A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation
Abstract
:1. Introduction
2. Results
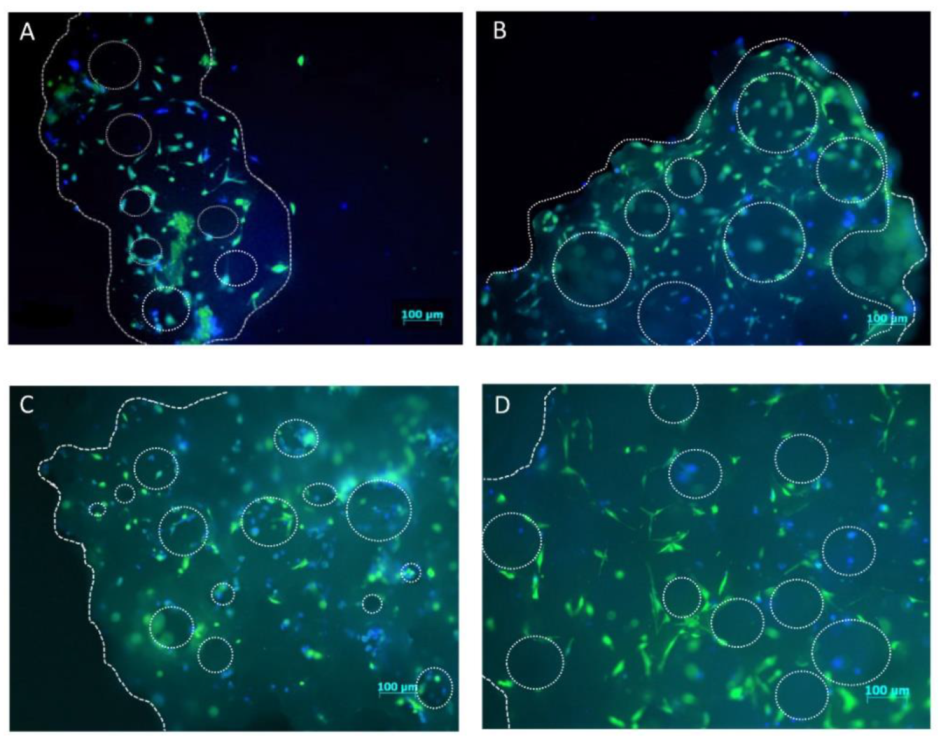
2.1. Effect of Cryopreservation on hMSCs Metabolic Activity
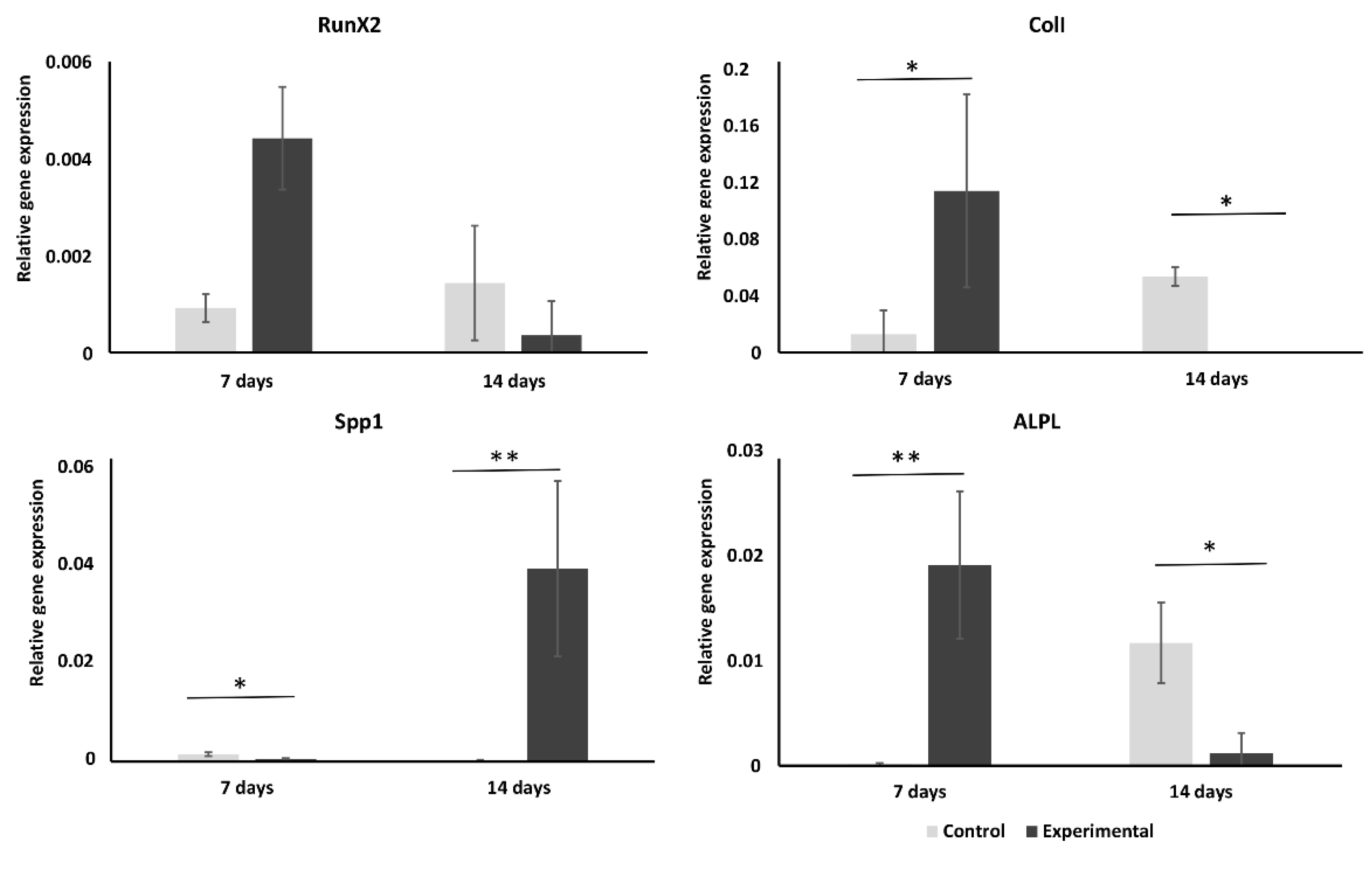
2.2. Cryopreserved hMSCs Show Stronger Osteogenic Activity Than Control Cells
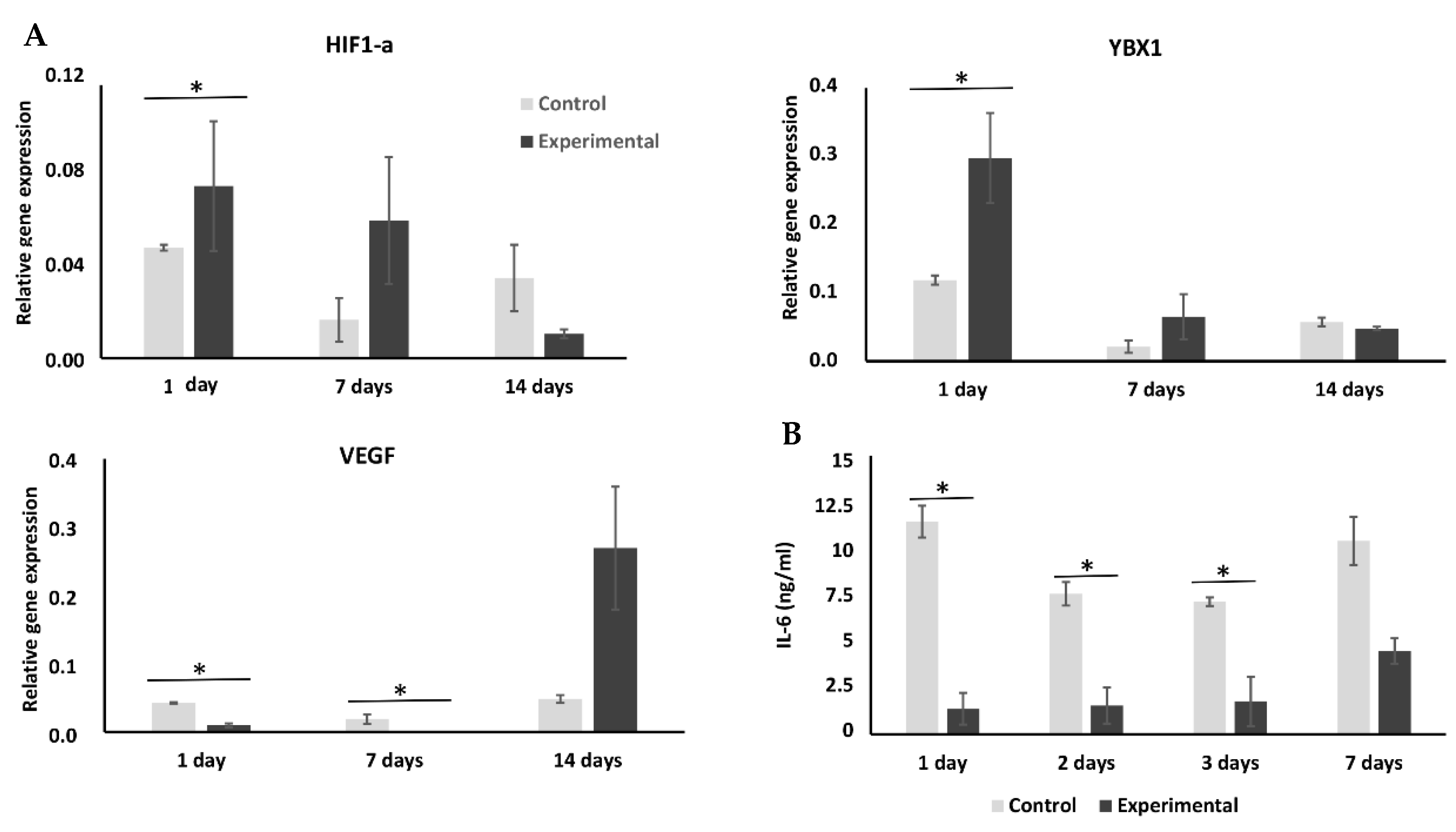
2.3. Cryopreservation Has an Effect on Expression of Hypoxia-Related Genes
2.4. Cryopreservation Enhances YBX1 Expression but Has No Effect on Wnt/Smad Pathways
2.5. Effect of Cryopreservation on Interleukin Expression
3. Discussion
4. Materials and Methods
4.2. Effect of Cryopreservation on Cell Activity
4.3. Cryopreservation and Thawing of hMSCs on β-TCP-Scaffold
4.4. Effect of Storage Temperature
4.5. Cell Metabolic Activity Measurements
4.6. hMSCs Distribution on Scaffold Granules
4.7. Osteogenic Differentiation
4.8. Gene Expression Analysis
4.9. Alkaline Phosphatase Expression Assay
4.10. ELISA
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannoudis, P.V.; Atkins, R. Management of long-bone non-unions. Injury 2007, 38 (Suppl. 2), S1–S2. [Google Scholar] [CrossRef]
- Ashman, O.; Phillips, A.M. Treatment of non-unions with bone defects: Which option and why? Injury 2013, 44, S43–S45. [Google Scholar] [CrossRef]
- Kinaci, A.; Neuhaus, V.; Ring, D.C. Trends in bone graft use in the United States. Orthopedics 2014, 37, e783–e788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous bone graft: Properties and techniques. J. Orthop. Trauma 2010, 24 (Suppl. 1), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Rigal, S.; Merloz, P.; Le Nen, D.; Mathevon, H.; Masquelet, A.-C. Bone transport techniques in posttraumatic bone defects. Orthop. Traumatol. Surg. Res. 2012, 98, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Malizos, K.N.; Zalavras, C.G.; Soucacos, P.N.; Beris, A.E.; Urbaniak, J.R. Free vascularized fibular grafts for reconstruction of skeletal defects. J. Am. Acad. Orthop. Surg. 2004, 12, 360–369. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Meier, S.; Nau, C.; Bonig, H.; Marzi, I. Safety and feasibility of cell-based therapy of autologous bone marrow-derived mononuclear cells in plate-stabilized proximal humeral fractures in humans. J. Transl. Med. 2016, 14, 314. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.W.; Choi, J.R.; Wan Safwani, W.K.Z. Biobanking of Human Mesenchymal Stem Cells: Future Strategy to Facilitate Clinical Applications. Adv. Exp. Med. Biol. 2016, 951, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ginis, I.; Grinblat, B.; Shirvan, M.H. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng. Part C Methods 2012, 18, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Yalvaç, M.E.; Ramazanoglu, M.; Tekguc, M.; Bayrak, O.F.; Shafigullina, A.K.; Salafutdinov, I.I.; Blatt, N.L.; Kiyasov, A.P.; Sahin, F.; Palotás, A.; et al. Human tooth germ stem cells preserve neuro-protective effects after long-term cryo-preservation. Curr. Neurovasc. Res. 2010, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Woods, E.J.; Perry, B.C.; Hockema, J.J.; Larson, L.; Zhou, D.; Goebel, W.S. Optimized cryopreservation method for human dental pulp-derived stem cells and their tissues of origin for banking and clinical use. Cryobiology 2009, 59, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Gonda, K.; Shigeura, T.; Sato, T.; Matsumoto, D.; Suga, H.; Inoue, K.; Aoi, N.; Kato, H.; Sato, K.; Murase, S.; et al. Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast. Reconstr. Surg. 2008, 121, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, F.; Lei, L.; Li, Y.; Xiao, Y. Application of autologous cryopreserved bone marrow mesenchymal stem cells for periodontal regeneration in dogs. Cells Tissues Organs 2009, 190, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Shu, C.; Cui, L.; Liu, W.; Cao, Y. Tissue-engineered bone formation with cryopreserved human bone marrow mesenchymal stem cells. Cryobiology 2008, 56, 209–215. [Google Scholar] [CrossRef]
- Hernández-Tapia, L.G.; Fohlerová, Z.; Žídek, J.; Alvarez-Perez, M.A.; Čelko, L.; Kaiser, J.; Montufar, E.B. Effects of Cryopreservation on Cell Metabolic Activity and Function of Biofabricated Structures Laden with Osteoblasts. Materials 2020, 13, 1966. [Google Scholar] [CrossRef]
- Mutsenko, V.; Knaack, S.; Lauterboeck, L.; Tarusin, D.; Sydykov, B.; Cabiscol, R.; Ivnev, D.; Belikan, J.; Beck, A.; Dipresa, D.; et al. Effect of ‘in air’ freezing on post-thaw recovery of Callithrix jacchus mesenchymal stromal cells and properties of 3D collagen-hydroxyapatite scaffolds. Cryobiology 2020, 92, 215–230. [Google Scholar] [CrossRef]
- Lindquist, J.A.; Mertens, P.R. Cold shock proteins: From cellular mechanisms to pathophysiology and disease. Cell Commun. Signal. 2018, 16, 63. [Google Scholar] [CrossRef] [Green Version]
- Hunt, C.J. Cryopreservation of Human Stem Cells for Clinical Application: A Review. Transfus. Med. Hemother. 2011, 38, 107–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogozhykh, O.; Prokopyuk, V.; Prokopyuk, O.; Kuleshova, L.; Goltsev, A.; Figueiredo, C.; Pogozhykh, D. Towards biobanking technologies for natural and bioengineered multicellular placental constructs. Biomaterials 2018, 185, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Malpique, R.; Osório, L.M.; Ferreira, D.S.; Ehrhart, F.; Brito, C.; Zimmermann, H.; Alves, P.M. Alginate encapsulation as a novel strategy for the cryopreservation of neurospheres. Tissue Eng. Part C Methods 2010, 16, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Kuleshova, L.L.; Gouk, S.S.; Hutmacher, D.W. Vitrification as a prospect for cryopreservation of tissue-engineered constructs. Biomaterials 2007, 28, 1585–1596. [Google Scholar] [CrossRef]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved mesenchymal stem cells regain functional potency following a 24-h acclimation period. J. Transl. Med. 2019, 17, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Han, P.; Bartels, D.M. Temperature Dependence of Oxygen Diffusion in H2O and D2O. J. Phys. Chem. 1996, 100, 5597–5602. [Google Scholar] [CrossRef]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef]
- Wagegg, M.; Gaber, T.; Lohanatha, F.L.; Hahne, M.; Strehl, C.; Fangradt, M.; Tran, C.L.; Schönbeck, K.; Hoff, P.; Ode, A.; et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS ONE 2012, 7, e46483. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.-G.; Zhao, X.; Yang, Q.; Li, Y.; Ge, C.; Zhao, G.; Franceschi, R.T. Physical and functional interactions between Runx2 and HIF-1α induce vascular endothelial growth factor gene expression. J. Cell. Biochem. 2011, 112, 3582–3593. [Google Scholar] [CrossRef] [Green Version]
- Rauen, T.; Frye, B.C.; Wang, J.; Raffetseder, U.; Alidousty, C.; En-Nia, A.; Floege, J.; Mertens, P.R. Cold shock protein YB-1 is involved in hypoxia-dependent gene transcription. Biochem. Biophys. Res. Commun. 2016, 478, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Kretov, D.A.; Clément, M.-J.; Lambert, G.; Durand, D.; Lyabin, D.N.; Bollot, G.; Bauvais, C.; Samsonova, A.; Budkina, K.; Maroun, R.C.; et al. YB-1, an abundant core mRNA-binding protein, has the capacity to form an RNA nucleoprotein filament: A structural analysis. Nucleic Acids Res. 2019, 47, 3127–3141. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.M.; Veinotte, C.J.; Cheng, H.; Grunewald, T.G.P.; Negri, G.L.; Somasekharan, S.P.; Corkery, D.P.; Tirode, F.; Mathers, J.; Khan, D.; et al. Translational Activation of HIF1α by YB-1 Promotes Sarcoma Metastasis. Cancer Cell 2015, 27, 682–697. [Google Scholar] [CrossRef] [Green Version]
- Kuçi, Z.; Bönig, H.; Kreyenberg, H.; Bunos, M.; Jauch, A.; Janssen, J.W.G.; Škifić, M.; Michel, K.; Eising, B.; Lucchini, G.; et al. Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: A multicenter survey. Haematologica 2016, 101, 985–994. [Google Scholar] [CrossRef]
- Bader, P.; Kuçi, Z.; Bakhtiar, S.; Basu, O.; Bug, G.; Dennis, M.; Greil, J.; Barta, A.; Kállay, K.M.; Lang, P.; et al. Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM). Bone Marrow Transplant. 2018, 53, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Bonig, H.; Kuçi, Z.; Kuçi, S.; Bakhtiar, S.; Basu, O.; Bug, G.; Dennis, M.; Greil, J.; Barta, A.; Kállay, K.M.; et al. Children and Adults with Refractory Acute Graft-versus-Host Disease Respond to Treatment with the Mesenchymal Stromal Cell Preparation “MSC-FFM”-Outcome Report of 92 Patients. Cells 2019, 8, 1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuçi, Z.; Seiberth, J.; Latifi-Pupovci, H.; Wehner, S.; Stein, S.; Grez, M.; Bönig, H.; Köhl, U.; Klingebiel, T.; Bader, P.; et al. Clonal analysis of multipotent stromal cells derived from CD271+ bone marrow mononuclear cells: Functional heterogeneity and different mechanisms of allosuppression. Haematologica 2013, 98, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leppik, L.; Gempp, A.; Kuçi, Z.; Kuçi, S.; Bader, P.; Bönig, H.; Marzi, I.; Henrich, D. A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation. Int. J. Mol. Sci. 2022, 23, 1425. https://doi.org/10.3390/ijms23031425
Leppik L, Gempp A, Kuçi Z, Kuçi S, Bader P, Bönig H, Marzi I, Henrich D. A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation. International Journal of Molecular Sciences. 2022; 23(3):1425. https://doi.org/10.3390/ijms23031425
Chicago/Turabian StyleLeppik, Liudmila, Anna Gempp, Zyrafete Kuçi, Selim Kuçi, Peter Bader, Halvard Bönig, Ingo Marzi, and Dirk Henrich. 2022. "A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation" International Journal of Molecular Sciences 23, no. 3: 1425. https://doi.org/10.3390/ijms23031425
APA StyleLeppik, L., Gempp, A., Kuçi, Z., Kuçi, S., Bader, P., Bönig, H., Marzi, I., & Henrich, D. (2022). A New Perspective for Bone Tissue Engineering: Human Mesenchymal Stromal Cells Well-Survive Cryopreservation on β-TCP Scaffold and Show Increased Ability for Osteogenic Differentiation. International Journal of Molecular Sciences, 23(3), 1425. https://doi.org/10.3390/ijms23031425







