Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Selenides
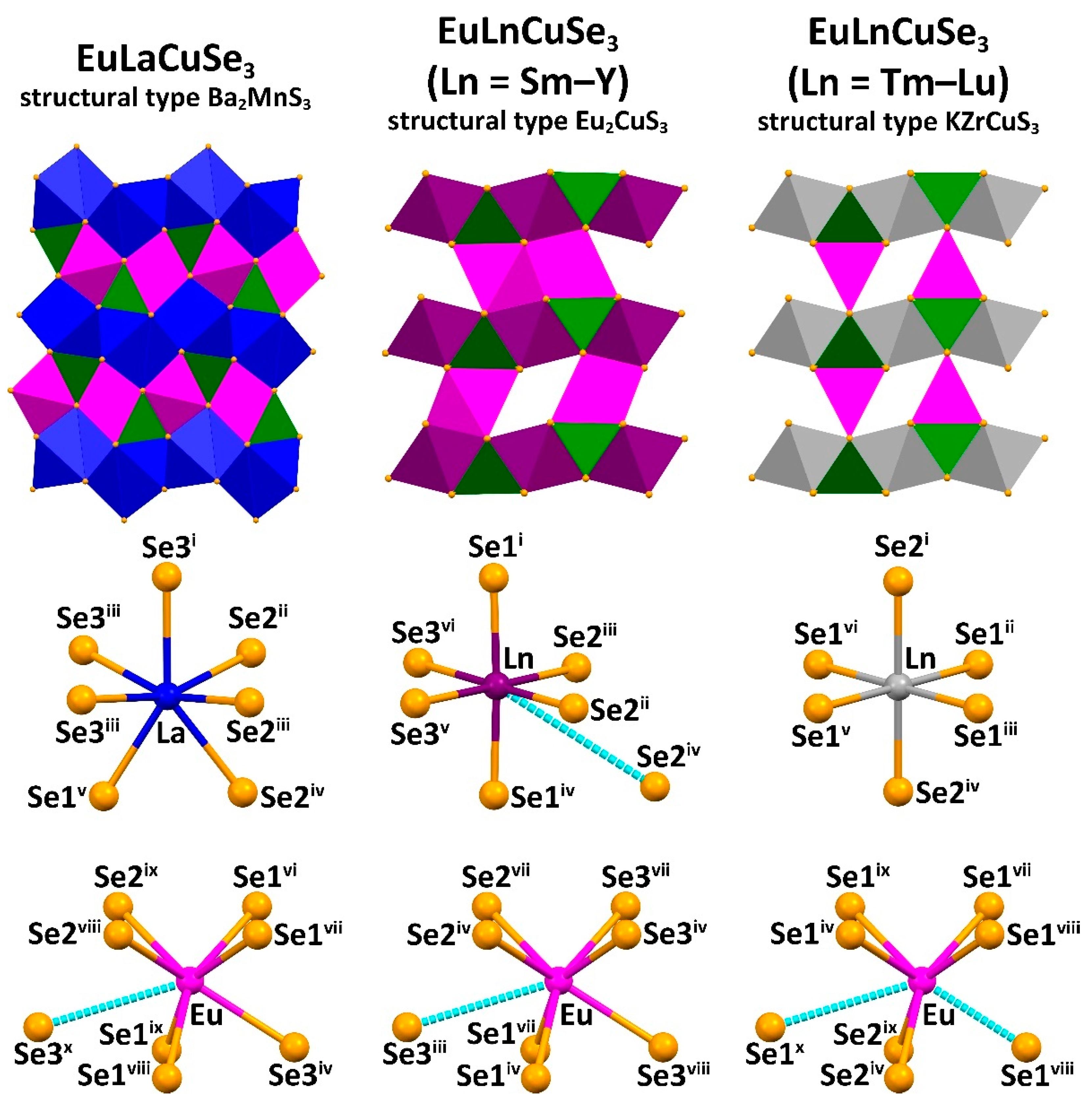
2.2. Crystal Structures
2.3. Elastic Properties
2.4. Phonon, Raman and IR Spectra
2.5. Band Structure and Optical Properites
2.6. Magnetic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Methods
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulay, L.D.; Kaczorowski, D.; Pietraszko, A. Crystal structure and magnetic properties of YbCuPbSe3. J. Alloys Compd. 2006, 413, 26–28. [Google Scholar] [CrossRef]
- Kuo, S.-M.; Chang, Y.-M.; Chung, I.; Jang, J.-I.; Her, B.-H.; Yang, S.-H.; Ketterson, J.B.; Kanatzidis, M.G.; Hsu, K.-F. New metal chalcogenides Ba4CuGa5Q12 (Q = S, Se) displaying strong infrared nonlinear optical response. Chem. Mater. 2013, 25, 2427–2433. [Google Scholar] [CrossRef]
- Huang, F.Q.; Mitchell, K.; Ibers, J.A. New layered materials: Syntheses, structures, and optical and magnetic properties of CsGdZnSe3, CsZrCuSe3, CsUCuSe3, and BaGdCuSe3. Inorg. Chem. 2001, 40, 5123–5126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Wu, T.; Bu, X.; Feng, P. Three dimensional open framework built from Cu-S icosahedral clusters and its photocatalytic property. J. Am. Chem. Soc. 2008, 130, 15238–15239. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Zheng, Y.-J.; Chen, Y.-K.; Yu, J.-S.; Wu, L.-M. Ba5Cu8In2S12: A quaternary semiconductor with a unique 3D copper-rich framework and ultralow thermal conductivity. Chem. Commun. 2017, 53, 2590–2593. [Google Scholar] [CrossRef] [Green Version]
- Repins, I.L.; Stanbery, B.J.; Young, D.L.; Li, S.S.; Metzger, W.K.; Perkins, C.L.; Shafarman, W.N.; Beck, M.E.; Chen, L.; Kapur, V.K.; et al. Comparison of device performance and measured transport parameters in widely varying Cu (In, Ga)(Se, S) solar cells. Prog. Photovolt. Res. Appl. 2006, 14, 25–43. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wei, S.H.; Zunger, A.; Katayama-Yoshida, H. Defect physics of the CuInSe2 chalcopyrite semiconductor. Phys. Rev. B 1998, 57, 9642–9656. [Google Scholar] [CrossRef]
- Koscielski, L.A.; Ibers, J.A. The structural chemistry of quaternary chalcogenides of the type AMM’Q3. Z. Anorg. Allg. Chem. 2012, 638, 2585–2593. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Molokeev, M.S.; Chernyshev, V.A.; Aleksandrovsky, A.S.; Krylov, A.S.; Krylova, S.N.; Velikanov, D.A.; Grigoriev, M.V.; Maximov, N.G.; Shestakov, N.P.; et al. Synthesis, structure, and properties of EuScCuS3 and SrScCuS3. J. Solid State Chem. 2021, 296, 121926. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Chernyshev, V.A.; Velikanov, D.A.; Aleksandrovsky, A.S.; Shestakov, N.P.; Molokeev, M.S.; Grigoriev, M.V.; Andreev, O.V.; Garmonov, A.A.; Matigorov, A.V.; et al. Regularities of the property changes in the compounds EuLnCuS3 (Ln = La-Lu). J. Alloys Compd. 2021, 874, 159968. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Solovyov, L.A.; Chernyshev, V.A.; Aleksandrovsky, A.S.; Andreev, O.V.; Krylova, S.N.; Krylov, A.S.; Velikanov, D.A.; Molokeev, M.S.; Maximov, N.G.; et al. Synthesis, structure, and properties of EuErCuS3. J. Alloys Compunds 2019, 805, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Sengar, B.S.; Garg, V.; Siddharth, G.; Kumar, A.; Pandey, S.K.; Dubey, M.; Atuchin, V.V.; Kumar, S.; Mukherjee, S. Improving the Cu2ZnSn(S,Se)4-based photovoltaic conversion efficiency by back-contact modification. IEEE Trans. Electron. Devices 2021, 68, 2748–2752. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, K.; Xia, P.; Bai, Q. The structural and electronic properties of Cu(In1–xBx)Se2 as a new photovoltaic material. RSC Adv. 2015, 5, 85431–85435. [Google Scholar] [CrossRef]
- Li, S.; Ma, R.; Zhang, X.; Li, X.; Zhao, W.; Zhu, H. Copper yttrium selenide: A potential photovoltaic absorption material for solar cells. Mater. Des. 2017, 118, 163–167. [Google Scholar] [CrossRef]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as thermoelectric materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- Zhao, K.; Blichfeld, A.B.; Eikeland, E.; Qiu, P.; Ren, D.; Bo, B.I.; Shi, X.; Chen, L. Extremely low thermal conductivity and high thermoelectric performance in liquid-like Cu2Se1–xSx polymorph materials. J. Mater. Chem. 2017, 5, 18148–18156. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.-R.; Qin, Y.; Deng, T.; Song, Q.; Jiang, B.; Liu, R.; Qiu, P.; Shi, X.; Chen, L. Copper chalcogenide thermoelectric materials. Sci. China Mater. 2019, 62, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Benaadad, M.; Nafidi, A.; Melkoud, S.; Khan, M.S.; Soubane, D. First-Principles investigations of structural, optoelectronic and thermoelectric properties of Cu-based chalcogenides compounds. J. Mater. Sci. 2021, 56, 15882–15897. [Google Scholar] [CrossRef]
- Sengar, B.S.; Garg, V.; Awasthi, V.; Aaryashree; Kumar, S.; Mukherjee, C.; Gupta, M.; Mukherjee, S. Growth and characterization of dual ion beam sputtered Cu2ZnSn (S, Se)4 thin films for cost-effective photovoltaic application. Sol. Energy 2016, 139, 1–12. [Google Scholar] [CrossRef]
- Lee, Y.S.; Gershon, T.; Gunawan, O.; Todorov, T.K.; Gokmen, T.; Virgus, Y.; Guha, S. Cu2ZnSnSe4 thin-film solar cells by thermal co-evaporation with 11.6% efficiency and improved minority carrier diffusion length. Adv. Energy Mater. 2015, 5, 1401372. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Beltran-Suito, R.; Hlukhyy, V.; Schmidt, J.; Menezes, P.W.; Driess, M. Crystalline copper selenide as a reliable non-noble electro(pre)catalyst for overall water. ChemSusChem 2020, 13, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Grey, I.E.; Steinfink, H. Crystal structure of Ba2MnSe3. Linear antiferromagnetism in Ba2MnX3 (X = S, Se). Inorg. Chem. 1971, 10, 691–696. [Google Scholar]
- Mansuetto, M.F.; Keane, P.M.; Ibers, J.A. Synthesis, structure, and conductivity of the new group IV chalcogenides KCuZrQ3 (Q = S, Se, Te). J. Solid State Chem. 1992, 101, 257–264. [Google Scholar] [CrossRef]
- Liu, C.; Hou, P.; Chai, W.; Tian, J.; Zheng, X.; Shen, Y.; Zhi, M.; Zhou, C.; Liu, Y. Hydrazine-Hydrothermal syntheses, characterizations and photoelectrochemical properties of two quaternary chalcogenidoantimonates(III) BaCuSbQ3 (Q = S, Se). J. Alloys Compd. 2016, 679, 420–425. [Google Scholar] [CrossRef]
- Christuk, A.E.; Wu, P.; Ibers, J.A. New quaternary chalcogenides BaLnMQ3 (Ln = Rare Earth; M = Cu, Ag; Q = S, Se). Structures and grinding-lnduced phase transition in BaLaCuQ3. J. Solid State Chem. 1994, 110, 330–336. [Google Scholar] [CrossRef]
- Strobel, S.; Schleid, T. Three structure types for strontium copper (I) lanthanide (III) selenides SrCuMSe3 (M = La, Gd, Lu). J. Alloys Compd. 2006, 418, 80–85. [Google Scholar] [CrossRef]
- Strobel, S.; Schleid, T. Quaternary strontium copper (I) lanthanoid (III) selenides with cerium and praseodymium: SrCuCeSe3 and SrCuPrSe3, unequal brother and sister. Z. Naturforsch. 2004, 59b, 985–991. [Google Scholar] [CrossRef]
- Maier, S.; Prakash, J.; Berthebaud, D.; Perez, O.; Bobev, S.; Gascoin, F. Crystal structures of the four new quaternary copper (I)-selenides A0.5CuZrSe3 and ACuYSe3 (A = Sr, Ba). J. Solid State Chem. 2016, 242, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ibers, J.A. Synthesis and characterization of a series of quaternary chalcogenides BaLnMQ3 (Ln = rare earth, M = coinage metal, Q = Se or Te). J. Solid State Chem. 1999, 147, 366–371. [Google Scholar] [CrossRef]
- Wu, P.; Christuk, A.E.; Ibers, J.A. New quaternary chalcogenides BaLnMQ3 (Ln = Rare Earth or Sc; M = Cu, Ag; Q = S, Se). Structure and property variation vs. rare-earth element. J. Solid State Chem. 1994, 110, 337–344. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Maier, S.; Steinberg, S. Eu2CuSe3 revisited by means of experimental and quantum-chemical techniques. Eur. J. Inorg. Chem. 2021, 15, 1510–1517. [Google Scholar] [CrossRef]
- Pal, K.; Xia, Y.; Shen, J.; He, J.; Luo, Y.; Kanatzidis, M.G.; Wolverton, C. Accelerated discovery of a large family of quaternary chalcogenides with very low lattice thermal conductivity. Comput. Mater. 2021, 7, 82. [Google Scholar] [CrossRef]
- Gulay, L.D.; Shemet, V.Y.; Olekseynuk, I.D. Crystal structures of the compounds YCuPbSe3, Y3CuSnSe7 and Y3Cu0.685Se6. J. Alloys Compd. 2004, 385, 160–168. [Google Scholar] [CrossRef]
- Pal, K.; Hua, X.; Xia, Y.; Wolverton, C. Unraveling the structure-valence-property relationships in AMM′q3 chalcogenides with promising thermoelectric performance. ACS Appl. Energy Mater. 2019, 3, 2110–2119. [Google Scholar] [CrossRef]
- Hao, S.; Ward, L.; Luo, Z.; Ozolins, V.; Dravid, V.P.; Kanatzidis, M.G.; Wolverton, C. Design strategy for high-performance thermoelectric materials: The prediction of electron-doped KZrCuSe3. Chem. Mater. 2019, 31, 3018–3024. [Google Scholar] [CrossRef]
- Ishtiyak, M.; Jana, S.; Karthikeyan, R.; Mamindla, R.; Tripathy, B.; Malladi, S.K.; Niranjan, M.; Prakash, J. Syntheses of five new layered quaternary chalcogenides SrScCuSe3, SrScCuTe3, BaScCuSe3, BaScCuTe3, and BaScAgTe3: Crystal structures, thermoelectric properties, and electronic structures. Inorg. Chem. Front. 2021, 8, 4086–4101. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D. Crystal structures of the RCuPbSe3 (R = Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu) compounds. J. Alloys Compd. 2005, 387, 160–164. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D.; Wołcyrz, M.; Stpien-Damm, J.; Pietraszko, A. Investigation of the Ho2Se3–Cu2Se–PbSe and Er2Se3–Cu2Se–PbSe systems at 870 K. J. Alloys Compd. 2006, 416, 173–178. [Google Scholar] [CrossRef]
- Gulay, L.D.; Olekseyuk, I.D. Structure types of the Pb-containing compounds of the systems R2X3–Cu2X–PbX (R-Rare earth element, X = S, Se). Visnyk Lviv. Univ. 2007, 48, 54–60. [Google Scholar]
- Gulay, L.D.; Wołcyrz, M.; Olekseyuk, I.D. Investigation of the Tb2Se3–Cu2Se–PbSe and Dy2Se3–Cu2Se–PbSe systems at 870 K. Pol. J. Chem. 2006, 80, 805–815. [Google Scholar]
- Gulay, L.D.; Wołcyrz, M.; Pietraszko, A.; Olekseyuk, I.D. Investigation of the Tm2Se3–Cu2Se–PbSe and Lu2Se3–Cu2Se–PbSe systems at 870 K. Pol. J. Chem. 2006, 80, 1703–1714. [Google Scholar] [CrossRef]
- Eberle, M.A. Darstellung und Charakterisierung quaternärer Seltenerdmetall-Verbindungen in Kombination mit Kupfer und Schwefel. Ph.D. Thesis, Stuttgart University, Stuttgart, Germany, 16 December 2016. [Google Scholar]
- Lemoine, P.; Carré, D.; Guittard, M. Structure du sulfure d’europium et de cuivre Eu2CuS3. Acta Crystallog. 1986, 42, 390–391. [Google Scholar] [CrossRef]
- Zhao, L.-D.; Tan, G.; Hao, S.; He, J.; Pei, Y.; Chi, H.; Wang, H.; Gong, S.; Xu, H.; Dravid, V.P.; et al. Ultrahigh power factor and thermoelectric performance in holedoped single crystal SnSe. Science 2016, 351, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-Performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Matsunaga, T.; Yamada, N.; Kojima, R.; Shamoto, S.; Sato, M.; Tanida, H.; Uruga, T.; Kohara, S.; Takata, M.; Zalden, P.; et al. Phase-Change materials: Vibrational softening upon crystallization and its impact on thermal properties. Adv. Funct. Mater. 2011, 21, 2232–2239. [Google Scholar] [CrossRef] [Green Version]
- Rugut, E.; Joubert, D.; Jones, G. Lattice dynamics and thermoelectric properties of YCuSe2. Mater. Today Commun. 2019, 21, 100617. [Google Scholar] [CrossRef]
- Ijjaali, I.; Mitchell, K.; Ibers, J.A. Preparation and structure of the light rare-earth copper selenides LnCuSe2 (Ln = La, Ce, Pr, Nd, Sm). J. Solid State Chem. 2004, 177, 760–764. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Andreev, O.V. Phase equilibria in systems DyCuS2–EuS and Cu2S–Dy2S3–EuS. Russ. J. Inorg. Chem. 2018, 63, 1494–1500. [Google Scholar] [CrossRef]
- Sikerina, N.V. Regularities of Phase Equilibria in SrS–Ln2S3–Cu2S Systems, Preparation and Structure of SrLnCuS3 Compounds. Ph.D. Thesis, University of Tyumen, Tyumen, Russia, 13 December 2005. [Google Scholar]
- Ruseikina, A.V.; Andreev, O.V. Phase equilibria in the Cu2S–La2S3–EuS system. Russ. J. Inorg. Chem. 2017, 62, 610–618. [Google Scholar] [CrossRef]
- Solovieva, A.V. Regularities of Phase Equilibria in AIIS–FeS, AIIS–FeS–Ln2S3, AIIS–Cu2S–Ln2S3 (AII = Mg, Ca, Sr, Ba; Ln = La–Lu) Systems. Ph.D. Thesis, University of Tyumen, Tyumen, Russia, 11 May 2005. [Google Scholar]
- Gulay, L.D.; Shemet, V.Y.; Olekseyuk, I.D.; Stępień-Damm, J.; Pietraszko, A.; Koldun, L.V.; Filimonyuk, J.O. Investigation of the R2S3–Cu2S–PbS (R = Y, Dy, Gd, Ho and Er) systems. J. Alloys Compd. 2007, 431, 77–84. [Google Scholar] [CrossRef]
- Andreev, O.V.; Ruseikina, A.V.; Solov’ev, L.A. Phase diagrams of sections in the EuS–Cu2S–Nd2S3 System. Russ. J. Inorg. Chem. 2011, 56, 792–797. [Google Scholar] [CrossRef]
- Aliyeva, R.A.; Bayramova, S.T.; Aliyev, O.M. Phase equilibriums in quasi-ternary system PbS–Cu2S–La2S3. Chem. Probl. 2008, 3, 503–508. [Google Scholar]
- Bayramova, S.T.; Aliyeva, S.I.; Ajdarova, D.S.; Aliyev, O.M. Phase equilibriums in quasi-ternary system Cu2S-PbS-Gd2S3 by sections CuGdS2–PbS and Cu2S–PbCuGdS3. Chem. Probl. 2015, 4, 424–427. [Google Scholar]
- Ruseikina, A.V.; Solov’ev, L.A.; Andreev, O.V. Crystal structures of α- and β-EuPrCuS3. Russ. J. Inorg. Chem. 2013, 58, 1231–1236. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Solov’ev, L.A. Crystal structures of α- and β-SrCeCuS3. Russ. J. Inorg. Chem. 2016, 61, 482–487. [Google Scholar] [CrossRef]
- Andreev, O.V.; Atuchin, V.V.; Aleksandrovsky, A.S.; Denisenko, Y.G.; Zakharov, B.A.; Tyutyunnik, A.P.; Habibullayev, N.N.; Velikanov, D.A.; Ulybin, D.A.; Shpindyuk, D.D. Synthesis, structure, and properties of EuLnCuSe3 (Ln = Nd, Sm, Gd, Er). Crystals 2022, 12, 17. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Kochedigov, V.A.; Zakiryanova, I.D.; Korzun, I.V. Research of thermal dissociation of interaction products of rare-earth element oxides with the components of atmospheric air. Anal. Control 2005, 9, 58–63. [Google Scholar]
- Hussen, G.A.; Buttrey, D.J.; DeSanto, P.; Abd-Elgaber, A.A.; Roshdy, H.; Myhoud, A. Formation and characterization of samarium oxide generated from different precursors. Thermochim. Acta 2003, 402, 27–36. [Google Scholar] [CrossRef]
- Abu-Zied, B.M.; Mohamed, A.-R.N.; Asiri, A.M. Effect of Thermal Treatment on the Formation, Textural and Electrical Conductivity Properties of Nanocrystalline Tb4O7. J. Nanosci. Nanotechnol. 2015, 15, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Evarestov, R.A.; Bandura, A.V. First-Principles calculations on the four phases of BaTiO3. J. Comput. Chem. 2012, 33, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, H.C. First principles study of zone centre phonons in rare-earth pyrochlore titanates, RE2Ti2O7 (RE = Gd, Dy, Ho, Er, Lu; Y). Vib. Spectrosc. 2012, 62, 180–187. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Solovyov, L.A.; Grigoriev, M.V.; Andreev, O.V. Crystal structure variations in the series SrLnCuS3 (Ln = La, Pr, Sm, Gd, Er and Lu). Acta Cryst. 2019, 75, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Wakeshima, M.; Furuuchi, F.; Hinatsu, Y. Crystal structures and magnetic properties of novel rare-earth copper sulfides, EuRCuS3 (R = Y, Gd–Lu). J. Phys. Condens. Matter 2004, 16, 5503–5518. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Demchuk, Z.A. Crystal structure and properties of AHoCuS3 (A = Sr or Eu). Russ. J. Inorg. Chem. 2017, 62, 27–32. [Google Scholar] [CrossRef]
- Azarapin, N.O.; Aleksandrovsky, A.S.; Atuchin, V.V.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Mukherjee, S.; Oreshonkov, A.S.; Andreev, O.V. Synthesis, structural and spectroscopic properties of orthorhombic compounds BaLnCuS3 (Ln = Pr, Sm). J. Alloys Compd. 2020, 832, 153134. [Google Scholar] [CrossRef]
- Furuuchi, F.; Wakeshima, M.; Hinatsu, Y. Magnetic properties and (151) Eu Mossbauer effects of mixed valence europium copper sulfide Eu2CuS3. J. Solid State Chem. 2004, 177, 3853–3858. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Zhao, Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal anisotropy index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef] [Green Version]
- Tuxworth, A.J.; Wang, C.-H.; Evans, J.S.O. Evans, synthesis, characterisation and properties of rare earth oxyselenides A4O4Se3 (A = Eu, Gd, Tb, Dy, Ho, Er, Yb and Y). Dalton Trans. 2015, 44, 3009–3019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eick, H.A. The crystal structure and lattice parameters of some rare earth mono-seleno oxides. Acta Cryst. 1960, 13, 161. [Google Scholar] [CrossRef]
- Oreshonkov, A.S.; Denisenko, Y.G. Structural features of Y2O2SO4 via DFT calculations of electronic and vibrational properties. Materials 2021, 14, 3246. [Google Scholar] [CrossRef]
- Landi, S., Jr.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Azarapin, N.O.; Atuchin, V.V.; Maximov, N.G.; Aleksandrovsky, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; Krylov, A.S.; Burkhanova, T.M.; Mukherjee, S.; et al. Synthesis, structure, melting and optical properties of three complex orthorhombic sulfides BaDyCuS3, BaHoCuS3 and BaYbCuS3. Mater. Res. Bull. 2021, 140, 111314. [Google Scholar] [CrossRef]
- Neel, L. Magnetism and local molecular field. Science 1971, 174, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Yusuf, S.M. The phenomenon of negative magnetization and its implications. Phys. Rep. 2015, 556, 1–34. [Google Scholar] [CrossRef]
- Dönni, A.; Pomjakushin, V.Y.; Zhang, L.; Yamaura, K.; Belik, A.A. Origin of negative magnetization phenomena in (Tm1–xMnx)MnO3: A neutron diffraction study. Phys. Rev. 2020, B101, 054442. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. The Principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 1929, 51, 1010–1026. [Google Scholar] [CrossRef]
- Solovyov, L.A. Full-Profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- Brennan, T.D.; Ibers, J.A. LaPbCuS3: Cu(I) insertion into the a-La2S3 framework. J. Solid State Chem. 1992, 97, 377–382. [Google Scholar] [CrossRef]
- Pennington, W.T. DIAMOND—Visual Crystal Structure Information System. J. Appl. Crystallogr. 1999, 32, 1028–1029. [Google Scholar] [CrossRef] [Green Version]
- Brown, I.D.; Altermatt, D. Bond-Valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. 1985, B41, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Velikanov, D.A. High-Sensitivity measurements of the magnetic properties of materials at cryogenic temperatures. Inorg. Mater. Appl. Res. 2020, 11, 801–808. [Google Scholar] [CrossRef]
- Velikanov, D.A. Magnetometer with a Superconducting Quantum Interferometric Sensor, RF Pat. 2481591. 2013. Available online: http://www.freepatent.ru/patents/2481591 (accessed on 19 October 2020).
- Velikanov, D.A. Vibration Magnetometer. RF Pat. 2341810. 2008. Available online: http://www.freepatent.ru/patents/2341810 (accessed on 19 October 2020).
- Crystal. Available online: http://www.crystal.unito.it/index.php (accessed on 19 June 2021).
- Energy-Consistent Pseudopotentials of the Stuttgart/Cologne Group. Available online: http://www.tc.uni-koeln.de/PP/clickpse.en.html (accessed on 19 June 2018).











| EuLaCuSe3 | EuSmCuSe3 | EuGdCuSe3 | EuTbCuSe3 | EuDyCuSe3 | |
| Space group | Pnma | Pnma | Pnma | Pnma | Pnma |
| Structural type | Ba2MnS3 | Eu2CuS3 | Eu2CuS3 | Eu2CuS3 | Eu2CuS3 |
| a (Å) | 8.4659(5) | 10.7634(5) | 10.6746(11) | 10.6027(15) | 10.5628(8) |
| b (Å) | 4.2140(3) | 4.1138(2) | 4.0966(4) | 4.0843(6) | 4.0768(3) |
| c (Å) | 16.6636(11) | 13.3860(7) | 13.3993(14) | 13.3926(19) | 13.3915(11) |
| V (Å3) | 594.47(7) | 592.71(5) | 585.94(11) | 579.97(14) | 576.67(8) |
| Dcalc (g cm−3) | 6.60401 | 6.75257 | 6.90762 | 6.99871 | 7.07976 |
| R factors | RDDM = 0.058 | RDDM = 0.040 | RDDM = 0.060 | RDDM = 0.042 | RDDM = 0.058 |
| Rexp = 0.053 | Rexp = 0.036 | Rexp = 0.042 | Rexp = 0.039 | Rexp = 0.053 | |
| RBragg = 0.044 | RBragg = 0.026 | RBragg = 0.031 | RBragg = 0.028 | RBragg = 0.024 | |
| Impurity | – | 1% SmCuSeO | 1.2% Gd4Se3O4 | 1.3% Tb2SeO2 | 2.8% Dy4Se3O4 |
| EuHoCuSe3 | EuYCuSe3 | EuTmCuSe3 | EuYbCuSe3 | EuLuCuSe3 | |
| Space group | Pnma | Pnma | Cmcm | Cmcm | Cmcm |
| Structural type | Eu2CuS3 | Eu2CuS3 | KZrCuS3 | KZrCuS3 | KZrCuS3 |
| a (Å) | 10.5182(8) | 10.5659(13) | 4.05721(17) | 4.0490(2) | 4.0434(2) |
| b (Å) | 4.0714(3) | 4.0800(5) | 13.3784(6) | 13.3699(7) | 13.3694(6) |
| c (Å) | 13.3898(10) | 13.4006(16) | 10.4477(5) | 10.4124(6) | 10.3954(5) |
| V (Å3) | 573.40(8) | 577.69(12) | 567.09(4) | 563.67(5) | 561.94(5) |
| Dcalc (g cm−3) | 7.14826 | 6.22125 | 7.27467 | 7.36674 | 7.41257 |
| R factors | RDDM = 0.057 | RDDM = 0.061 | RDDM = 0.046 | RDDM = 0.047 | RDDM = 0.045 |
| Rexp = 0.038 | Rexp = 0.050 | Rexp = 0.038 | Rexp = 0.040 | Rexp = 0.040 | |
| RBragg = 0.029 | RBragg = 0.041 | RBragg = 0.021 | RBragg = 0.022 | RBragg = 0.019 | |
| Impurity | 2.2% Ho2SeO2 | 2.2% Y2SeO2 | 4.9% Tm4Se3O4 | 4.1% Yb4Se3O4 | 4.3% Lu4Se3O4 |
| La | Ce | Pr | Nd | Sm | Gd | Tb | Dy | Ho | Y | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Space group | Pnma | Cmcm | ||||||||||||
| Structural type | Ba2MnS3 | Eu2CuS3 | KZrCuS3 | |||||||||||
| B (GPa) | 77.2 | 77.6 | 66.6 | 67.1 | 68.2 | 68.5 | 69.1 | 69.1 | 69.2 | 70.0 | 70.9 | 71.4 | 71.7 | 72.0 |
| G (GPa) | 35.6 | 31.6 | 32.0 | 31.9 | 31.2 | 30.1 | 30.3 | 27.8 | 24.3 | 30.4 | 25.1 | 26.9 | 28.1 | 28.8 |
| HV (GPa) | 4.8 | 4.9 | 4.7 | 4.6 | 4.3 | 4.0 | 4.0 | 3.4 | 2.7 | 4.0 | 2.8 | 3.1 | 3.4 | 3.5 |
| AU | 0.29 | 0.31 | 0.59 | 0.67 | 1.07 | 1.62 | 1.68 | 3.04 | 6.39 | 1.92 | 6.46 | 4.69 | 3.83 | 3.45 |
| Calc. BGB3LYP (eV) 1 | 2.63 | 2.63 | 2.38 | 2.38 | 2.42 | 2.45 | 2.48 | 2.49 | 2.51 | 2.52 | 2.54 | 2.56 | 2.59 | |
| Calc. BGPBE0 (eV) 1 | 2.78 | 2.79 | 2.50 | 2.51 | 2.53 | 2.56 | 2.58 | 2.59 | 2.61 | 2.62 | 2.65 | 2.67 | 2.69 | |
| Calc. BGPBE (eV) 1 | 1.25 | 1.25 | 1.00 | 1.01 | 1.04 | 1.06 | 1.07 | 1.08 | 1.10 | 1.04 | 1.12 | 1.13 | 1.14 | 1.15 |
| Calc. BGPBE (eV) [32] | 0.94 | 0.91 | 0.95 | 0.98 | 1.04 | 0.96 | 1.11 | 1.02 | 1.04 | 1.07 | 1.06 | 1.09 | – | 1.13 |
| Exp. BGindirect (eV) 1 | 1.19 | – | – | – | – | 1.01 | 0.93 | 0.93 | – | 0.80 | – | 1.24, 1.70 | – | 2.06 |
| Exp. BGKubelka-Munk (eV) 1 | 1.35 | – | – | – | 1.04, 1.76 | 1.11, 1.82 | 0.93, 1.88 | 1.09, 1.60 | 1.14, 1.87 | 1.03 | – | 1.16 | – | 1.38 |
| Exp. BGdirect (eV) 1 | 1.54 | – | – | – | 1.95 | 2.01 | 1.97 | 1.87 | 2.05 | 1.19 | – | 1.07 | – | 2.09 |
| La | Sm | Gd | Tb | Dy | Ho | Y | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|---|
| Space group | Pnma | Cmcm | ||||||||
| Structural type | Ba2MnS3 | Eu2CuS3 | KZrCuS3 | |||||||
| χ·106 (m3 mol−1) | 0.326 | 0.330 | 0.628 | 0.887 | 0.884 | 0.886 | 0.325 | 0.644 | 0.430 | 0.373 |
| Exp. μ296K (μB) | 7.86 | 7.87 | 10.86 | 12.90 | 12.94 | 12.95 | 7.85 | 10.99 | 9.00 | 8.38 |
| Exp. μ15K (μB) | 7.5 | – | – | 10.7 | 11.0 | 12.3 | 10.9 | 12.0 | 8.8 | 14.0 |
| Calc. μ (μB) | 7.937 | 7.982 | 11.225 | 12.550 | 13.279 | 13.248 | 7.937 | 10.962 | 9.142 | 7.937 |
| Exp. C296K (K m3 kmol−1) | 0.0970 | 0.0972 | 0.1853 | 0.2616 | 0.2631 | 0.2634 | 0.0969 | 0.1899 | 0.1272 | 0.1102 |
| Exp. C15K (K m3 kmol−1) | 0.09 | – | – | 0.19 | 0.19 | 0.24 | 0.19 | 0.22 | 0.12 | 0.32 |
| Calc. C (K m3 kmol−1) | 0.09900 | 0.1001 | 0.1980 | 0.24750 | 0.27709 | 0.27578 | 0.09900 | 0.18883 | 0.13133 | 0.09900 |
| Arrangement 1 | Ferro | – | – | Ferri | Ferri | Ferri | Ferro | Ferri | Ferro | Ferro |
| θp (K) | 0.2 | – | – | −1.3 | −0.7 | −0.4 | 3.3 | 1.1 | 4.5 | 3.1 |
| Tb | Dy | Ho | Tm | |
|---|---|---|---|---|
| Space group | Pnma | Cmcm | ||
| Structural type | Eu2CuS3 | KZrCuS3 | ||
| C (K m3 kmol−1) | 0.19 | 0.19 | 0.24 | 0.22 |
| 1/χ0 (kmol m−3) | 13 | 12 | 2.8 | 3.8 |
| σ (K m3 kmol) | 7.5 | 29 | 1.0 | 55 |
| θ (K) | 5.8 | 4.7 | 6.1 | 2.1 |
| Tc (K) | 6.0 | 5.5 | 6.2 | 4.5 |
| La | Tb | Dy | Ho | Y | Er | Tm | Yb | Lu | |
|---|---|---|---|---|---|---|---|---|---|
| Space group | Pnma | Cmcm | |||||||
| Structural type | Ba2MnS3 | Eu2CuS3 | KZrCuS3 | ||||||
| Tc for X = S (K) | 2.4 [10] | 4.9 [68] | 4.6 [68] | 4.8 [10] | 4.5 [68] | 4.8 [11] | 4.8 [68] | 5.5 [68] | 5.4 [68] |
| Tc for X = Se (K) | ~1 | 6.0 | 5.5 | 6.2 | ~3 | 4.7 [60] | 4.5 | ~4.3 | ~3 |
| Arrangement for X = S 1 | Ferro | Ferri | Ferri | Ferri | Ferro | Ferri | Ferri | Ferro | Ferro |
| Arrangement for X = Se 1 | Ferro | Ferri | Ferri | Ferri | Ferro | Ferri | Ferri | Ferro | Ferro |
| Compound (Mass) | Calculated (%) | Found (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eu | Ln | Cu | Se | O | Eu | Ln | Cu | Se | O | |
| EuLaCuSe3 (591.29) | 25.70 | 23.49 | 10.75 | 40.06 | – | 25.65 | 23.40 | 10.72 | 40.23 | – |
| EuSmCuSe3 (602.75) | 25.21 | 24.95 | 10.54 | 39.30 | – | 25.44 | 24.68 | 10.64 | 39.19 | 0.05 |
| EuGdCuSe3 (609.64) | 24.93 | 25.79 | 10.42 | 38.86 | – | 24.65 | 26.31 | 10.29 | 38.67 | 0.08 |
| EuTbCuSe3 (611.31) | 24.86 | 26.00 | 10.39 | 38.75 | – | 24.55 | 26.65 | 10.25 | 38.45 | 0.10 |
| EuDyCuSe3 (614.89) | 24.71 | 26.43 | 10.34 | 38.52 | – | 23.99 | 27.62 | 10.05 | 38.15 | 0.19 |
| EuHoCuSe3 (617.32) | 24.62 | 26.72 | 10.29 | 38.37 | – | 24.05 | 27.76 | 10.07 | 37.96 | 0.16 |
| EuYCuSe3 (541.29) | 28.07 | 16.42 | 11.74 | 43.76 | – | 27.47 | 17.43 | 11.47 | 43.38 | 0.25 |
| EuTmCuSe3 (621.32) | 24.46 | 27.19 | 10.23 | 38.12 | – | 23.24 | 29.24 | 9.72 | 37.48 | 0.32 |
| EuYbCuSe3 (625.43) | 24.30 | 27.67 | 10.16 | 37.87 | – | 23.31 | 29.37 | 9.75 | 37.31 | 0.26 |
| EuLuCuSe3 (627.35) | 24.22 | 27.89 | 10.13 | 37.76 | – | 23.16 | 29.68 | 9.70 | 37.18 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriev, M.V.; Solovyov, L.A.; Ruseikina, A.V.; Aleksandrovsky, A.S.; Chernyshev, V.A.; Velikanov, D.A.; Garmonov, A.A.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; et al. Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies. Int. J. Mol. Sci. 2022, 23, 1503. https://doi.org/10.3390/ijms23031503
Grigoriev MV, Solovyov LA, Ruseikina AV, Aleksandrovsky AS, Chernyshev VA, Velikanov DA, Garmonov AA, Molokeev MS, Oreshonkov AS, Shestakov NP, et al. Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies. International Journal of Molecular Sciences. 2022; 23(3):1503. https://doi.org/10.3390/ijms23031503
Chicago/Turabian StyleGrigoriev, Maxim V., Leonid A. Solovyov, Anna V. Ruseikina, Aleksandr S. Aleksandrovsky, Vladimir A. Chernyshev, Dmitriy A. Velikanov, Alexander A. Garmonov, Maxim S. Molokeev, Aleksandr S. Oreshonkov, Nikolay P. Shestakov, and et al. 2022. "Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies" International Journal of Molecular Sciences 23, no. 3: 1503. https://doi.org/10.3390/ijms23031503
APA StyleGrigoriev, M. V., Solovyov, L. A., Ruseikina, A. V., Aleksandrovsky, A. S., Chernyshev, V. A., Velikanov, D. A., Garmonov, A. A., Molokeev, M. S., Oreshonkov, A. S., Shestakov, N. P., Matigorov, A. V., Volkova, S. S., Ostapchuk, E. A., Kertman, A. V., Schleid, T., & Safin, D. A. (2022). Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies. International Journal of Molecular Sciences, 23(3), 1503. https://doi.org/10.3390/ijms23031503









