The Effect of RADA16-I and CDNF on Neurogenesis and Neuroprotection in Brain Ischemia-Reperfusion Injury
Abstract
:1. Introduction
2. Results
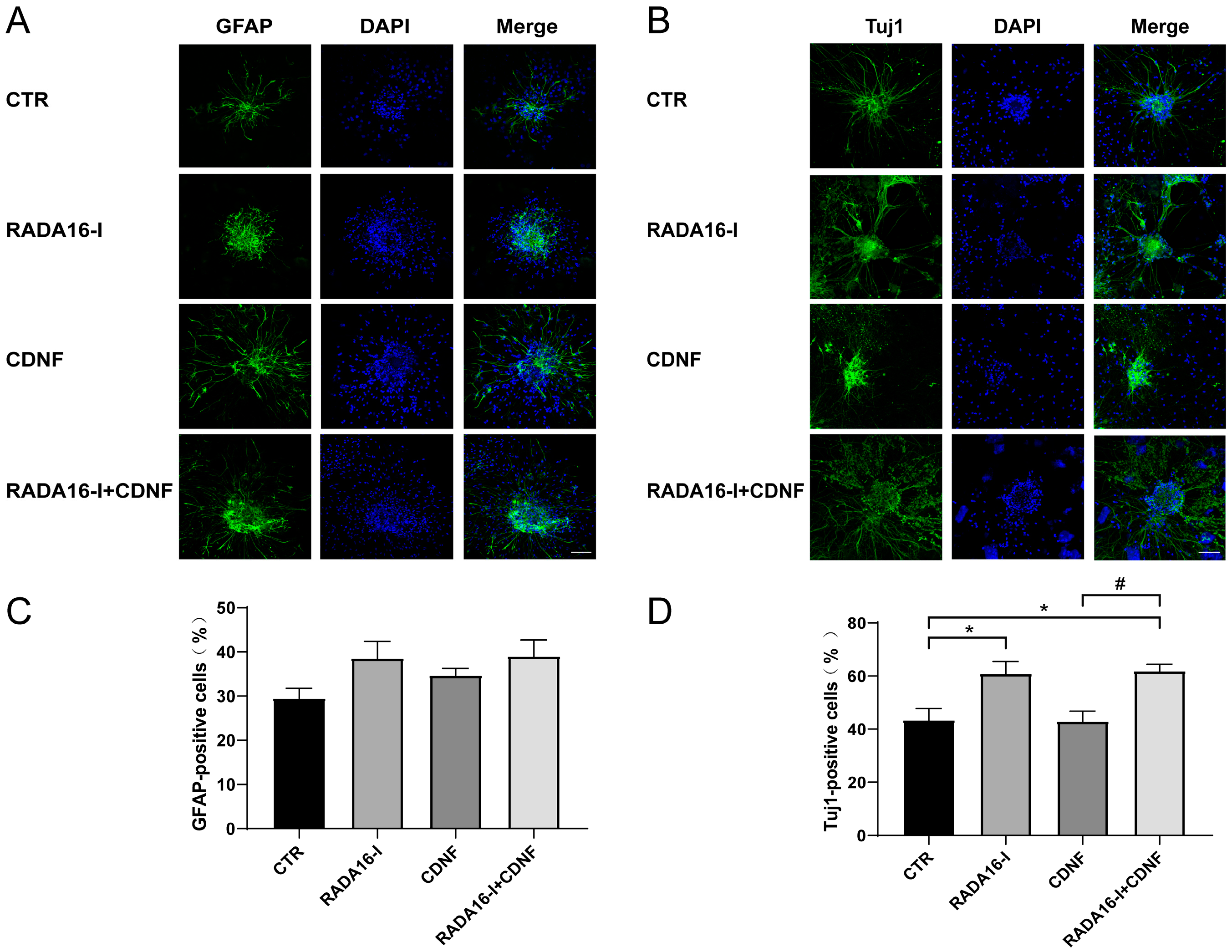
2.1. CDNF, but Not RADA16-I, Promotes the Proliferation of Cultured NSCs
2.2. RADA16-I, but Not CDNF, Promotes the Neural Differentiation of Cultured NSCs
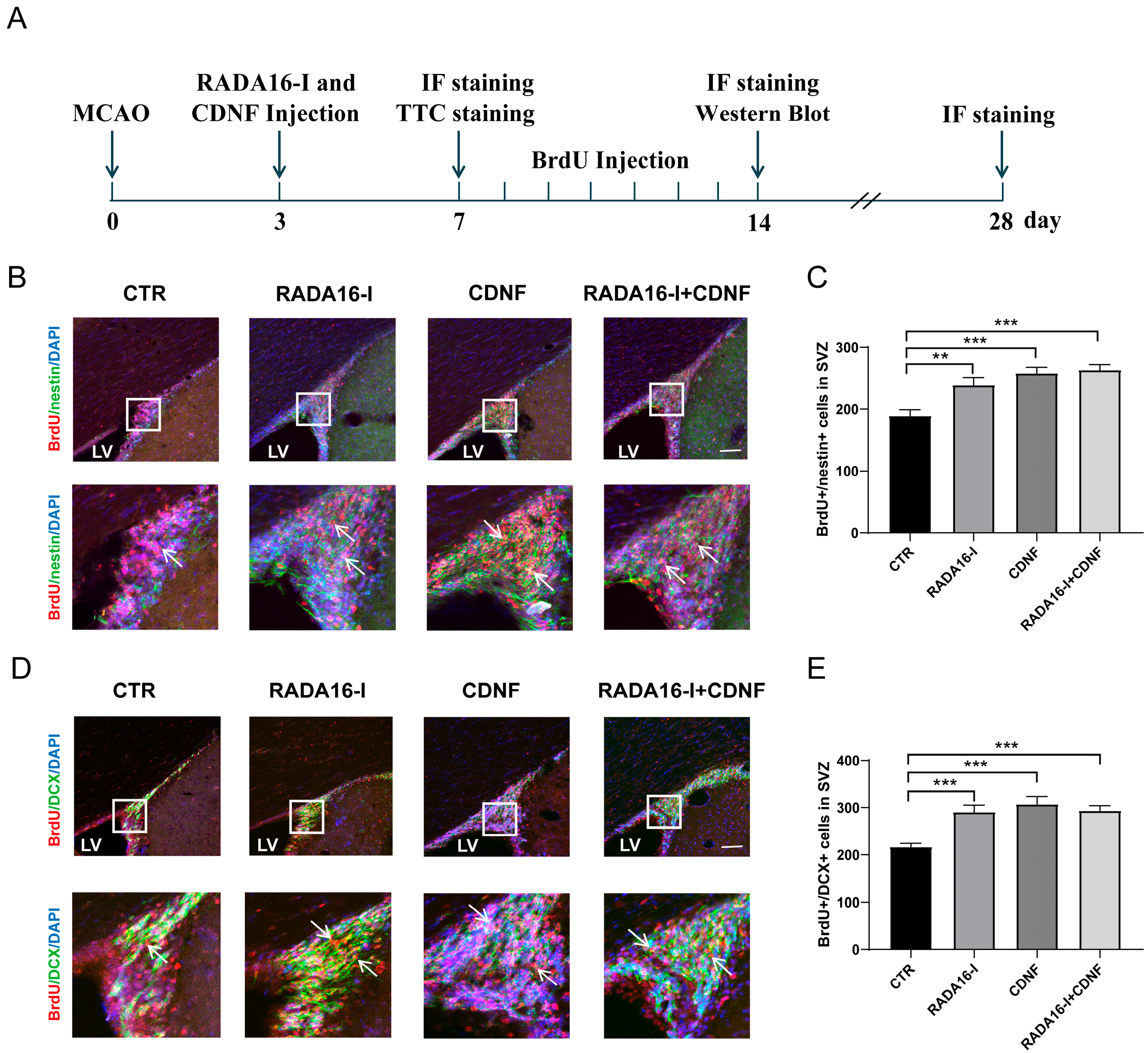
2.3. RADA16-I and CDNF Promote the Proliferation of NSCs and Neuroblasts in the SVZ of Ischemia/Reperfusion Rats
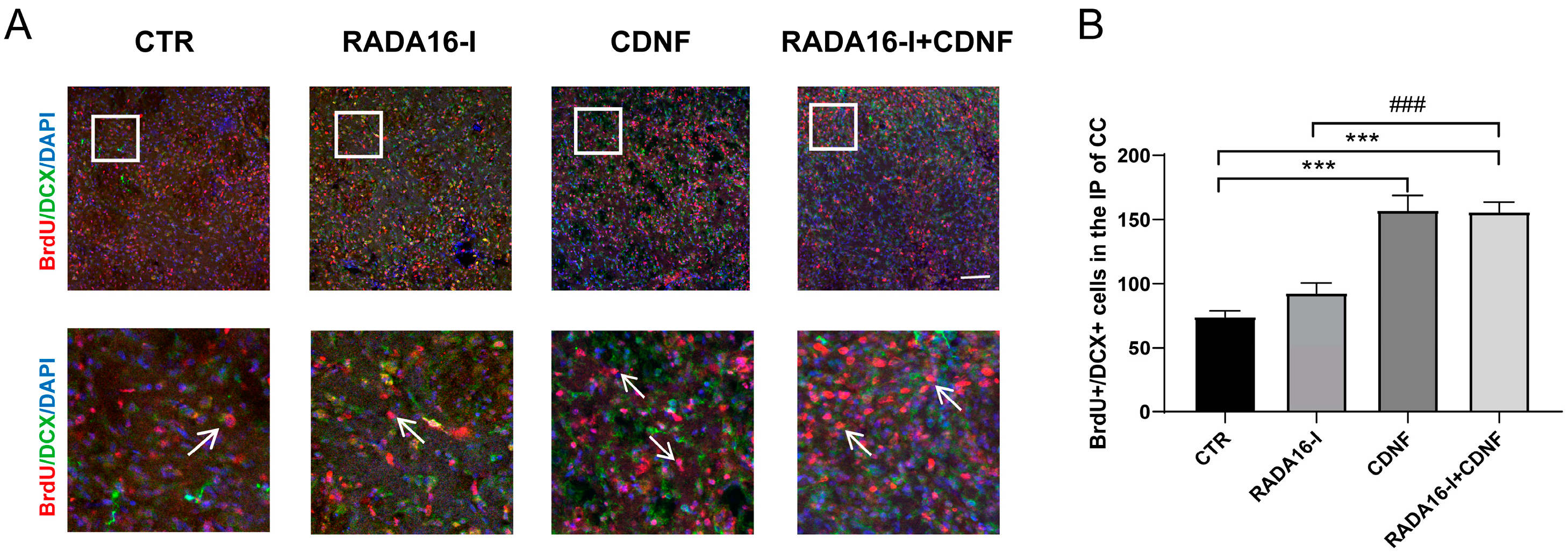
2.4. CDNF, but Not RADA16-I, Promotes the Migration of Neuroblasts in the SVZ into the Ischemic Penumbra Area of Ischemia/Reperfusion Rats
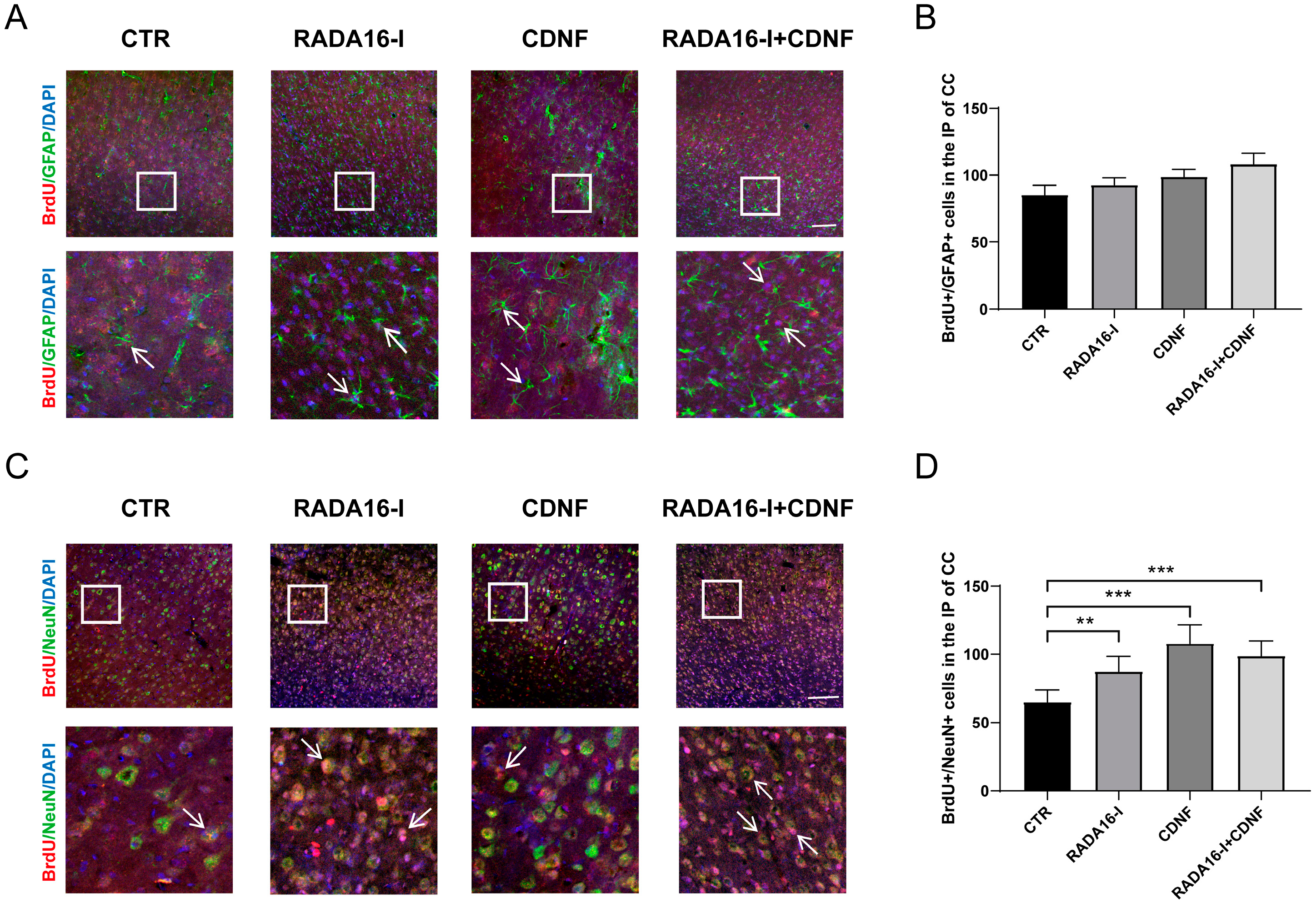
2.5. RADA16-I and CDNF Promote Neural Differentiation in Ischemia/Reperfusion Rats
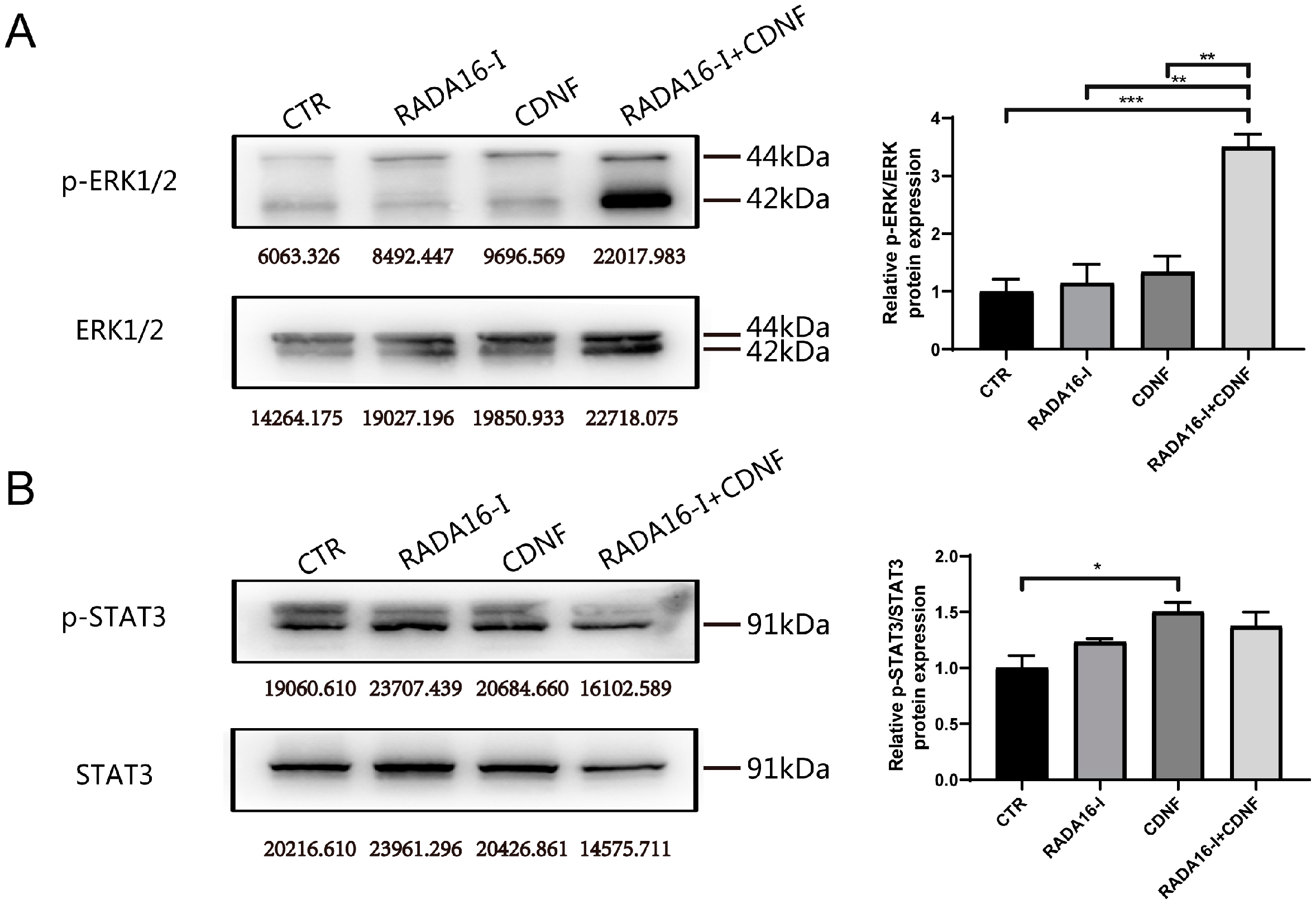
2.6. Pathways Involved in the Neurogenesis of CDNF and RADA16-I in Ischemia/Reperfusion Rats
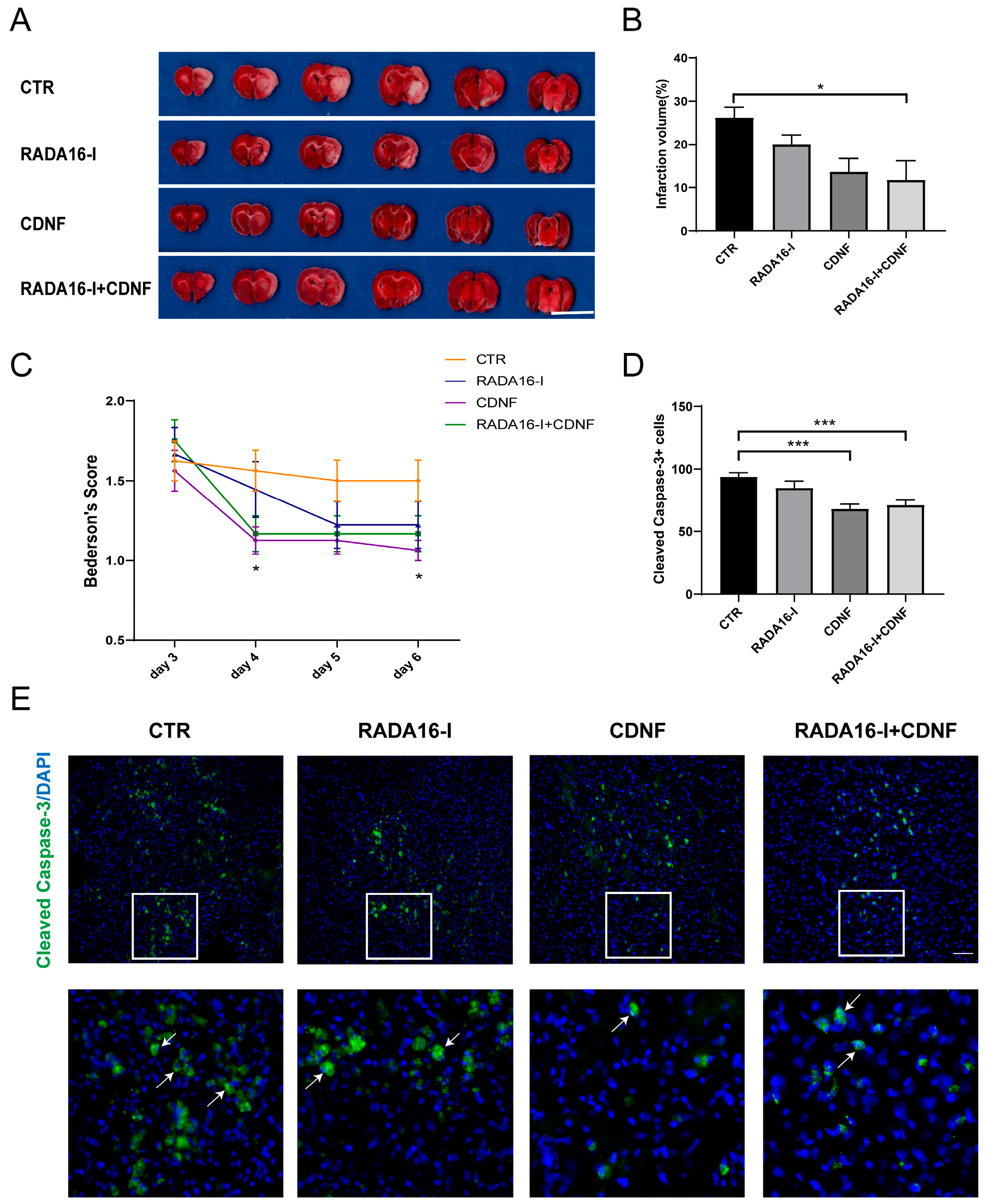
2.7. CDNF, but Not RADA16-I, Has a Neuroprotective Effect on Ischemia/Reperfusion Rats
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. NSC Culture
4.3. Immunocytochemistry
4.4. MCAO Model
4.5. Intracerebroventricular Microinjection
4.6. BrdU Labeling
4.7. Immunohistochemistry
4.8. Evaluation of Infarct Volume
4.9. Examination of Neurological Deficits
4.10. Western Blotting
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| DAPI | 4,6-diamidino-2-phenylindole dihydrochloride hydrate |
| CDNF | cerebral dopamine neurotrophic factor |
| NSCs | neural stem cells |
| LV | lateral ventricle |
| MCAO | middle cerebral artery occlusion |
| NTFs | neurotrophic factors |
| NGF | nerve growth factor |
| BDNF | brain-derived neurotrophic factor |
| GDNF | glial cell line-derived neurotrophic factor |
| OGD | oxygen-glucose deprivation |
| SVZ | subventricular zone |
| SGZ | subgranular zone |
| DG | dentate gyrus |
| MANF | mesencephalic astrocyte-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| CCA | common carotid artery |
| ECA | external carotid artery |
| ICA | internal carotid artery |
| MCA | middle cerebral artery |
| DCX | doublecortin |
| TTC | triphenyltetrazolium chloride |
| SEM | standard error of the mean |
| HRP | horseradish peroxidase |
References
- Schmidt, C.E.; Leach, J.B. Neural tissue engineering: Strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [Green Version]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Approaches to neural tissue engineering using scaffolds for drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Kornev, V.A.; Grebenik, E.A.; Solovieva, A.B.; Dmitriev, R.I.; Timashev, P.S. Hydrogel-assisted neuroregeneration approaches towards brain injury therapy: A state-of-the-art review. Comput. Struct. Biotechnol. J. 2018, 16, 488–502. [Google Scholar] [CrossRef]
- Yokoi, H.; Kinoshita, T.; Zhang, S.G. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef] [Green Version]
- Ortinau, S.; Schmich, J.; Block, S.; Liedmann, A.; Jonas, L.; Weiss, D.G.; Helm, C.A.; Rolfs, A.; Frech, M.J. Effect of 3D-scaffold formation on differentiation and survival in human neural progenitor cells. Biomed. Eng. Online 2010, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Ylä-Outinen, L.; Joki, T.; Varjola, M.; Skottman, H.; Narkilahti, S. Three-dimensional growth matrix for human embryonic stem cell-derived neuronal cells. J. Tissue Eng. Regen. Med. 2014, 8, 186–194. [Google Scholar] [CrossRef]
- Guo, J.; Leung, K.K.G.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.-H.; Zhang, W.; Pu, J.K.S.; Ng, G.K.P.; Wong, W.M.; et al. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 345–351. [Google Scholar] [CrossRef]
- Cheng, T.-Y.; Chen, M.-H.; Chang, W.-H.; Huang, M.-Y.; Wang, T.-W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34, 2005–2016. [Google Scholar] [CrossRef]
- Francis, N.L.; Bennett, N.K.; Halikere, A.; Pang, Z.P.; Moghe, P.V. Self-Assembling Peptide Nanofiber Scaffolds for 3-D Reprogramming and Transplantation of Human Pluripotent Stem Cell-Derived Neurons. ACS Biomater. Sci. Eng. 2016, 2, 1030–1038. [Google Scholar] [CrossRef]
- Lindholm, P.; Saarma, M. Novel CDNF/MANF family of neurotrophic factors. Dev. Neurobiol. 2010, 70, 360–371. [Google Scholar] [CrossRef]
- Hovland, D.N.; Boyd, R.B.; Butt, M.T.; Engelhardt, J.A.; Moxness, M.S.; Ma, M.H.; Emery, M.G.; Ernst, N.B.; Reed, R.P.; Zeller, J.R.; et al. Reprint: Six-Month Continuous Intraputamenal Infusion Toxicity Study of Recombinant Methionyl Human Glial Cell Line-Derived Neurotrophic Factor (r-metHuGDNF) in Rhesus Monkeys. Toxicol. Pathol. 2007, 35, 1013–1029. [Google Scholar] [CrossRef]
- Lang, A.E.; Gill, S.; Patel, N.K.; Lozano, A.; Nutt, J.G.; Penn, R.; Brooks, D.J.; Hotton, G.; Moro, E.; Heywood, P.; et al. Randomized controlled trial of intraputamenal glial cell line–derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466. [Google Scholar] [CrossRef]
- Petrova, P.S.; Raibekas, A.; Pevsner, J.; Vigo, N.; Anafi, M.; Moore, M.K.; Peaire, A.E.; Shridhar, V.; Smith, D.I.; Kelly, J.; et al. MANF—A new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. J. Mol. Neurosci. 2003, 20, 173–187. [Google Scholar] [CrossRef]
- Lindholm, P.; Voutilainen, M.H.; Laurén, J.; Peränen, J.; Leppänen, V.-M.; Andressoo, J.-O.; Lindahl, M.; Janhunen, S.; Kalkkinen, N.; Timmusk, T.; et al. Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 2007, 448, 73–77. [Google Scholar] [CrossRef]
- Voutilainen, M.H.; Bäck, S.; Pörsti, E.; Toppinen, L.; Lindgren, L.; Lindholm, P.; Peränen, J.; Saarma, M.; Tuominen, R.K. Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 9651–9659. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.-L.; Wang, L.-H.; Liu, X.-Y.; Zhang, Y.-X.; Hu, M.-Y.; Liu, L.; Fang, Y.-Y.; Mu, Y.; Zhao, Y.; Huang, S.-H.; et al. Cerebral Dopamine Neurotrophic Factor (CDNF) Has Neuroprotective Effects against Cerebral Ischemia That May Occur through the Endoplasmic Reticulum Stress Pathway. Int. J. Mol. Sci. 2018, 19, 1905. [Google Scholar] [CrossRef] [Green Version]
- Tseng, K.-Y.; Anttila, J.E.; Khodosevich, K.; Tuominen, R.K.; Lindahl, M.; Domanskyi, A.; Airavaara, M. MANF promotes differentiation and migration of neural progenitor cells with potential neural regenerative effects in stroke. Mol. Ther. 2018, 26, 238–255. [Google Scholar] [CrossRef] [Green Version]
- Nasrolahi, A.; Mahmoudi, J.; Karimipour, M.; Akbarzadeh, A.; Sadigh-Eteghad, S.; Salehi, R.; Farajdokht, F.; Farhoudi, M. Effect of cerebral dopamine neurotrophic factor on endogenous neural progenitor cell migration in a rat model of Parkinson’s disease. EXCLI J. 2019, 18, 139–153. [Google Scholar]
- Lin, C.-Q.; Chen, L.-K. Cerebral dopamine neurotrophic factor promotes the proliferation and differentiation of neural stem cells in hypoxic environments. Neural Regen. Res. 2020, 15, 2057–2062. [Google Scholar]
- Reynolds, B.A.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central-nervous-system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.A.; Temple, S. A self-renewing multipotential stem-cell in embryonic rat cerebral-cortex. Nature 1994, 372, 263–266. [Google Scholar] [CrossRef]
- Temple, S. The development of neural stem cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef]
- Kokaia, Z.; Lindvall, O. Neurogenesis after ischaemic brain insults. Curr. Opin. Neurobiol. 2003, 13, 127–132. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, Z.G.; Zhang, L.; Chopp, M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 2001, 105, 33–41. [Google Scholar] [CrossRef]
- Parent, J.M.; Vexler, Z.S.; Gong, C.; Derugin, N.; Ferriero, D.M. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002, 52, 802–813. [Google Scholar] [CrossRef]
- Kreuzberg, M.; Kanov, E.; Timofeev, O.; Schwaninger, M.; Monyer, H.; Khodosevich, K. Increased subventricular zone-derived cortical neurogenesis after ischemic lesion. Exp. Neurol. 2010, 226, 90–99. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D.G. Cns stem-cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Garca-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Jin, K.; Sun, Y.; Xie, L.; Peel, A.; Mao, X.O.; Batteur, S.; Greenberg, D.A. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol. Cell. Neurosci. 2003, 24, 171–189. [Google Scholar] [CrossRef]
- Sun, L.; Lee, J.; Fine, H.A. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J. Clin. Investig. 2004, 113, 1364–1374. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, Z.; Wang, L.; Wang, Y.; Gousev, A.; Zhang, L.; Ho, K.-L.; Morshead, C.; Chopp, M. Activated Neural Stem Cells Contribute to Stroke-Induced Neurogenesis and Neuroblast Migration toward the Infarct Boundary in Adult Rats. J. Cereb. Blood Flow Metab. 2004, 24, 441–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thored, P.; Arvidsson, A.; Cacci, E.; Ahlenius, H.; Kallur, T.; Darsalia, V.; Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Persistent Production of Neurons from Adult Brain Stem Cells During Recovery after Stroke. Stem. Cells 2006, 24, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-P.; Sailor, K.A.; Lang, B.T.; Park, S.-W.; Vemuganti, R.; Dempsey, R.J. Monocyte Chemoattractant Protein-1 Plays a Critical Role in Neuroblast Migration after Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2007, 27, 1213–1224. [Google Scholar] [CrossRef]
- Wang, T.-W.; Chang, K.-C.; Chen, L.-H.; Liao, S.-Y.; Yeh, C.-W.; Chuang, Y.-J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Zheng, Q.X.; Wu, Y.C.; Hao, D.J. Compatibility of Neural Stem Cells with Functionalized Self-assembling Peptide Scaffold In vitro. Biotechnol. Bioprocess Eng. 2010, 15, 545–551. [Google Scholar] [CrossRef]
- Taraballi, F.; Natalello, A.; Campione, M.; Villa, O.; Doglia, S.; Paleari, A.; Gelain, F. Glycine-spacers influence functional motifs exposure and self-assembling propensity of functionalized substrates tailored for neural stem cell cultures. Front. Neuroeng. 2010, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Ihrie, R.A.; Álvarez-Buylla, A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron 2011, 70, 674–686. [Google Scholar] [CrossRef] [Green Version]
- Tseng, K.-Y.; Danilova, T.; Domanskyi, A.; Saarma, M.; Lindahl, M.; Airavaara, M. MANF Is Essential for Neurite Extension and Neuronal Migration in the Developing Cortex. Eneuro 2017, 4, ENEURO.0214-17.2017. [Google Scholar] [CrossRef]
- Li, Q.; Chau, Y. Neural differentiation directed by self-assembling peptide scaffolds presenting laminin-derived epitopes. J. Biomed. Mater. Res. Part A 2010, 94, 688–699. [Google Scholar] [CrossRef]
- Ng, D.C.H.; Lin, B.H.; Lim, C.P.; Huang, G.; Zhang, T.; Poli, V.; Cao, X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J. Cell Biol. 2006, 172, 245–257. [Google Scholar] [CrossRef]
- Zhou, L.; Too, H.-P. Mitochondrial Localized STAT3 Is Involved in NGF Induced Neurite Outgrowth. PLoS ONE 2011, 6, e21680. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.Y.; Kulhanek, D.J.; Eckenstein, F.P. Differential Patterns of ERK and STAT3 Phosphorylation after Sciatic Nerve Transection in the Rat. Exp. Neurol. 2000, 166, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; MurphyUllrich, J.E.; Wells, A. Role for gelsolin in actuating epidermal growth factor receptor-mediated cell motility. J. Cell Biol. 1996, 134, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Anand-Apte, B.; Zetter, B.R.; Viswanathan, A.; Qiu, R.G.; Chen, J.; Ruggieri, R.; Symons, M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J. Biol. Chem. 1997, 272, 30688–30692. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Jacobson, K.; Schaller, M.D. MAP kinases and cell migration. J. Cell Sci. 2004, 117, 4619–4628. [Google Scholar] [CrossRef] [Green Version]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef]
- Airavaara, M.; Shen, H.; Kuo, C.-C.; Peränen, J.; Saarma, M.; Hoffer, B.; Wang, Y. Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J. Comp. Neurol. 2009, 515, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Wang, X.-J.; Xie, S.-S.; Zhang, D.-L.; Wang, X.-P.; Li, B.-Q.; Ma, W.; Xin, H. A comparison of proliferative capacity and passaging potential between neural stem and progenitor cells in adherent and neurosphere cultures. Int. J. Dev. Neurosci. 2011, 29, 723–731. [Google Scholar] [CrossRef]
- Wang, X.-J.; Sun, T.; Kong, L.; Shang, Z.-H.; Yang, K.-Q.; Zhang, Q.-Y.; Jing, F.-M.; Dong, L.; Xu, X.-F.; Liu, J.-X.; et al. Gypenosides pre-treatment protects the brain against cerebral ischemia and increases neural stem cells/progenitors in the subventricular zone. Int. J. Dev. Neurosci. 2014, 33, 49–56. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ren, H.; Peng, A.; Cheng, H.; Chen, J.; Xia, X.; Liu, T.; Wang, X. The Effect of RADA16-I and CDNF on Neurogenesis and Neuroprotection in Brain Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 1436. https://doi.org/10.3390/ijms23031436
Liu X, Ren H, Peng A, Cheng H, Chen J, Xia X, Liu T, Wang X. The Effect of RADA16-I and CDNF on Neurogenesis and Neuroprotection in Brain Ischemia-Reperfusion Injury. International Journal of Molecular Sciences. 2022; 23(3):1436. https://doi.org/10.3390/ijms23031436
Chicago/Turabian StyleLiu, Xingyu, Haiyuan Ren, Ai Peng, Haoyang Cheng, Jiahao Chen, Xue Xia, Ting Liu, and Xiaojing Wang. 2022. "The Effect of RADA16-I and CDNF on Neurogenesis and Neuroprotection in Brain Ischemia-Reperfusion Injury" International Journal of Molecular Sciences 23, no. 3: 1436. https://doi.org/10.3390/ijms23031436
APA StyleLiu, X., Ren, H., Peng, A., Cheng, H., Chen, J., Xia, X., Liu, T., & Wang, X. (2022). The Effect of RADA16-I and CDNF on Neurogenesis and Neuroprotection in Brain Ischemia-Reperfusion Injury. International Journal of Molecular Sciences, 23(3), 1436. https://doi.org/10.3390/ijms23031436





