Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy
Abstract
1. Introduction
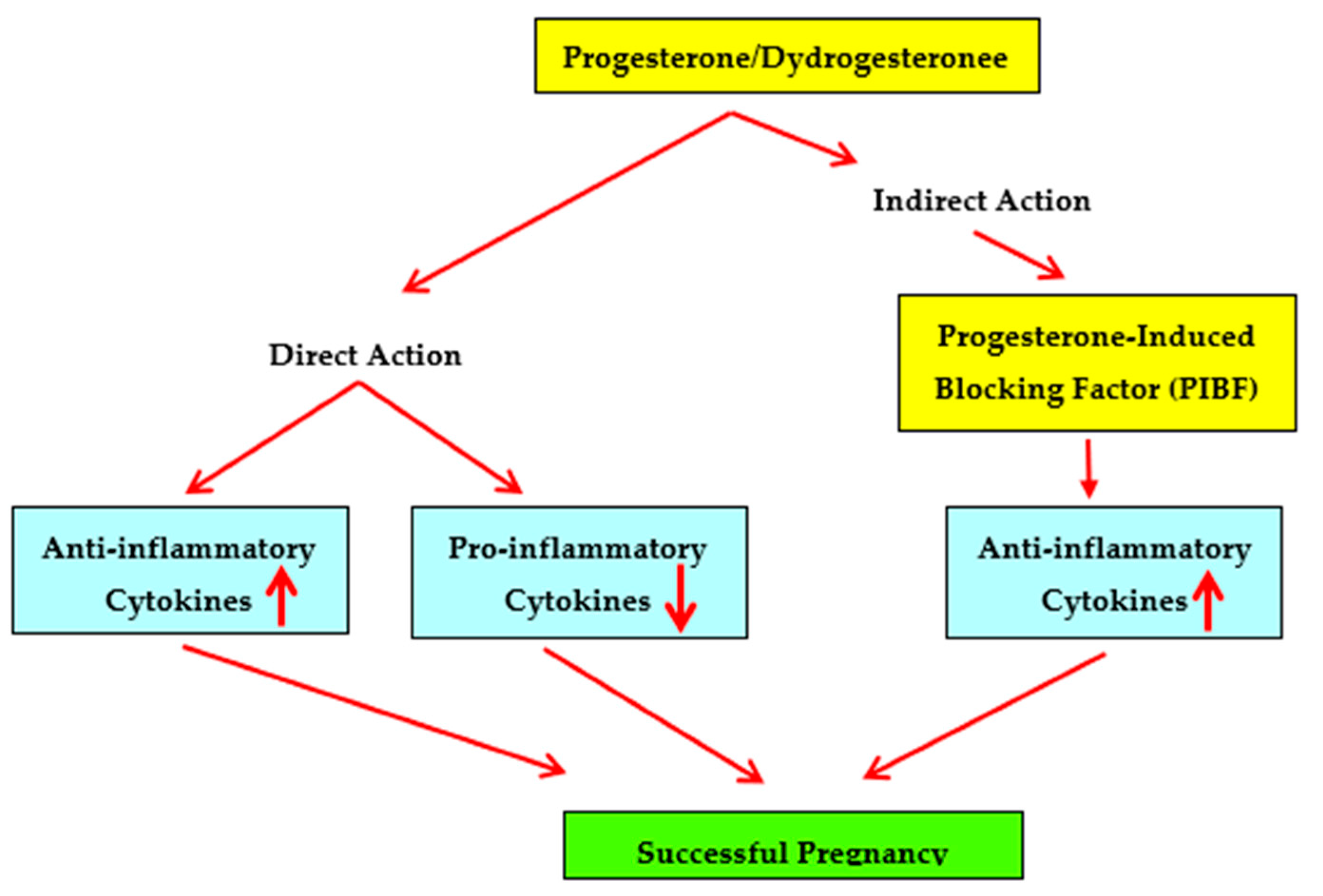
2. Immunosuppressive Capabilities of Progesterone
3. Cytokines and Pregnancy Complications
3.1. Recurrent Spontaneous Miscarriage
3.2. Pre-Eclampsia
3.3. Preterm Delivery
4. Supplementation with Progestogens
4.1. Supplementation with Progesterone
4.2. Supplementation with the Oral Progestogen Dydrogesterone
| Ref. No. | Type of Study | Outcome of Study |
|---|---|---|
| Supplementation with Progesterone | ||
| [92] | Meta-analysis of 14 trials (2003) | No difference in risk of miscarriage |
| [93] | Update of above study (2008) | Reduced rate of miscarriage |
| [94] | Critical evaluation of randomized, placebo-controlled trials | Increase in live-birth rate |
| [95] | Meta-analysis of 12 trials | Reduction in number of miscarriages as compared to placebo |
| [97] | Randomized, placebo-controlled study on women with uRSM * | No evidence of improved live-birth rate |
| Supplementation with Progesterone | ||
| [111] | Placebo-controlled study on dydrogesterone supplementation in women with uRSM | Fewer miscarriages as compared to placebo |
| [112] | Prospective, open, randomized study on women with uRSM | Significant reduction in miscarriage |
| [113] | Randomized, double-blind, placebo-controlled study | Significant decrease in number of miscarriages, increase in mean gestational age at delivery |
| [114] | Meta-analyses of studies on dydrogesterone supplementation | Significant reduction in odds for miscarriage |
| [115] | Meta-analysis of 13 studies on dydrogesterone supplementation | Significantly higher pregnancy rate |
| [116] | Systemic review and meta-analysis | Significant reduction in rate of miscarriage |
5. Progesterone-Induced Blocking Factor (PIBF)
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IFN | Interferon |
| IL | Interleukin |
| PBMC | Peripheral blood mononuclear cells |
| PE | Pre-eclampsia |
| PIBF | Progesterone-induced blocking factor |
| PR | Progesterone receptor |
| PTD | Preterm labor |
| RSM | Recurrent spontaneous miscarriage |
| Th | T helper |
| TNF | Tumor necrosis factor |
References
- Siiteri, P.K.; Febres, F.; Clemens, L.E.; Chang, R.J.; Gondos, B.; Stites, D. Progesterone and maintenance of pregnancy: Is progesterone nature’s immunosuppressant? Ann. N. Y. Acad. Sci. 1977, 286, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Watnick, A.S.; Russo, R.A. Survival of skin homografts in uteri of pregnant and progesterone-estrogen treated rats. Proc. Soc. Exp. Biol. Med. 1968, 128, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.J.; Bazer, F.W.; Segerson, E.C. Skin graft survival in the uterine lumen of ewes treated with progesterone. Am. J. Reprod. Immunol. Microbiol. 1986, 12, 48–54. [Google Scholar] [CrossRef]
- Black, W.G.; Simon, J.; McNutt, S.H.; Casida, L.E. Investigations on the physiological basis for the differential response of estrous and pseudopregnant rabbit uteri to induced infection. Am. J. Vet. Res. 1953, 14, 318–323. [Google Scholar]
- Rowson, L.E.A.; Lamming, G.E.; Fry, R.M. The relationship between ovarian hormones and uterine infection. Vet. Rec. 1953, 65, 335–340. [Google Scholar]
- Hansen, P.J. Regulation of uterine immune function by progesterone—Lessons from the sheep. J. Reprod. Immunol. 1998, 40, 63–79. [Google Scholar] [CrossRef]
- Jones, L.A.; Kreem, S.; Shweash, M.; Paul, A.; Alexander, J.; Roberts, C.W. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J. Immunol. 2010, 185, 4525–4534. [Google Scholar] [CrossRef]
- Menzies, F.M.; Henriquez, F.L.; Alexander, J.; Roberts, C.W. Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology 2011, 134, 281–291. [Google Scholar] [CrossRef]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front Immunol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Butts, C.L.; Shukair, S.A.; Duncan, K.M.; Bowers, E.; Horn, C.; Belyavskaya, E.; Tonelli, L.; Sternberg, E.M. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int. Immunol. 2007 19, 287–296. [CrossRef]
- Jones, L.A.; Anthony, J.P.; Henriquez, F.L.; Lyons, R.E.; Nickdel, M.B.; Carter, K.C.; Alexander, J.; Roberts, C.W. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology 2008, 125, 59–69. [Google Scholar] [CrossRef]
- Su, L.; Sun, Y.; Ma, F.; Lü, P.; Huang, H.; Zhou, J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol. Lett. 2009, 125, 151–1555. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadou, N.; Tucker, L.; Anderson, D.J. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J. Immunol. 1999, 162, 7510–7518. [Google Scholar] [PubMed]
- Thiele, K.; Hierweger, A.M.; Riquelme, J.I.A.; Solano, M.E.; Lydon, J.P.; Arck, P.C. Impaired progesterone-responsiveness of CD11c(+) dendritic cells affects the generation of CD4(+) regulatory T cells and is associated with intrauterine growth restriction in mice. Front. Endocrinol. 2019, 10, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Csabai, T.; Pallinger, E.; Kovacs, A.F.; Miko, E.; Bognar, Z.; Szekeres-Bartho, J. Altered immune response and implantation failure in progesterone-induced blocking factor-deficient mice. Front. Immunol. 2020, 11, 349–356. [Google Scholar] [CrossRef]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-related immune modulation of pregnancy and labor. Front. Endocrinol. 2019, 10, 198–210. [Google Scholar] [CrossRef]
- Li, X.; O’Malley, B.W. Unfolding the action of progesterone receptors. J. Biol. Chem. 2003, 278, 39261–39264. [Google Scholar] [CrossRef]
- Mulac-Jericevic, B.; Mullinax, R.A.; DeMayo, F.J.; Lydon, J.P.; Conneely, O.M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000, 289, 1751–1754. [Google Scholar] [CrossRef]
- Chien, E.J.; Liao, C.F.; Chang, C.P.; Pu, H.F.; Lu, L.M.; Shie, M.C.; Hsieh, D.J.; Hsu, M.T. The non-genomic effects on Na(+)/H(+)-exchange 1 by progesterone and 20alpha-hydroxyprogesterone in human T cells. J. Cell Physiol. 2007, 211, 544–550. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Barakonyi, A.; Miko, E.; Polgar, B.; Palkovics, T. The role of γ/δ T cells in the feto-maternal relationship. Semin. Immunol. 2000, 13, 229–233. [Google Scholar] [CrossRef]
- Arruvito, L.; Giulianelli, S.; Flores, A.C.; Paladino, N.; Barboza, M.; Lanari, C.; Fainboim, L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J. Immunol. 2008, 180, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Saunders, P.T.; Moffet-King, A.; Grrome, N.O.; Critchley, H.O. Steroid receptor expression in uterine natural killer cells. J. Clin. Endocrinol. Metabol. 2003, 88, 440–449. [Google Scholar] [CrossRef]
- Chien, H.; Guo, W.; Li, P.; Zhao, G.; Fan, H.; Hu, Y.; Hou, Y. Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am. J. Reprod. Immunol. 2012, 67, 463–473. [Google Scholar]
- Gellersen, B.; Brosens, J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: A decidualizing affair. J. Endocrinol. 2003, 178, 357–372. [Google Scholar] [CrossRef]
- Florio, P.; Rossi, M.; Viganò, P.; Luisi, S.; Torricelli, M.; Torres, P.B.; Di Blasio, A.M.; Petraglia, F. Interleukin 1beta and progesterone stimulate activin a expression and secretion from cultured human endometrial stromal cells. Reprod. Sci. 2007, 14, 29–36. [Google Scholar] [CrossRef]
- Hantak, A.M.; Bagchi, I.C.; Bagchi, M.K. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 2014, 58, 139–146. [Google Scholar] [CrossRef]
- Vota, D.; Aguero, M.; Grasso, E.; Hauk, V.; Gallino, L.; Soczewski, E.; Pérez Leirós, C.; Ramhorst, R. Progesterone and VIP cross-talk enhances phagocytosis and anti-inflammatory profile in trophoblast-derived cells. Mol. Cell. Endocrinol. 2017, 443, 146–154. [Google Scholar] [CrossRef]
- Fujiwara, H. Immune cells contribute to systemic cross-talk between the embryo and mother during early pregnancy in cooperation with the endocrine system. Reprod. Med. Biol. 2006, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Verma, R.; Nair, R.R.; Budhwar, S.; Khanna, A.; Agrawal, N.R.; Sinha, R.; Birendra, R.; Rajender, S.; Singh, K. Altered crosstalk of estradiol and progesterone with myeloid-derived suppressor cells and Th1/Th2 cytokines in early miscarriage is associated with early breakdown of maternal-fetal tolerance. Am. J. Reprod. Immunol. 2019, 81, e13081. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hou, H.; Zhao, W.; Wang, J.; Peng, Q. Administration of exogenous progesterone protects against Brucella abortus infection-induced inflammation in pregnant mice. J. Infect. Dis. 2021, 224, 532–543. [Google Scholar] [CrossRef]
- Kirshenbaum, M.; Orvieto, R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J. Assist. Reprod. Genet. 2019, 36, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.S.; Dhama, K.; Chakraborty, S.; Samad, H.A.; Latheef, S.K.; Sharun, K.; Khurana, S.K.; Tiwari, R.; Bhatt, P.K.V.; Chaicumpa, W. Role of antisperm antibodies in infertility, pregnancy, and potential for contraceptive and antifertility vaccine designs: Research progress and pioneering vision. Vaccines 2019, 7, 116. [Google Scholar]
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Choubey, D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J. Interferon Cytokine Res. 2011, 10, 695–703. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The cytokines of asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T helper cell subsets and related human immunological disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. T cell subpopulations. Chem. Immunol. Allergy 2014, 100, 155–164. [Google Scholar]
- Mosmann, T.R.; Kobie, J.J.; Lee, F.E.; Quataert, S.A. T helper cytokine patterns: Defined subsets, random expression, and external modulation. Immunol. Res. 2009, 45, 173–184. [Google Scholar] [CrossRef]
- Kim, C.J.; Romero, R.; Chaemsaithong, P.; Kim, J.S. Chronic inflammation of the placenta: Definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015, 213, S53–S69. [Google Scholar] [CrossRef]
- Tan, H.X.; Yang, S.L.; Li, M.Q.; Wang, H.Y. Autophagy suppression of trophoblast cells induces pregnancy loss by activating decidual NK cytotoxicity and inhibiting trophoblast invasion. Cell Commun. Signal. 2020, 18, 73–80. [Google Scholar] [CrossRef]
- Chaouat, G.; Menu, E.; Clark, D.A.; Dy, M.; Minkowski, M.; Wegmann, T.G. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J. Reprod. Fertil. 1990, 89, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Haimovici, F.; Hill, J.A.; Anderson, D.J. The effects of soluble products of activated lymphocytes and macrophages on blastocyst implantation events in vitro. Biol. Reprod. 1991, 44, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg., R.; Luyten, C.; Vercruysse, L.; Keith, J.C., Jr.; Van Assche, F.A. Cytotoxic effects of tumour necrosis factor (TNF)-α and interferon -γ on cultured human trophoblast are modulated by fibronectin. Mol. Hum. Reprod. 2000, 6, 635–641. [Google Scholar] [CrossRef][Green Version]
- Kwak-Kim, J.; Bao, S.; Lee, S.K.; Kim, J.W.; Gilman-Sachs, A. Immunological modes of pregnancy loss: Inflammation, immune effectors, and stress. Am. J. Reprod. Immunol. 2014, 72, 129–140. [Google Scholar] [CrossRef]
- Hill, J.A.; Polgar, K.; Anderson, D.J. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA 1995, 273, 1933–1936. [Google Scholar] [CrossRef]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta, M.; Farhat, R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 713–718. [Google Scholar] [CrossRef]
- Makhseed, M.; Raghupathy, R.; Azizieh, F.; Al-Azemi, M.M.; Hassan, N.A.; Bandar, A. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am. J. Reprod. Immunol. 1999, 42, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Hassan, N.; Al-Azemi, M.; Al-Shamali, E. Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions. Cell Immunol. 1999, 196, 122–130. [Google Scholar] [CrossRef]
- Makhseed, M.; Raghupathy, R.; Azizieh, F.; Omu, A.; Al-Shamali, E.; Ashkanani, L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod. 2001, 16, 2219–2226. [Google Scholar] [CrossRef]
- Marzi, M.; Vigano, A.; Trabattoni, D.; Villa, M.L.; Salvaggio, A.; Clerici, E.; Clerici, M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 1996, 106, 127–133. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Lombardelli, L.; Logiodice, F.; Kullolli, O.; Romagnani, S.; Le Bouteiller, P. T helper cell mediated-tolerance towards fetal allograft in successful pregnancy. Clin. Mol. Allergy 2015, 13, 9–18. [Google Scholar] [CrossRef]
- Banerjee, P.; Ghosh, S.; Dutta, M.; Subramani, E.; Khalpada, J.; Roychoudhury, S.; Chakravarty, B.; Chaudhury, K. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS ONE 2013, 8, e80940. [Google Scholar] [CrossRef]
- Malik, R.; Kumar, V. Hypertension in pregnancy. Adv. Exp. Med. Biol. 2017, 956, 375–393. [Google Scholar] [PubMed]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Robertson, W.B.; Brosens, I.; Dixon, H.G. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J. Pathol. Bacteriol. 1967, 93, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Equils, O.; Kellogg, C.; McGregor, J.; Gravett, M.; Neal-Perry, G.; Gabay, C. The role of the IL-1 system in pregnancy and the use of IL-1 system markers to identify women at risk for pregnancy complications. Biol. Reprod. 2020, 103, 684–694. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Corradetti, A.; Giannubilo, S.R. Placental cytokines in the pathogenesis of preeclampsia and HELLP syndrome. Curr. Womens Health Rev. 2008, 4, 280–285. [Google Scholar] [CrossRef]
- Weel, I.C.; Romão-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peraçoli, J.C.; Borges, V.T.; Araujo, J.P., Jr.; Peraçoli, M.T. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef]
- Sakai, M.; Tsuda, H.; Tanebe, K.; Sasaki, Y.; Saito, S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am. J. Reprod. Immunol. 2002, 47, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sheibak, N.; Mahmoudzadeh-Sagheb, H.; Moudi, B.; Heidari, Z. Elevated immunoexpression of interferon-gamma in placenta tissue samples from pregnancies complicated with preeclampsia compared to the placenta previa. Pregnancy Hypertens 2020, 22, 175–180. [Google Scholar] [CrossRef]
- Xu, J.; Gu, Y.; Sun, J.; Zhu, H.; Lewis, D.F.; Wang, Y. Reduced CD200 expression is associated with altered Th1/Th2 cytokine production in placental trophoblasts from preeclampsia. Am. J. Reprod. Immunol. 2018, 79, e12763. [Google Scholar] [CrossRef]
- Szarka, A.; Rigó, J., Jr.; Lázár, L.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59–66. [Google Scholar] [CrossRef]
- Saito, S.; Umekage, H.; Sakamoto, Y.; Sakai, M.; Tanebe, K.; Sasaki, Y.; Morikawa, H. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am. J. Reprod. Immunol. 1999, 41, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Rolinski, J.; Leszczynska-Goarzelak, B.; Oleszczuk, J. The expressions of intracellular cytokines in the lymphocytes of preeclamptic patients. Am. J. Reprod. Immunol. 2002, 48, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.T.; Schondorf, T.; Gohring, U.J.; Kurbacher, C.M.; Pinto, I.; Breidenbach, M.; Mallmann, P.; Kolhagen, H.; Engel, H. Cytokine expression in peripheral blood lymphocytes indicates a switch to T(HELPER) cells in patients with preeclampsia. J. Reprod. Immunol. 2002, 54, 133–142. [Google Scholar] [CrossRef]
- Luppi, P.; Deloia, J.A. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin. Immunol. 2006, 118, 268–275. [Google Scholar] [CrossRef]
- Boij, R.; Svensson, J.; Nilsson-Ekdahl, K.; Sandholm, K.; Lindahl, T.L.; Palonek, E.; Garle, M.; Berg, G.; Ernerudh, J.; Jenmalm, M.; et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am. J. Reprod. Immunol. 2012, 68, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Orange, S.; Horvath, J.; Hennessy, A. Preeclampsia is associated with a reduced interleukin-10 production from peripheral blood mononuclear cells. Hypertens Pregnancy 2003, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.Y.; Raghupathy, R. IL-10 and pregnancy complications. Clin. Exp. Obstet. Gynecol. 2017, 44, 252–258. [Google Scholar] [PubMed]
- Jonsson, Y.; Rubèr, M.; Matthiesen, L.; Berg, G.; Nieminen, K.; Sharma, S.; Ernerudh, J.; Ekerfelt, C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J. Reprod. Immunol. 2006, 70, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Azizieh, F.; Raghupathy, R.; Makhseed, M. Maternal cytokine production patterns in women with pre-eclampsia. Am. J. Reprod. Immunol. 2005, 54, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Cytokines as key players in the pathophysiology of preeclampsia. Med. Princ. Pract. 2013, 22 (Suppl. 1), 8–19. [Google Scholar] [CrossRef]
- Da Fonseca, E.B.; Damião, R.; Moreira, D.A. Preterm birth prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 40–49. [Google Scholar] [CrossRef]
- Lien, Y.C.; Zhang, Z.; Barila, G.; Green-Brown, A.; Elovitz, M.A.; Simmons, R.A. Intrauterine inflammation alters the transcriptome and metabolome in placenta. Front. Physiol. 2020, 11, 592689. [Google Scholar] [CrossRef]
- Makhseed, M.; Raghupathy, R.; El-Shazly, S.; Azizieh, F.; Al-Harmi, J.A.; Al-Azemi, M.M. Pro-inflammatory maternal cytokine profile in preterm delivery. Am. J. Reprod. Immunol. 2003, 49, 308–318. [Google Scholar] [CrossRef]
- Ashford, K.; Chavan, N.R.; Wiggins, A.T.; Sayre, M.M.; McCubbin, A.; Critchfield, A.S.; O’Brien, J. Comparison of serum and cervical cytokine levels throughout pregnancy between preterm and term births. AJP Rep. 2018, 8, e113–e120. [Google Scholar] [CrossRef]
- Park, H.; Park, K.H.; Kim, Y.M.; Kook, S.Y.; Jeon, S.J.; Yoo, H.N. Plasma inflammatory and immune proteins as predictors of intra-amniotic infection and spontaneous preterm delivery in women with preterm labor: A retrospective study. BMC Pregnancy Childbirth 2018, 18, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Denney, J.M.; Nelson, E.; Wadhwa, P.; Waters, T.; Mathew, L.; Goldenberg, R.L.; Culhane, J.F. Cytokine profiling: Variation in immune modulation with preterm birth vs. uncomplicated term birth identifies pivotal signals in pathogenesis of preterm birth. J. Perinat. Med. 2020, 49, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, I.; Zaragoza, D.B.; Guilbert, L.; Robertson, S.A.; Mitchell, B.F.; Olson, D.M. Inflammatory processes in preterm and term parturition. J. Reprod. Immunol. 2008, 79, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.Y.; Park, J.W.; Ryu, A.; Lee, S.Y.; Cho, S.H.; Park, K.H. Prediction of impending preterm delivery based on sonographic cervical length and different cytokine levels in cervicovaginal fluid in preterm labor. J. Obstet. Gynaecol. Res. 2016, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, S.; Makhseed, M.; Azizieh, F.; Raghupathy, R. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am. J. Reprod. Immunol. 2004, 52, 45–52. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Dudley, D.J. Immunoendocrinology of preterm labor: The link between corticotropin-releasing hormone and inflammation. Am. J. Obstet. Gynecol. 1999, 180 Pt 3, S251–S256. [Google Scholar] [CrossRef]
- Choi, B.C.; Polgar, K.; Xiao, L.; Hill, J.A. Progesterone inhibits in-vitro embryotoxic Th1 cytokine production to trophoblast in women with recurrent pregnancy loss. Hum. Reprod. 2000, 15, 46–59. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Raghupathy, R.; Saito, S.; Szekeres-Bartho, J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front. Immunol. 2021, 12, 717808. [Google Scholar] [CrossRef]
- Schindler, A.E. Progestogens for treatment and prevention of pregnancy disorders. Horm. Mol. Biol. Clin. Investig. 2010, 3, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Oates-Whitehead, R.M.; Haas, D.M.; Carrier, J.A. Progestogen for preventing miscarriage. Cochrane Database Syst. Rev. 2003, 4, CD003511. [Google Scholar]
- Haas, D.M.; Ramsey, P.S. Progestogen for preventing miscarriage. Cochrane Database Syst. Rev. 2008, 16, CD003511. [Google Scholar]
- Coomarasamy, A.; Devall, A.J.; Brosens, J.J.; Quenby, S.; Stephenson, M.D.; Sierra, S.; Christiansen, O.B.; Small, R.; Brewin, J.; Roberts, T.E.; et al. Micronized vaginal progesterone to prevent miscarriage: A critical evaluation of randomized evidence. Am. J. Obstet. Gynecol. 2020, 223, 167–176. [Google Scholar] [CrossRef]
- Haas, D.M.; Hathaway, T.J.; Ramsey, P.S. Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst. Rev. 2019, 2019, CD003511. [Google Scholar] [CrossRef]
- Carp, H.J.A. Progestogens and pregnancy loss. Climacteric 2018, 21, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Coomarasamy, A.; Williams, H.; Truchanowicz, E.; Seed, P.T.; Small, R.; Quenby, S.; Gupta, P.; Dawood, F.; Koot, Y.E.; Atik, R.B.; et al. PROMISE: First-trimester progesterone therapy in women with a history of unexplained recurrent miscarriages—A randomised, double-blind, placebo-controlled, international multicentre trial and economic evaluation. Health Technol. Assess. 2016, 20, 1–92. [Google Scholar] [CrossRef] [PubMed]
- Carp, H. Immunotherapy for recurrent pregnancy loss. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Schmouder, V.M.; Prescott, G.M.; Franco, A.; Fan-Havard, P. The rebirth of progesterone in the prevention of preterm labor. Ann. Pharmacother. 2013, 47, 527–536. [Google Scholar] [CrossRef]
- Dodd, J.M.; Crowther, C.A.; Cincotta, R.; Flenady, V.; Robinson, J.S. Progesterone supplementation for preventing preterm birth: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2005, 84, 526–533. [Google Scholar] [CrossRef]
- Matei, A.; Saccone, G.; Vogel, J.P.; Armson, A.B. Primary and secondary prevention of preterm birth: A review of systematic reviews and ongoing randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 224–239. [Google Scholar] [CrossRef]
- Wiegratz, I.; Kuhl, H. Metabolic and clinical effects of progestogens. Eur. J. Contracept. Reprod. Health Care 2006 11, 153–161. [CrossRef]
- Schindler, A.E. Progestogen effects on various organs and their functions. Gynecol. Endocrinol. 2007, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J.R.; Schweppe, K.W.; Thijssen, J.H. Classification and pharmacology of progestins. Maturitas 2003, 46, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R.; Al Mutawa, E.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Modulation of cytokine production by dydrogesterone in lymphocytes from women with recurrent miscarriage. BJOG 2005, 112, 1096–1101. [Google Scholar] [CrossRef]
- AbdulHussain, G.; Azizieh, F.; Makhseed, M.; Raghupathy, R. Effects of progesterone, dydrogesterone and estrogen on the production of Th1/Th2/Th17 cytokines by lymphocytes from women with recurrent spontaneous miscarriage. J. Reprod. Immunol. 2020, 140, 103132. [Google Scholar] [CrossRef]
- Xu, W.M.; Xiao, Z.N.; Wang, X.B.; Huang, Y. IL-17 induces fetal loss in a CBA/J×BALB/c mouse model, and an anti-IL-17 antibody prevents fetal loss in a CBA/J×DBA/2 mouse model. Am. J. Reprod. Immunol. 2016, 75, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Hao, C.F.; Yi-Lin; Yin, G.J.; Bao, S.H.; Qiu, L.H.; Lin, Q.D. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J. Reprod. Immunol. 2010, 84, 164–170. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, N.; Lin, J.; Wang, C.; Pan, X.; Chen, L.; Li, D.; Wang, L. Distinct pattern of Th17/Treg cells in pregnant women with a history of unexplained recurrent spontaneous abortion. Biosci. Trends 2018, 12, 157–167. [Google Scholar] [CrossRef]
- Raghupathy, R.; Szekeres-Bartho, J. Dydrogesterone and the immunology of pregnancy. Horm. Mol. Biol. Clin. Investig. 2016, 27, 63–71. [Google Scholar] [CrossRef]
- El-Zibdeh, M.Y. Dydrogesterone in the reduction of recurrent spontaneous abortion. J. Steroid. Biochem. Mol. Biol. 2005, 97, 431–434. [Google Scholar] [CrossRef]
- Pandian, R.U. Dydrogesterone in threatened miscarriage: A Malaysian experience. Maturitas 2009, 65, S47–S50. [Google Scholar] [CrossRef]
- Kumar, A.; Begum, N.; Prasad, S.; Aggarwal, S.; Sharma, S. Oral dydrogesterone treatment during early pregnancy to prevent recurrent pregnancy loss and its role in modulation of cytokine production: A double-blind, randomized, parallel, placebo-controlled trial. Fertil. Steril. 2014, 102, 1357–1363.e3. [Google Scholar] [CrossRef] [PubMed]
- Carp, H. A systematic review of dydrogesterone for the treatment of recurrent miscarriage. Gynecol. Endocrinol. 2015, 31, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lu, Q. Efficacy of dydrogesterone on treating recurrent miscarriage and its influence on immune factors: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 10971–10985. [Google Scholar] [CrossRef] [PubMed]
- Saccone, G.; Schoen, C.; Franasiak, J.M.; Scott, R.T., Jr.; Berghella, V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: A systematic review and meta-analysis of randomized, controlled trials. Fertil. Steril. 2017, 107, 430–438.e3. [Google Scholar] [CrossRef]
- Schindler, A.E. Present and future aspects of dydrogesterone in prevention or treatment of pregnancy disorders: An outlook. Horm. Mol. Biol. Clin. Investig. 2016, 27, 49–53. [Google Scholar] [CrossRef]
- Schindler, A.E. New data about preeclampsia: Some possibilities of prevention. Gynecol. Endocrinol. 2018, 34, 636–637. [Google Scholar] [CrossRef]
- Hudic, I.; Schindler, A.E.; Szekeres-Bartho, J.; Stray-Pedersen, B. Dydrogesterone and pre-term birth. Horm. Mol. Biol. Clin. Investig. 2016, 27, 81–83. [Google Scholar] [CrossRef]
- Mohamad Razi, Z.R.; Schindler, A.E. Review on role of progestogen (dydrogesterone) in the prevention of gestational hypertension. Horm. Mol. Biol. Clin. Investig. 2016, 27, 73–76. [Google Scholar] [CrossRef]
- Tskhay, V.; Schindler, A.; Shestakova, M.; Klimova, O.; Narkevich, A. The role of progestogen supplementation (dydrogesterone) in the prevention of preeclampsia. Gynecol. Endocrinol. 2020, 36, 698–701. [Google Scholar] [CrossRef]
- Ali, A.B.; Ahmad, M.F.; Kwang, N.B.; Shan, L.P.; Shafie, N.M.; Omar, M.H. Dydrogesterone support following assisted reproductive technique (ART) reduces the risk of pre-eclampsia. Horm. Mol. Biol. Clin. Investig. 2016, 27, 93–96. [Google Scholar] [CrossRef]
- Stute, P. Dydrogesterone indications beyond menopausal hormone therapy: An evidence review and woman’s journey. Gynecol. Endocrinol. 2021, 37, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.G.; Patki, A.; Pexman-Fieth, C. Dydrogesterone use in early pregnancy. Gynecol. Endocrinol. 2016, 32, 97–106. [Google Scholar] [CrossRef]
- Stanczyk, F.Z. Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev. Endocr. Metab. Disord. 2002, 3, 211–224. [Google Scholar] [CrossRef]
- Maxson, W.S.; Hargrove, J.T. Bioavailability of oral micronized progesterone. Fertil. Steril. 1985, 44, 622–626. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al-Azemi, M. Modulation of cytokine production by the dydrogesterone metabolite dihydrodydrogesterone. Am. J. Reprod. Immunol. 2015, 74, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Szekeres-Bartho, J.; Kilaŕ, F.; Falkay, G.; Csernus, V.; Török, A.; Pacsa, A.S. The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: I. Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am. J. Reprod. Immunol. Microbiol 1985, 9, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Kozma, N.; Halasz, M.; Polgar, B.; Poehlmann, T.G.; Markert, U.R.; Palkovics, T.; Keszei, M.; Par, G.; Kiss, K.; Szeberenyi, J.; et al. Progesterone-induced blocking factor activates STAT6 via binding to a novel IL-4 receptor. J. Immunol. 2006, 176, 819–826. [Google Scholar] [CrossRef]
- Szekeres-Bartho, J.; Wegmann, T.G. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J. Reprod. Immunol. 1996, 31, 81–95. [Google Scholar] [CrossRef]
- Raghupathy, R.; Al-Mutawa, E.; Al-Azemi, M.; Makhseed, M.; Azizieh, F.; Szekeres-Bartho, J. Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J. Reprod. Immunol. 2009, 80, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.; Hansen, P.J.; Mulac Jericevic, B.; Piccinni, M.P.; Szekeres-Bartho, J. Progesterone during pregnancy: Endocrine-immune cross talk in mammalian species and the role of stress. Am. J. Reprod. Immunol. 2007, 58, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.N.; Witcher, A.C.; Comley, K.; Cunningham, M.W., Jr.; Ibrahim, T.; Cornelius, D.C.; LaMarca, B.; Amaral, L.M. Progesterone-induced blocking factor improves blood pressure, inflammation, and pup weight in response to reduced uterine perfusion pressure (RUPP). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R719–R727. [Google Scholar] [CrossRef]
- Lim, M.K.; Ku, C.W.; Tan, T.C.; Lee, Y.H.J.; Allen, J.C.; Tan, N.S. Characterisation of serum progesterone and progesterone-induced blocking factor (PIBF) levels across trimesters in healthy pregnant women. Sci. Rep. 2020, 10, 3840. [Google Scholar] [CrossRef] [PubMed]
- Check, J.H.; Levin, E.; Bollendorf, A.; Locuniak, J. Miscarriage in the first trimester according to the presence or absence of the progesterone-induced blocking factor at three to five weeks from conception in progesterone supplemented women. Clin. Exp. Obstet. Gynecol. 2005, 32, 13–14. [Google Scholar]
- Liang, Q.; Tong, L.; Xiang, L.; Shen, S.; Pan, C.; Liu, C.; Zhang, H. Correlations of the expression of γδ T cells and their co-stimulatory molecules TIGIT, PD-1, ICOS and BTLA with PR and PIBF in the peripheral blood and decidual tissues of women with unexplained recurrent spontaneous abortion. Clin. Exp. Immunol. 2021, 203, 55–65. [Google Scholar] [CrossRef]
- Kalinka, J.; Szekeres-Bartho, J. The impact of dydrogesterone supplementation on hormonal profile and progesterone-induced blocking factor concentrations in women with threatened abortion. Am. J. Reprod. Immunol. 2005, 53, 166–171. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghupathy, R.; Szekeres-Bartho, J. Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy. Int. J. Mol. Sci. 2022, 23, 1333. https://doi.org/10.3390/ijms23031333
Raghupathy R, Szekeres-Bartho J. Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy. International Journal of Molecular Sciences. 2022; 23(3):1333. https://doi.org/10.3390/ijms23031333
Chicago/Turabian StyleRaghupathy, Raj, and Julia Szekeres-Bartho. 2022. "Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy" International Journal of Molecular Sciences 23, no. 3: 1333. https://doi.org/10.3390/ijms23031333
APA StyleRaghupathy, R., & Szekeres-Bartho, J. (2022). Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy. International Journal of Molecular Sciences, 23(3), 1333. https://doi.org/10.3390/ijms23031333





