, B.M.; et al. Pharmacological, Mechanistic, and Pharmacokinetic Assessment of Novel Melatonin-Tamoxifen Drug Conjugates as Breast Cancer Drugs. Mol. Pharmacol. 2019, 96, 272–296. [Google Scholar] [CrossRef]
- Selfridge, J.M.; Gotoh, T.; Schiffhauer, S.; Liu, J.; Stauffer, P.E.; Li, A.; Capelluto, D.G.; Finkielstein, C.V. Chronotherapy: Intuitive, sound, founded but not broadly applied. Drugs 2016, 76, 1507–1521. [Google Scholar] [CrossRef]
- Bermúdez-Guzmán, L.; Blanco-Saborío, A.; Ramírez-Zamora, J.; Lovo, E. The Time for Chronotherapy in Radiation Oncology. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Haus, E.; Halberg, F.; Loken, M.; Kim, Y. Circadian Rhythmometry of Mammalian Radiosensitivity; Academic Press, Inc.: Cambridge, MA, USA, 1974. [Google Scholar]
- Dijk, D.-J.; Duffy, J.F. Novel Approaches for Assessing Circadian Rhythmicity in Humans: A Review. J. Biol. Rhythm. 2020, 35, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, R.; Node, K.; Akashi, M. Estimation methods for human circadian phase by use of peripheral tissues. Hypertens. Res. 2016, 39, 623–627. [Google Scholar] [CrossRef]
- Lee, K.-M.; Jung, D.-Y.; Hwang, H.; Kim, W.-H.; Lee, J.-Y.; Kim, T.-Y.; Im, S.-A.; Lee, K.-H.; Spiegel, D.; Hahm, B.-J. Late chronotypes are associated with neoadjuvant chemotherapy-induced nausea and vomiting in women with breast cancer. Chronobiol. Int. 2017, 34, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Ma, Q.; Rorsales-Corral, S.; de Almeida Chuffa, L.G. Melatonin inhibits Warburg-dependent cancer by redirecting glucose oxidation to the mitochondria: A mechanistic hypothesis. Cell. Mol. Life Sci. 2020, 77, 2527–2542. [Google Scholar] [CrossRef] [PubMed]
- Klevecz, R.R.; Braly, P.S. Circadian and Ultradian Cytokinetic Rhythms of Spontaneous Human Cancer. Ann. N. Y. Acad. Sci. 1991, 618, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Suo, Y.; Fu, Y.; Zhang, F.; Ding, N.; Pang, K.; Xie, C.; Weng, X.; Tian, M.; He, H.; et al. In vivo flow cytometry reveals a circadian rhythm of circulating tumor cells. Light Sci. Appl. 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Geerdink, M.; Walbeek, T.J.; Beersma, D.G.M.; Hommes, V.; Gordijn, M.C.M. Short Blue Light Pulses (30 Min) in the Morning Support a Sleep-Advancing Protocol in a Home Setting. J. Biol. Rhythm. 2016, 31, 483–497. [Google Scholar] [CrossRef]
- Ángeles-Castellanos, M.; Amaya, J.M.; Salgado-Delgado, R.; Buijs, R.M.; Escobar, C. Scheduled Food Hastens Re-Entrainment More Than Melatonin Does after a 6-h Phase Advance of the Light-Dark Cycle in Rats. J. Biol. Rhythm. 2011, 26, 324–334. [Google Scholar] [CrossRef]
- Scheer, F.; Wright, K.P., Jr.; Kronauer, R.E.; Czeisler, C.A. Plasticity of the Intrinsic Period of the Human Circadian Timing System. PLoS ONE 2007, 2, e721. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Krusen, B.M.; Nguyen, H.C.B.; Teng, C.W.; Woodie, L.N.; Tackenberg, M.C.; Geisler, C.E.; Gaisinsky, J.; Peed, L.C.; Carpenter, B.J.; et al. REV-ERB nuclear receptors in the suprachiasmatic nucleus control circadian period and restrict diet-induced obesity. Sci. Adv. 2021, 7, eabh2007. [Google Scholar] [CrossRef]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553, 351–355. [Google Scholar] [CrossRef]




| a. Positive Circadian Proteins | |||
| Core Clock Protein | Most Relevant Isoforms | Role in Core Clock Network | Possible Roles in Breast Cancer |
| Aryl hydrocarbon receptor nuclear translocator like Alias: Brain and muscle ARNT-like | ARNTL (BMAL1) | BMAL1:CLOCK binds E-box to promote transcription of clock-controlled genes | Maintains circadian amplitude Enables hypoxia response Regulates fatty acid oxidation Decreases with senescence [24,29,33,34] |
| Clock circadian regulator Alias: Circadian locomotor output cycles kaput (mouse) homolog | CLOCK | BMAL1:CLOCK binds E-box to promote transcription of clock-controlled genes | Associated with BC risk/incidence Regulates circadian acetylation Protooncogenic c-MYC regulation Suppressor WEE1 regulation G2/M transition gating [3,27,35,36,37] |
| RAR related orphan receptor Alias: Retinoic acid receptor-related orphan receptor | RORA (RORα) | RORα binds RRE to promote transcription of BMAL1 | Antitumor activity Anti-inflammatory activity G1 completion gating [38,39] |
| b. Negative Circadian Proteins | |||
| Core Clock Protein | Most Relevant Isoforms | Role in Core Clock Network | Possible Roles in Breast Cancer |
| Period circadian regulator | PER1 PER2 PER3  | PER:CRY heterodimers suppress BMAL1:CLOCK-mediated transcription | Decreased expression in BC Predict response/outcomes in BC Inhibits cancer cell growth Inhibits tumorigenesis Required for ATM:CHEK2 DDR Required for radiation-induced clock gene upregulation, DDR Adipocyte differentiation G0 cell cycle exit [3,27,39,40,41,42,43,44,45,46,47,48,49,50] |
| Cryptochrome circadian regulator | CRY1 CRY2  | PER:CRY heterodimers suppress BMAL1:CLOCK-mediated transcription | Decreases with ↑BC stage Inhibits reactive tumor formation p53 Signaling, G2/M gating ER status of BC Shift work, BC incidence Reduces MYC tumor burden Counteracts hypoxia response Required for ATR:CHEK1 replication fork stress response [3,29,36,37,38,39,51] |
| Nuclear receptor subfamily 1 group D member 1 Alias: REV-ERBα | NR1D1 (REV-ERBα) | REV-ERBα binds RRE to suppress transcription of BMAL1 | Selective lethality to BC cells Cancer cell lethality Glucose consumption G1/S transition enabling [3,38,39,52] |
| Basic helix-loop-helix family member e41/42 Aliases: Differentially expressed in chondrocytes (DEC), SHARP, STRA13 | BHLHE40 (DEC1) BHLHE41 (DEC2)  | DEC is transcribed from E-box promoter, then binds E-box to prevent binding of BMAL1:CLOCK | BC tumor suppression, outcomes, receptor status Delayed S phase in BC cells Epithelial-to-mesenchymal transition [32,53,54] |
| a. Human Breast Cancer Association Studies | |||
| Study | Type | Exposure | Conclusion |
| Circadian disrupting exposures and breast cancer risk: a meta-analysis [75] | Meta-analysis | Shift work, short sleep duration, employment as flight attendant | Circadian disruption is associated with an increased breast cancer risk in women. (RR = 1.14; 95% CI 1.08–1.21). |
| Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies [76] | Two prospective cohort studies (NHS I and II) | Night shift work | Long term night shift work had a higher risk of breast cancer. Pronounced with shift work during young adulthood. (HR = 2.15, 95% CI: 1.23, 3.73 |
| Night-shift work and breast and prostate cancer risk: updating the evidence from epidemiological studies. Night-shift work and breast and prostate cancer risk: updating the evidence from epidemiological studies [77] | Meta-analysis | Night shift work | Risk is inconclusive and more studies are required |
| Evaluating the Association between Artificial Light-at-Night Exposure and Breast and Prostate Cancer Risk in Spain (MCC-Spain Study) [78] | Population based multi case-control study | Artificial light at night | Prostate and breast cancer were associated with high estimated exposure to outdoor light at night |
| Night Shift Work and Risk of Breast Cancer in Women [79] | Case-control | Night shift work | Positively associated night shift work with breast cancer. (OR = 8.58; 95% CI: 2.19–33.8) |
| Outdoor light at night at residences and breast cancer risk in Canada [80] | Population based case-control study | Outdoor light at night | Outdoor light at night has a small effect or no effect on breast cancer risk |
| NTP Cancer Hazard Assessment Report on Night Shift Work and Light at Night [81] | Systematic review | Night shift work, light at night | Likely causal relationship of persistent night shift work, particularly in young adults, to developing breast cancer |
| b. Studies Including Genetic Variants | |||
| Study | Type | Exposure | Outcome |
| Period3 structural variation: a circadian biomarker associated with breast cancer in young women [82] | Case-control study | PER3 variation | Increased risk of breast cancer in premenopausal women |
| Circadian genes and breast cancer susceptibility in rotating shift workers [83] | Prospective cohort | Shift work | Common variation in circadian genes play at most a small role in breast cancer risk among women of European ancestry. Neuronal PAS domain protein 2 (NPAS2) was strongly associated with breast cancer risk (p-value = 0.0005) |
| Breast cancer risk, night work, and circadian clock gene polymorphisms [84] | Population-based case-control study in France | Night shift work | Circadian clock gene variants modulate breast cancer risk. SNPs in RORA (rs1482057 and rs12914272) and in CLOCK were associated with breast cancer risk. |
| Circadian gene variants and breast cancer [85] | Epidemiological studies cited | Light at night | Circadian gene variants are significantly associated with breast cancer risk. BMAL1, BMAL2, CLOCK, NPAS2, CRY1, CRY2, PER1, PER3 and TIMELESS. |
| BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work [86] | Cohort study | Night shift work | Lower BRCA1 and BRCA2 expression were found in a group of shift workers. It may be one of the potential factors related to the higher risk of breast cancer. |
| Time of Radiotherapy for Breast Cancer | ||||
|---|---|---|---|---|
| Cancer Cite and Study | Type | Timing | Radiation | Endpoints and Findings |
| Breast Genetic Variants Predict Optimal Timing of Radiotherapy to Reduce Side-effects in Breast Cancer Patients | Prospective cohort (n = 343) | Before vs. after 12:00 pm (≥66% of total dose) | 50 Gy in 25 fractions, 40 Gy in 15 fractions | Acute skin toxicity, late skin toxicity, clock gene alleles: Morning radiation increased acute and late breast erythema Effect of radiation time on late toxicity depended on PER3, NOCT alleles (p = 0.03) (Late preference) |
|
Breast Comparison of acute skin reaction following morning versus late afternoon radiotherapy in patients with breast cancer who have undergone curative surgical resection | Retrospective (n = 395) | Before 10:00 am vs. after 15:00 | 50.4 Gy in 28 fractions before 2003, 50 Gy in 25 fractions | Acute skin reaction, survival, treatment failure: Afternoon radiation increased grade 2+ acute skin toxicity (p = 0.0088) No difference in treatment failure or survival outcomes (Early preference) |
| Patient Factors | Treatment Factors |
|---|---|
| Circadian phase, biomarkers * | Time of day, narrow ranges with significant gaps |
| Chronotype | Primary cancer site |
| Clock gene analysis | Disease stage, grade, mutations |
| Demographics, exposure history | Radiation dose, modality, fractionation |
| Radiation toxicity, tumor control | Consistency of radiation times, carryover effects |
| Microbiome analysis | Adjuvant/definitive therapies, immune suppression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, N.; Lombardo, J.; Matlack, L.; Smith, A.; Hines, K.; Shi, W.; Simone, N.L. Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology. Int. J. Mol. Sci. 2022, 23, 1331. https://doi.org/10.3390/ijms23031331
Nelson N, Lombardo J, Matlack L, Smith A, Hines K, Shi W, Simone NL. Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology. International Journal of Molecular Sciences. 2022; 23(3):1331. https://doi.org/10.3390/ijms23031331
Chicago/Turabian StyleNelson, Nicolas, Joseph Lombardo, Lauren Matlack, Alexandria Smith, Kamryn Hines, Wenyin Shi, and Nicole L. Simone. 2022. "Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology" International Journal of Molecular Sciences 23, no. 3: 1331. https://doi.org/10.3390/ijms23031331
APA StyleNelson, N., Lombardo, J., Matlack, L., Smith, A., Hines, K., Shi, W., & Simone, N. L. (2022). Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology. International Journal of Molecular Sciences, 23(3), 1331. https://doi.org/10.3390/ijms23031331






