10-Dehydrogingerdione Attenuates Tramadol-Induced Nephrotoxicity by Modulating Renal Oxidative Stress, Inflammation and Apoptosis in Experimental Rats: Role of HO-1 Activation and TLR4/NF-κB/ERK Inhibition
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Kidney Weight, and Kidney Weight to Body Weight Ratio
2.2. Serum Kidney Function Parameters
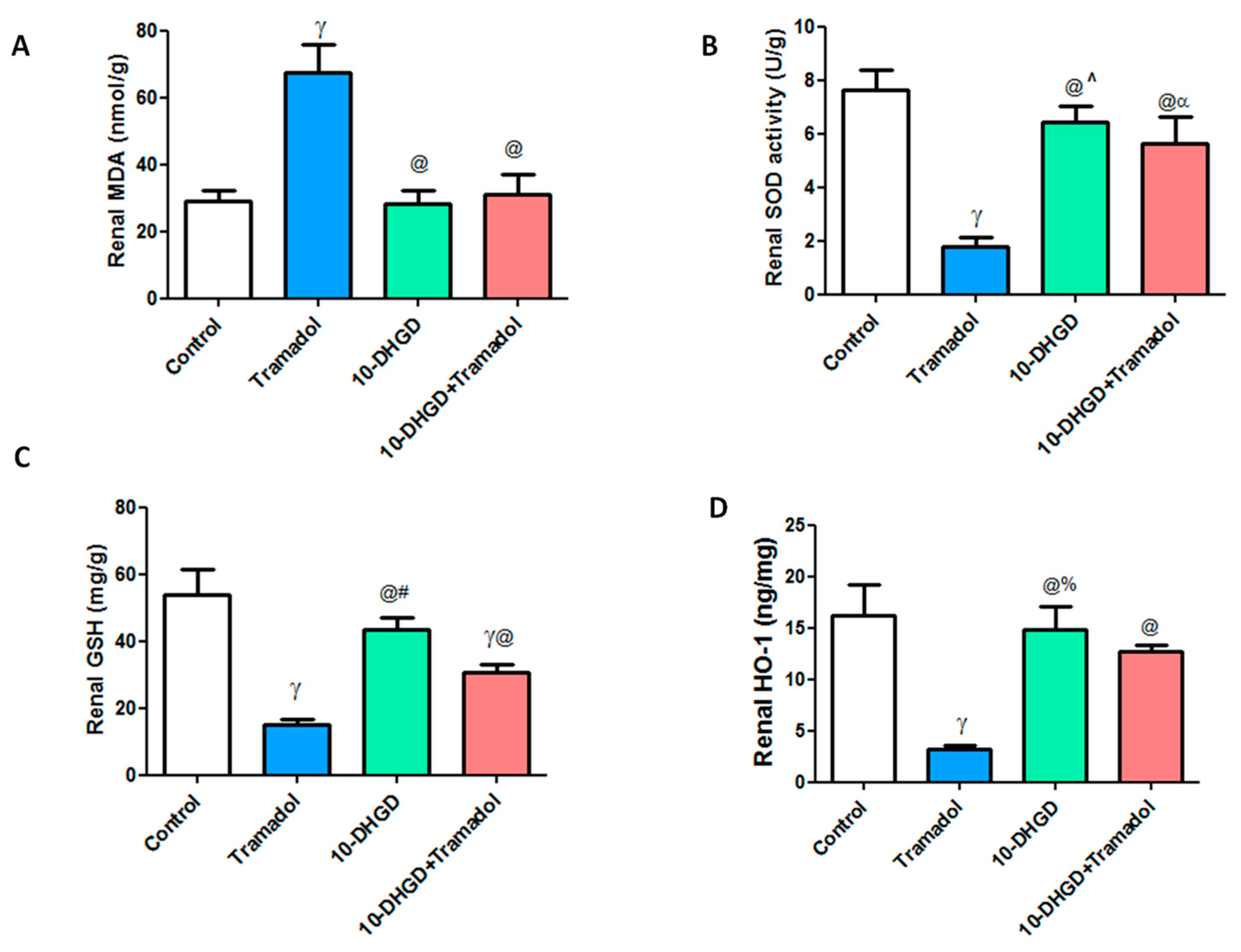
2.3. Renal Oxidative and Antioxidant Markers
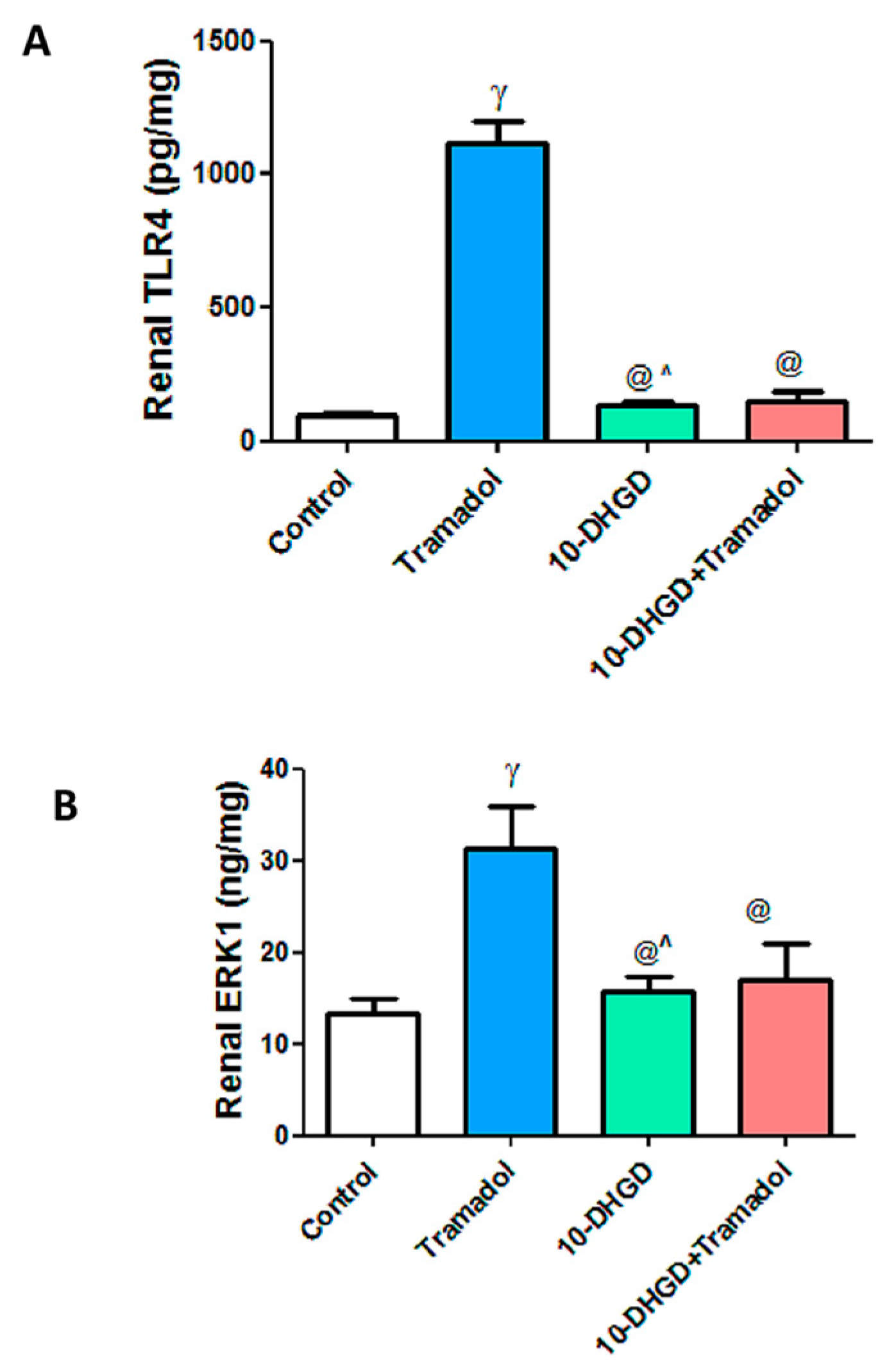
2.4. Renal Toll-like Receptor-4 and Extracellular Signal-Regulated Protein Kinase-1
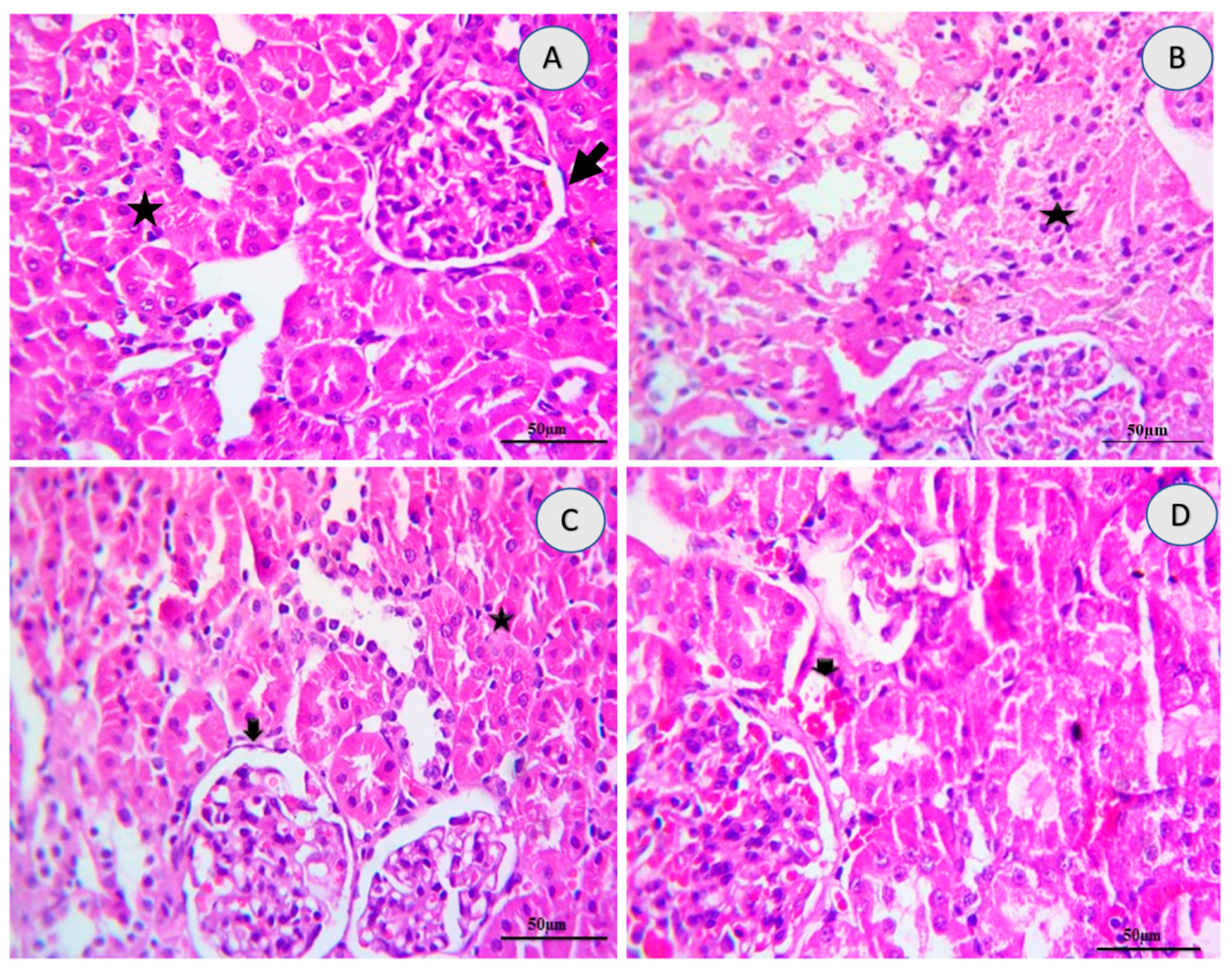
2.5. Renal Histology
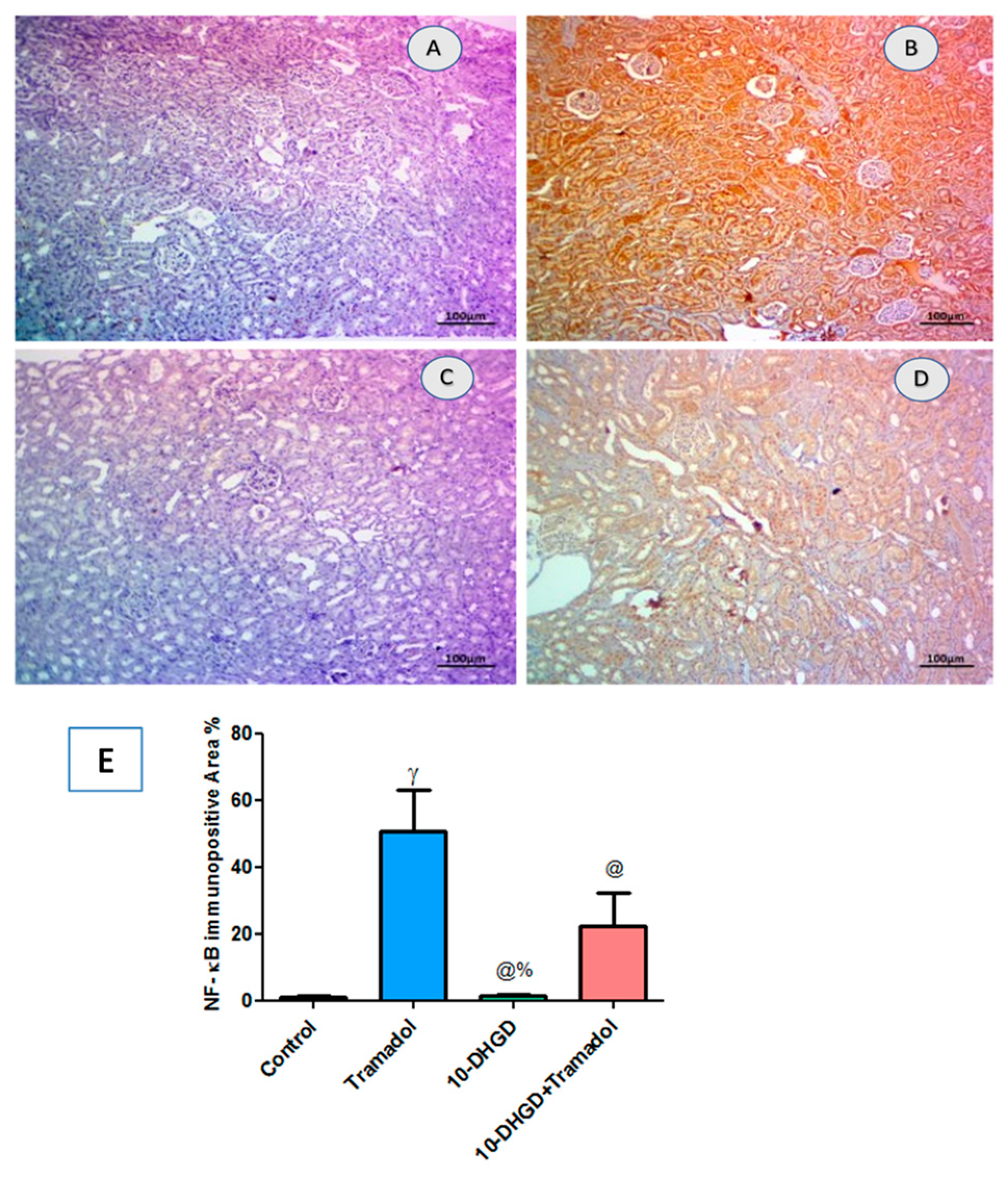
2.6. Renal NF-κB
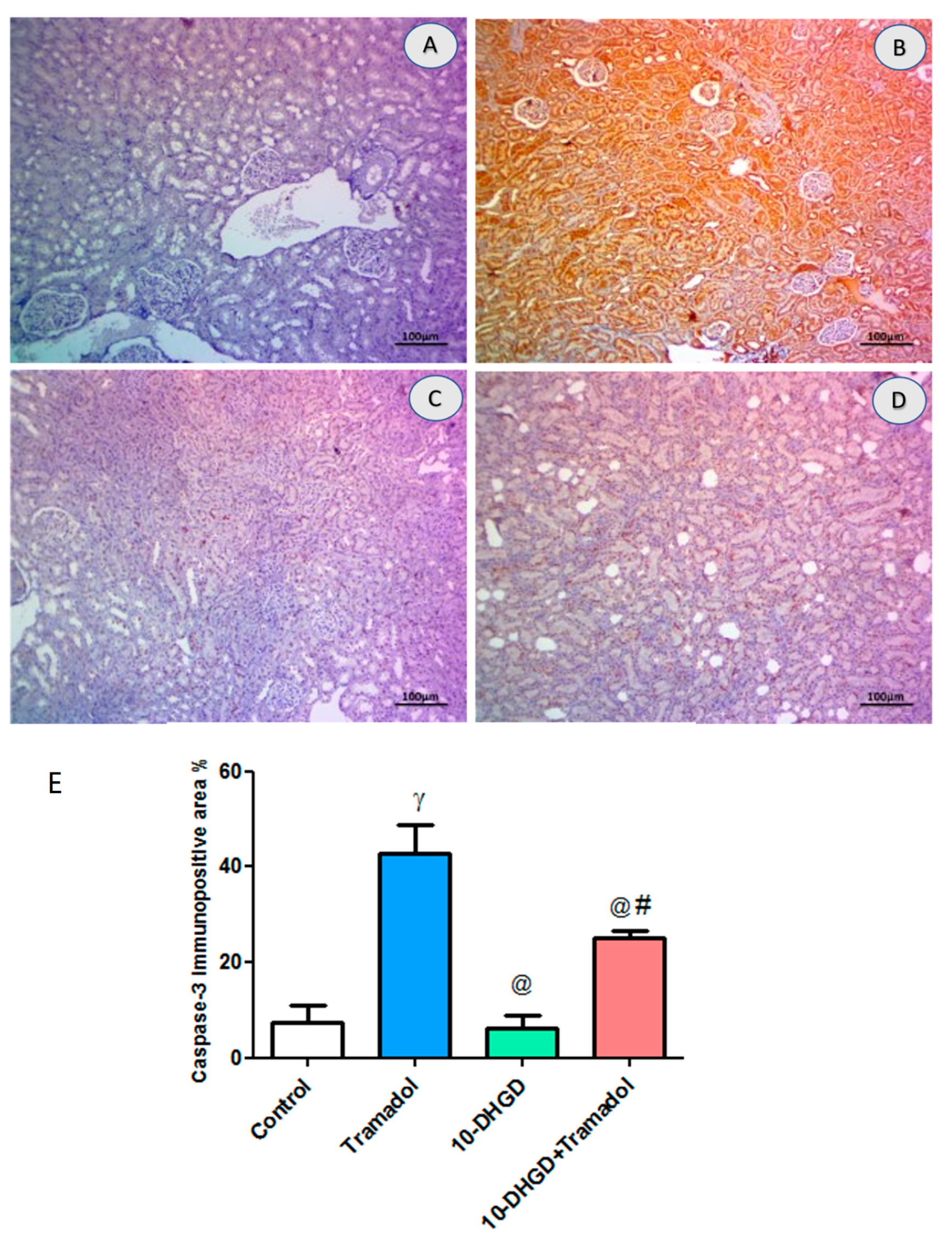
2.7. Renal Apoptotic Marker (Caspase-3)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. Experimental Design
4.4. Sampling
4.5. Biochemical Measurements
4.6. Enzyme Linked Immunosorbent Assay (ELISA)
4.7. Histopathological Examinations
4.8. Immunohistochemistry Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodieux, F.; Vutskits, L.; Posfay-Barbe, K.M.; Habre, W.; Piguet, V.; Desmeules, J.A.; Samer, C.F. When the safe alternative is not that safe: Tramadol prescribing in children. Front. Pharmacol. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Balhara, Y.P.S.; Parmar, A.; Sarkar, S. Use of tramadol for management of opioid use disorders: Rationale and recommendations. J. Neurosci. Rural Pract. 2018, 9, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pergolizzi, J.V.; LeQuang, J.A.; Berger, G.K.; Raffa, R.B. The basic pharmacology of opioids informs the opioid discourse about misuse and abuse: A review. Pain Ther. 2017, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthiesen, T.; Wöhrmann, T.; Coogan, T.; Uragg, H. The experimental toxicology of tramadol: An overview. Toxicol. Lett. 1998, 95, 63–71. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Hill, L.; Osler, W.; O’Keefe, S. Effect of tramadol and morphine on pain and gastrointestinal motor function in patients with chronic pancreatitis. Dig. Dis. Sci. 1999, 44, 1107–1116. [Google Scholar] [CrossRef]
- Tarn, D.M.; Wenger, A.; Good, J.S.; Hoffing, M.; Scherger, J.E.; Wenger, N.S. Do physicians communicate the adverse effects of medications that older patients want to hear? Drugs Ther. Perspect. 2015, 31, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Hong, H.; Senior, J.; Tong, W. Biomarkers for drug-induced liver injury. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Blanco, V.E.; Hernandorena, C.V.; Scibona, P.; Belloso, W.; Musso, C.G. Acute kidney injury pharmacokinetic changes and its impact on drug prescription. Healthcare 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Abbas, R.N.; Jouda, J.; Alshammary, A.G.; Jumaa, M.S. Toxicity of liver and kidney induced by different concentrations of tramadol in young and adult mice. Ann. Trop. Med. Health 2020, 23, 128–133. [Google Scholar] [CrossRef]
- McCarberg, B.H.; Barkin, R.L. Long-acting opioids for chronic pain: Pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am. J. Ther. 2001, 8, 181–186. [Google Scholar] [CrossRef]
- Bameri, B.; Shaki, F.; Ahangar, N.; Ataee, R.; Samadi, M.; Mohammadi, H. Evidence for the involvement of the dopaminergic system in seizure and oxidative damage induced by tramadol. Int. J. Toxicol. 2018, 37, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Zheng, Q.S.; Pan, J.; Zheng, R.L. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin. Pharmacol. Toxicol. 2004, 95, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-W.; Lu, J.; Wang, X.-H.; Fu, S.-K.; Li, Q.; Lin, F.-Q. Neuronal apoptosis in morphine addiction and its molecular mechanism. Int. J. Clin. Exp. Med. 2013, 6, 540. [Google Scholar]
- Nath, K.A. Heme oxygenase-1 and acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2014, 23, 17. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Migita, C.T. Mechanism of heme degradation by heme oxygenase. J. Inorg. Biochem. 2000, 82, 33–41. [Google Scholar] [CrossRef]
- Schuller, D.J.; Wilks, A.; de Montellano, P.R.O.; Poulos, T.L. Crystal structure of human heme oxygenase-1. Nat. Struct. Biol. 1999, 6, 860–867. [Google Scholar]
- Uddin, M.J.; Kim, E.H.; Hannan, M.; Ha, H. Pharmacotherapy against oxidative stress in chronic kidney disease: Promising small molecule natural products targeting Nrf2-HO-1 signaling. Antioxidants 2021, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef]
- Wenzel, P.; Rossmann, H.; Müller, C.; Kossmann, S.; Oelze, M.; Schulz, A.; Arnold, N.; Simsek, C.; Lagrange, J.; Klemz, R. Heme oxygenase-1 suppresses a pro-inflammatory phenotype in monocytes and determines endothelial function and arterial hypertension in mice and humans. Eur. Heart J. 2015, 36, 3437–3446. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, R.M.; Hughes, S.M.; Ledgerwood, E.C. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic. Biol. Med. 2012, 53, 1522–1530. [Google Scholar] [CrossRef]
- Wu, W.; McKown, L.; Gauthier, A.; Jones, W.; Raffa, R. Metabolism of the analgesic drug, tramadol hydrochloride, in rat and dog. Xenobiotica 2001, 31, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Bassiony, M.M.; Salah El-Deen, G.M.; Yousef, U.; Raya, Y.; Abdel-Ghani, M.M.; El-Gohari, H.; Atwa, S.A. Adolescent tramadol use and abuse in Egypt. Am. J. Drug Alcohol Abus. 2015, 41, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.R.; Masson, G.S.; Ebenezer, P.J.; Del Piero, F.; Francis, J. Role of TLR4 in lipopolysaccharide-induced acute kidney injury: Protection by blueberry. Free Radic. Biol. Med. 2014, 71, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.O.R.; He, Y.; Bertagnolli, M.; Mai-Vo, T.-A.; Fernandes, R.O.; Boudreau, F.; Cloutier, A.; Luu, T.M.; Nuyt, A.M. TLR (Toll-Like Receptor) 4 antagonism prevents left ventricular hypertrophy and dysfunction caused by neonatal hyperoxia exposure in rats. Hypertension 2019, 74, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zeng, M.; Li, M.; Kan, Y.; Li, B.; Xu, R.; Wu, Y.; Wang, S.; Zheng, X.; Feng, W. Protopine protects mice against Lps-induced acute kidney injury by inhibiting apoptosis and inflammation via the Tlr4 signaling pathway. Molecules 2020, 25, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, S.-T.; Tseng, S.-T. Oxidative stress markers in type 2 diabetes patients with diabetic nephropathy. Clin. Exp. Nephrol. 2017, 21, 283–292. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Bartok, E.; Rieger, A.; Franchi, L.; Núñez, G.; Hornung, V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011, 187, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Park, G.-S.; Lee, S.Y.; Kim, J.Y.; Kim, Y.K. The conformation and CETP inhibitory activity of [10]-dehydrogingerdione isolated from Zingiber officinale. Arch. Pharmacal Res. 2011, 34, 727–731. [Google Scholar] [CrossRef]
- Fish, R.; Danneman, P.J.; Brown, M.; Karas, A. Anesthesia and Analgesia in Laboratory Animals; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S.; Raine, L.; Fanger, H. Use of biotin-avidin-peroxi dase conplex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody techniques. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Groups | Initial Body Weight (g) | Final Body Weight (g) | Change in Body Weight | Kidney Weight (mg) | Kidney Weight to Body Weight Ratio (%) |
|---|---|---|---|---|---|
| Control | 149.8 ± 7.59 | 200.9 ± 14.5 | 51.1 | 1.410 ± 0.17 | 0.67 ± 0.097 |
| Tramadol | 159.2 ± 6.76 | 227.4 ± 13.09 | 68.2 | 1.357 ± 0.13 | 0.62 ± 0.071 |
| 10-DHGD | 151.8 ± 6.49 | 211 ± 12.94 | 59.2 | 1.502 ± 0.11 | 0.710 ± 0.07 |
| 10-DHGD+Tramadol | 161.4 ± 6.35 | 222.6 ± 11.65 | 61.2 | 1.560 ± 0.14 | 0.715 ± 0.085 |
| Parameters | Control | Tramadol | 10-DHGD | 10-DHGD+Tramadol |
|---|---|---|---|---|
| Creatinine (mg/dL) | 0.713 ± 0.053 | 1.453 ± 0.252 γ | 0.733 ± 0.040 @^ | 0.993 ± 0.0637 @ |
| Urea (mg/dL) | 46.67 ± 3.251 | 61.33 ± 5.49 γ | 50.00 ± 1.23 @ | 53.00 ± 3.240 © |
| Uric acid (mg/dL) | 1.138 ± 0.121 | 4.263 ± 1.207 γ | 1.673 ± 0.234 @ | 2.187 ± 0.297 @ |
| Lesions | Control | 10-DHGD | Tramadol | Tramadol+10-DHGD |
|---|---|---|---|---|
| Inflammation (nephritis) | 0 | 0 | 2 | 0 |
| Necrosis/Degeneration | 0 | 0 | 3 | 1 |
| Hemorrhages | 0 | 0 | 3 | 1 |
| Cystic Dilatation | 0 | 0 | 1 | 0 |
| Fibrosis | 0 | 0 | 1 | 0 |
| Edema | 0 | 0 | 2 | 0 |
| Tubular Casts | 0 | 0 | 3 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnagar, G.M.; Elseweidy, M.M.; Mahmoud, Y.K.; Elkomy, N.M.I.M.; Althafar, Z.M.; Alnomasy, S.F.; Al-Gabri, N.A.; Shawky, M. 10-Dehydrogingerdione Attenuates Tramadol-Induced Nephrotoxicity by Modulating Renal Oxidative Stress, Inflammation and Apoptosis in Experimental Rats: Role of HO-1 Activation and TLR4/NF-κB/ERK Inhibition. Int. J. Mol. Sci. 2022, 23, 1384. https://doi.org/10.3390/ijms23031384
Elnagar GM, Elseweidy MM, Mahmoud YK, Elkomy NMIM, Althafar ZM, Alnomasy SF, Al-Gabri NA, Shawky M. 10-Dehydrogingerdione Attenuates Tramadol-Induced Nephrotoxicity by Modulating Renal Oxidative Stress, Inflammation and Apoptosis in Experimental Rats: Role of HO-1 Activation and TLR4/NF-κB/ERK Inhibition. International Journal of Molecular Sciences. 2022; 23(3):1384. https://doi.org/10.3390/ijms23031384
Chicago/Turabian StyleElnagar, Gehad M., Mohamed M. Elseweidy, Yasmin K. Mahmoud, Nesreen M. I. M. Elkomy, Ziyad M. Althafar, Sultan F. Alnomasy, Naif A. Al-Gabri, and Mohamed Shawky. 2022. "10-Dehydrogingerdione Attenuates Tramadol-Induced Nephrotoxicity by Modulating Renal Oxidative Stress, Inflammation and Apoptosis in Experimental Rats: Role of HO-1 Activation and TLR4/NF-κB/ERK Inhibition" International Journal of Molecular Sciences 23, no. 3: 1384. https://doi.org/10.3390/ijms23031384







