Intralesional Infiltrations of Arteriosclerotic Tissue Cells-Free Filtrate Reproduce Vascular Pathology in Healthy Recipient Rats
Abstract
:1. Significance
2. Introduction
3. Materials and Methods
3.1. Ethics and Consents
3.2. Collection and Processing of Samples
3.2.1. Collection of Human Samples
3.2.2. Cell-Free Filtrate Preparation
3.3. Induction of Skin Wound in Rats, Infiltration with Test Solutions and Subsequent Analyses
3.4. Statistical Analyses
4. Results
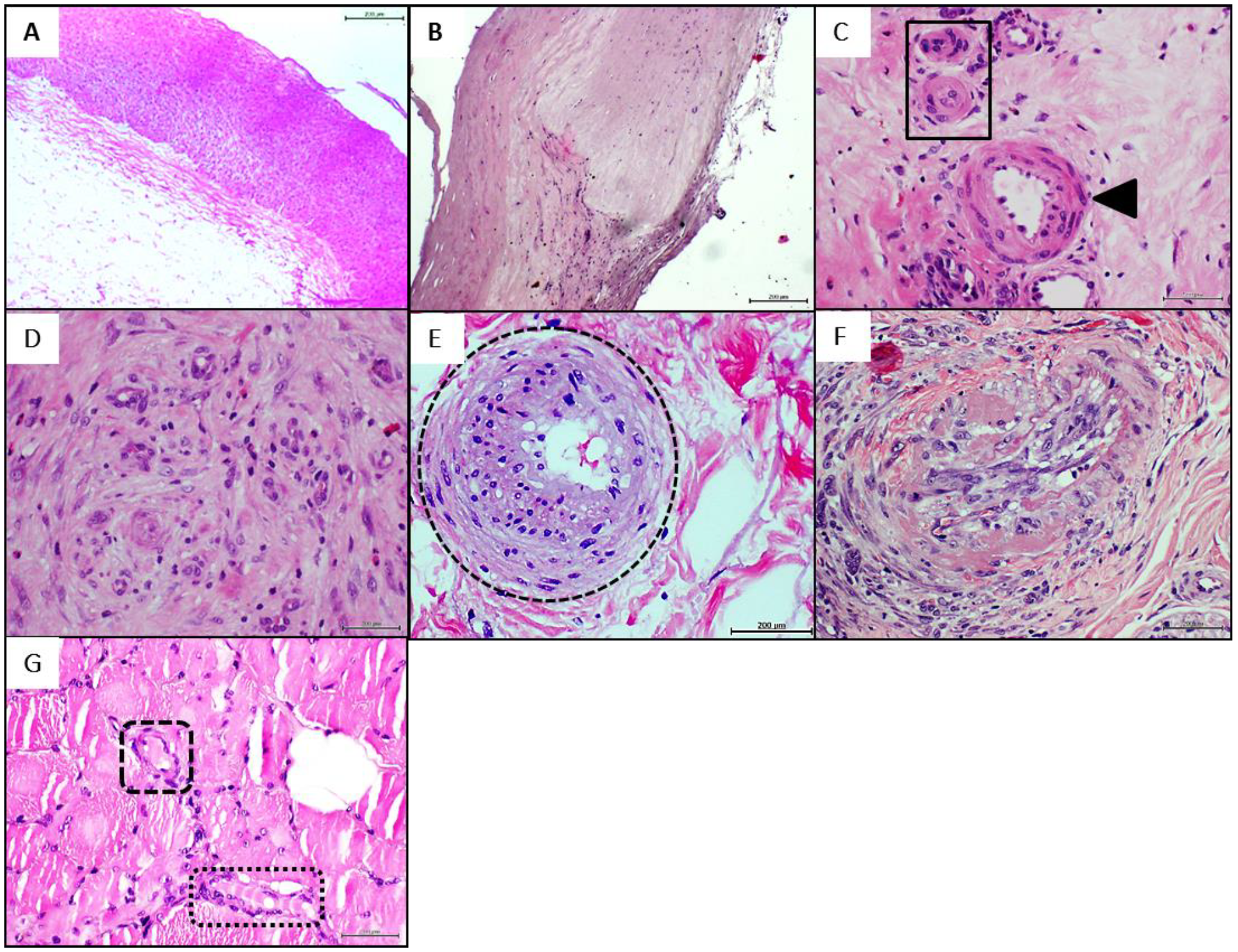
4.1. Donors’ Arteries Histopathological and Biochemical Characterization
4.2. Donors’ Homogenate Effect on Wound Healing Response, Nerves Integrity, and Inflammation
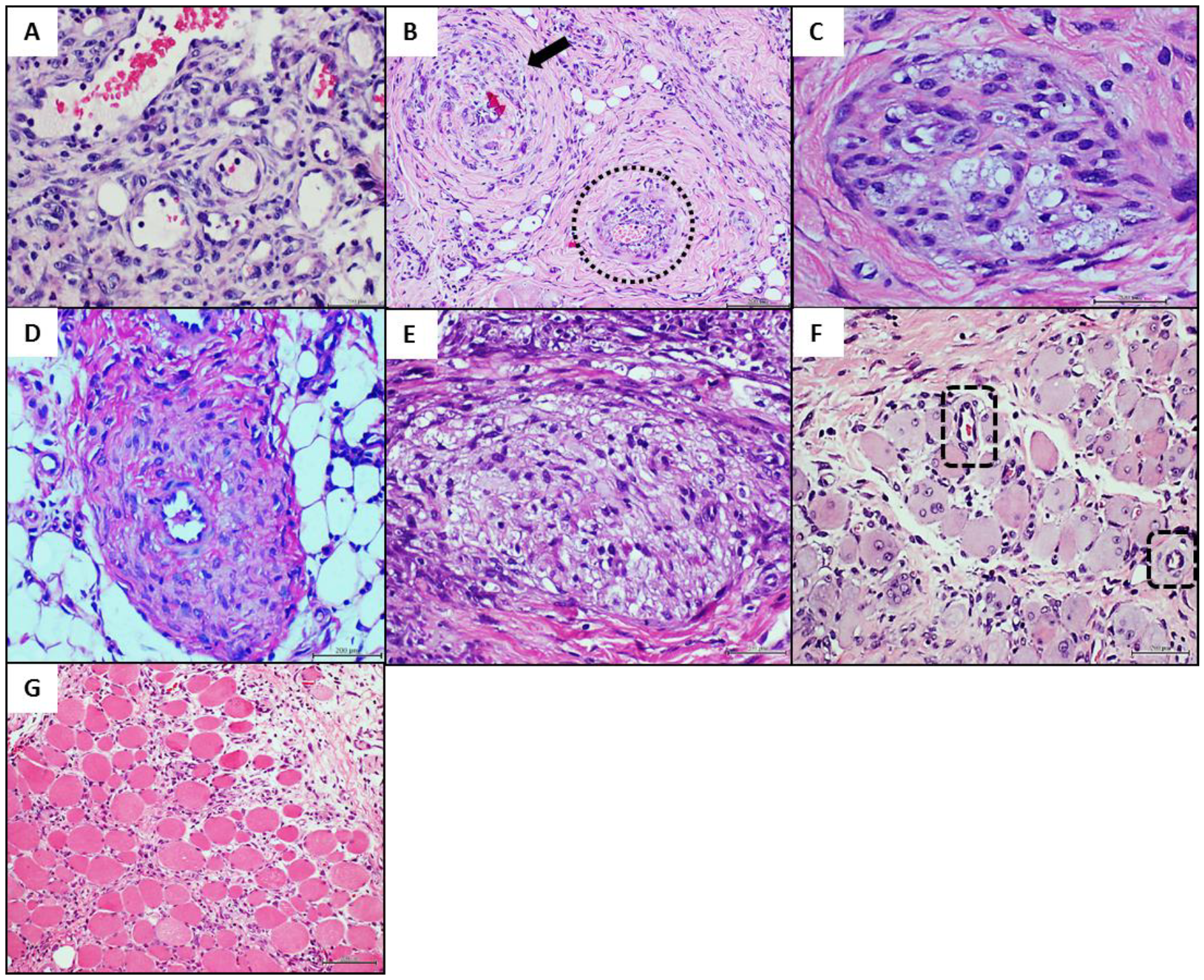
4.3. Donors’ Vascular Histopathologic Anomalies Are Recapitulated in the Rats’ Recipient Tissue
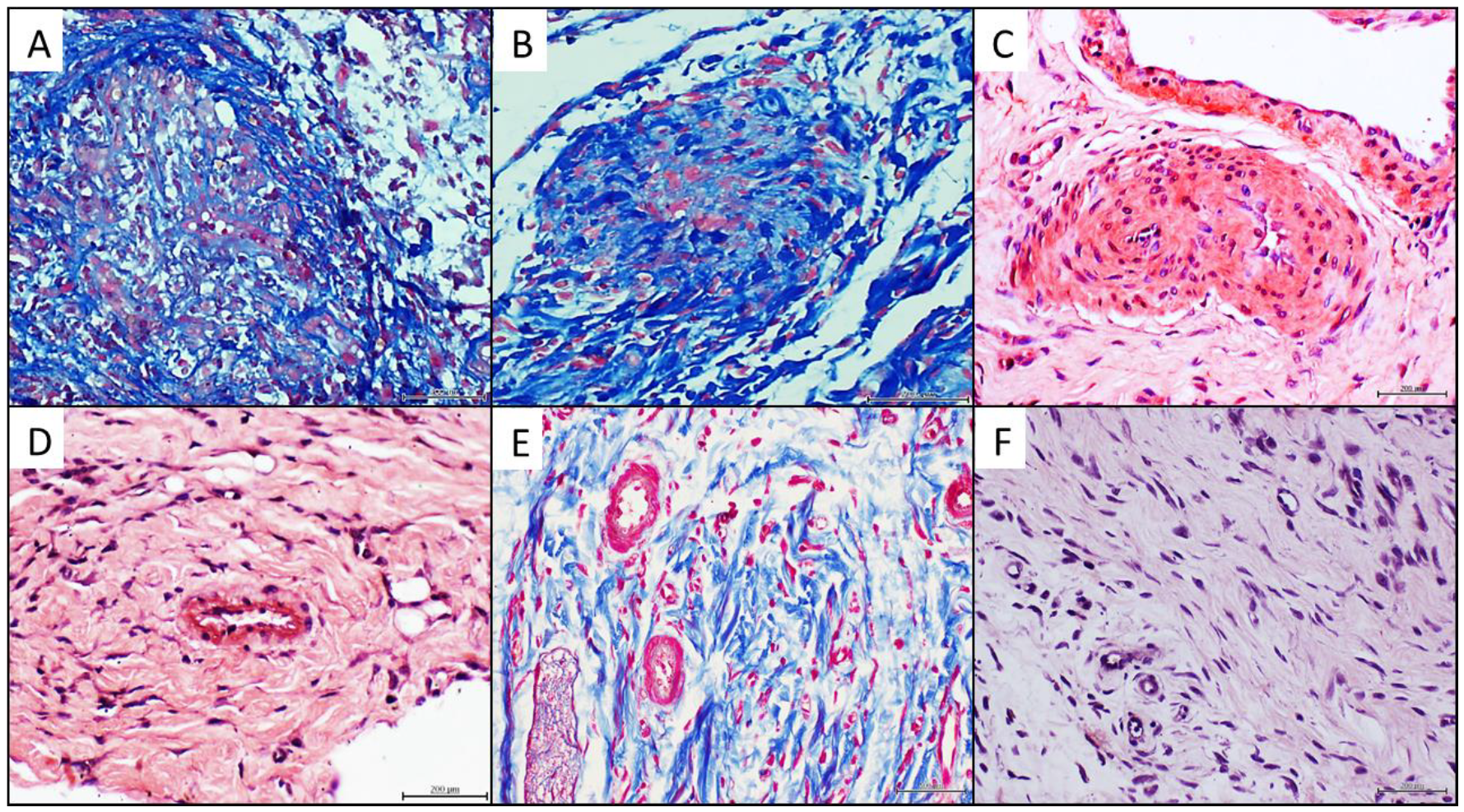
4.4. Histochemical and Immunohistochemical Characterization of the Arteriolar Remodeling
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kullo, J.I.; Rooke, T.W. Clinical Practice. Peripheral Artery Disease. N. Engl. J. Med. 2016, 374, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Gallino, A. Non-coronary atherosclerosis. Eur. Heart J. 2014, 35, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- Reinecke, H. Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur. Heart J. 2015, 36, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Conte, M.S. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasri, J. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J. Vasc. Surg. 2018, 68, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, S.; Armstrong, E.J. Diabetes mellitus: An important risk factor for peripheral vascular disease. Expert Rev. Cardiovasc. 2020, 18, 31–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olafsdottir, A.F. Excess risk of lower extremity amputations in people with type 1 diabetes compared with the general population: Amputations and type 1 diabetes. BMJ Open Diabetes Res. Care 2019, 7, e000602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.I.; Mena, C.; Sumpio, B.E. The Role of Lower Extremity Amputation in Chronic Limb-Threatening Ischemia. Int. J. Angiol. 2020, 29, 149–155. [Google Scholar] [CrossRef]
- McCarron, J.G.; Lee, M.D.; Wilson, C. The Endothelium Solves Problems That Endothelial Cells Do Not Know Exist. Trends Pharmacol. Sci. 2017, 38, 322–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalucka, J. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell 2020, 180, 764–779. [Google Scholar] [CrossRef]
- Kiseleva, R.Y. Targeting therapeutics to endothelium: Are we there yet? Drug Deliv. Transl. Res. 2018, 8, 883–902. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Mari, Y. Histological and Transcriptional Expression differences between Diabetic Foot and Pressure Ulcers. J. Diabetes Metab. 2013, 4, 296. [Google Scholar]
- Berlanga-Acosta, J.A. Intralesional infiltrations of cell-free filtrates derived from human diabetic tissues delay the healing process and recreate diabetes histopathological changes in healthy rats. Front. Clin. Diabetes Healthc. 2021, 2, 617741. [Google Scholar] [CrossRef]
- Gouin, J.P.; Kiecolt-Glaser, J.K. The impact of psychological stress on wound healing: Methods and mechanisms. Immunol. Allergy Clin. N. Am. 2011, 31, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Marucha, P.T.; Kiecolt-Glaser, J.K.; Favagehi, M. Mucosal wound healing is impaired by examination stress. Psychosom. Med. 1998, 60, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Narula, N. Pathology of Peripheral Artery Disease in Patients with Critical Limb Ischemia. J. Am. Coll. Cardiol. 2018, 72, 2152–2163. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Khoo, R.; Jansen, S. The Evolving Field of Wound Measurement Techniques: A Literature Review. Wounds 2016, 28, 175–181. [Google Scholar] [PubMed]
- Greenhalgh, D.G. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 1990, 136, 1235–1246. [Google Scholar]
- Wyffels, J.T.; Edsberg, L.E. Granulation tissue of chronic pressure ulcers as a predictive indicator of wound closure. Adv. Skin Wound Care 2011, 24, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J. Wound healing promotion in rats treated with EGF is dose dependent. Biotecnol. Apl. 1996, 13, 181–185. [Google Scholar]
- Labazi, H.; Trask, A.J. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol. Res. 2017, 123, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sachidanandam, K. Glycemic control prevents microvascular remodeling and increased tone in type 2 diabetes: Link to endothelin-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Boesch, J.M. Histological, electrophysiological and clinical effects of thermal radiofrequency therapy of the saphenous nerve and pulsed radiofrequency therapy of the sciatic nerve in dogs. Vet. Anaesth. Analg. 2019, 46, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Galkowska, H.; Wojewodzka, U.; Olszewski, W.L. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006, 14, 558–565. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—A review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlanga-Acosta, J. Expression of cell proliferation cycle negative regulators in fibroblasts of an ischemic diabetic foot ulcer. A clinical case report. Int. Wound J. 2013, 10, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Prevalent peripheral arterial disease and inflammatory burden. BMC Geriatr. 2016, 16, 213. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poznyak, A.V. NADPH Oxidases and Their Role in Atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef]
- Egana-Gorrono, L. Receptor for Advanced Glycation End Products (RAGE) and Mechanisms and Therapeutic Opportunities in Diabetes and Cardiovascular Disease: Insights from Human Subjects and Animal Models. Front. Cardiovasc. Med. 2020, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Sun, L. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc. Res. 2009, 82, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagishi, S. Advanced Glycation End Products: A Molecular Target for Vascular Complications in Diabetes. Mol. Med. 2015, 21, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Jandeleit-Dahm, K.; Cooper, M.E. The role of AGEs in cardiovascular disease. Curr. Pharm. Des. 2008, 14, 979–986. [Google Scholar] [CrossRef]
- Tian, K. CD36 in Atherosclerosis: Pathophysiological Mechanisms and Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Yue, H. CD36 Enhances Vascular Smooth Muscle Cell Proliferation and Development of Neointimal Hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Suvorava, T. Impact of eNOS-Dependent Oxidative Stress on Endothelial Function and Neointima Formation. Antioxid. Redox Signal. 2015, 23, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Ponnuswamy, P. ENOS protects from atherosclerosis despite relevant superoxide production by the enzyme in apoE mice. PLoS ONE 2012, 7, e30193. [Google Scholar]
- Melincovici, C.S. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Wang, X. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.P.; Beck, D.T.; Braith, R.W. Systemic plasma levels of nitrite/nitrate (NOx) reflect brachial flow-mediated dilation responses in young men and women. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Chronic nitrite treatment activates adenosine monophosphate-activated protein kinase-endothelial nitric oxide synthase pathway in human aortic endothelial cells. J. Funct. Foods 2021, 80, 104447. [Google Scholar] [CrossRef]
- Fan, W. Reduced Sirtuin1 signalling exacerbates diabetic mice hindlimb ischaemia injury and inhibits the protective effect of a liver X receptor agonist. J. Cell. Mol. Med. 2020, 24, 5476–5490. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Li, H.; Xia, N. The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging. Front. Physiol. 2019, 10, 1173. [Google Scholar] [CrossRef] [Green Version]
- Rafieian-Kopaei, M. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Tomczyk, M.M.; Dolinsky, V.W. The Cardiac Lipidome in Models of Cardiovascular Disease. Metabolites 2020, 10, 254. [Google Scholar] [CrossRef]
- Chevalier, J. Obstruction of Small Arterioles in Patients with Critical Limb Ischemia due to Partial Endothelial-to-Mesenchymal Transition. iScience 2020, 23, 101251. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Karvinen, H. Long-term VEGF-A expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011, 18, 1166–1172. [Google Scholar] [CrossRef] [Green Version]
- Fournet, M.; Bonte, F.; Desmouliere, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [Green Version]
- Nativel, M. Lower extremity arterial disease in patients with diabetes: A contemporary narrative review. Cardiovasc. Diabetol. 2018, 17, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H. Vascular Macrophages in Atherosclerosis. J. Immunol. Res. 2019, 2019, 4354786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L. CD36 and lipid metabolism in the evolution of atherosclerosis. Br. Med. Bull. 2018, 126, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, C.; Novials, A.; Parrizas, M. Exosomes and diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3107. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, S. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim. Biophys. Acta 2015, 1852, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Su, S.A. Emerging role of exosome-mediated intercellular communication in vascular remodeling. Oncotarget 2017, 8, 25700–25712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaverbeke, M.; Gal, D.; Holvoet, P. Functional Role of Cardiovascular Exosomes in Myocardial Injury and Atherosclerosis. Adv. Exp. Med. Biol. 2017, 998, 45–58. [Google Scholar] [PubMed]


| Antibody | Biological Implication | Reference |
|---|---|---|
| Anti-TNF-α (Ab6671) | TNF-α. Pro-inflammatory cytokine involved in the pathogenesis of arteriosclerosis | [28,29] |
| Anti-NOX2/gp91phox (Ab31092) | NADPH oxidase (NOX) activity producing reactive oxygen species, involved in arteriosclerosis development | [29,30] |
| Anti-RAGE (Ab16329) | RAGE is activated in hyperglycemia and hyperlipidemia, inducing pro-inflammatory responses and oxidative stress in a variety of vascular diseases | [29,31,32] |
| Anti-AGE (Ab23722) | Evidences indicate that interaction of AGE with their receptor (RAGE) elicits oxidative stress generation and as a result evokes proliferative, inflammatory, thrombotic, and fibrotic reactions in a variety of cells leading to vascular pathology | [33,34] |
| Anti-CD36 (Ab78054) | CD36 is a class B scavenger receptor and membrane glycoprotein playing a critical role in atherosclerotic onset and development | [35,36] |
| Anti-endothelial NOS phosphorylated in serine-1177 (Ab75639) | e-NOS plays a major role in angiogenesis, vascular homeostasis and protection. Failure of e-NOS function has been implicated in hypertension, thrombosis, atherogenesis, and abnormal vascular reactivity | [37,38] |
| Anti-VEGF receptor-2 (Ab2349) | VEGFR-2 is the major receptor for VEGF, expressed in vascular endothelial cells. It is implicated in angiogenesis, vascular cells survival, migration, and proliferation. Responsible for the conservation of a normal vascular anatomy | [39,40] |
| Sample | IL-6/mg Prot. (pg/mg) | IL-1β/mg Prot. (pg/mg) | AGE/mg Prot. (ng/mg) | MDA/mg Prot. (nmol/mg) | VCAM1 (ng/mL) | Sirtuin-1 (ng/mL) | Nitrite/Nitrate Ratio |
|---|---|---|---|---|---|---|---|
| Donor 1 Peripheral arterial disease | 65.24 | 33.99 | 5.635 | 0.313 | 2.15 | N.D. < 0.29 | 0.99 |
| Donor 2 Peripheral arterial disease | 3.99 | 17.53 | 1.022 | 0.464 | 2.15 | 0.78 | 0.56 |
| Healthy donor/Normal artery | 10.43 | 16.97 | 0.676 | 0.601 | 8.86 | 3.26 | 2.81 |
| Wound Closure (%) | Arterial Thickening (Wall to Lumen Ratio) | Nerve Fibres Damaged (%) | Inflammation | |
|---|---|---|---|---|
| Experiment 1 | ||||
| PAD artery 1 | 47.80 ± 6.40 (a) | 1.78 ± 0.39 (a) | 58.15 ± 14.87 (a) | 7.87 ± 1.83 (a) |
| Healthy artery | 53.78 ± 13.30 (a) | 0.37 ± 0.12 (b) | 53.47 ± 29.35 (a) | 5.57 ± 2.39 (b) |
| Saline | 52.45 ± 9.55 (a) | 0.34 ± 0.11 (b) | 40.80 ± 15.94 (b) | 2.03 ± 0.17 (c) |
| Experiment 2 | ||||
| PAD artery 2 | 68.08 ± 10.03 (b) | 1.15 ± 0.29 (a) | 73.98 ± 13.14 (a) | 2.86 ± 1.25 (a) |
| Healthy artery | 79.65 ± 9.12 (a) | 0.66 ± 0.18 (b) | 53.51 ± 24.60 (a,b) | 2.38 ± 1.12 (a) |
| Saline | 74.12 ± 7.65 (a,b) | 0.62 ± 0.35 (b) | 41.72 ± 19.60 (b) | 1.09 ± 0.27 (b) |
| Markers | Human Donor Samples | Recipient Rats | |||
|---|---|---|---|---|---|
| HAD | PAD | HAD | PAD | p Value | |
| AGE | 0.00 | 7.75 | 0.00 ± 0.00 | 0.26 ± 0.46 | 0.6016 |
| CD36 | 1.50 | 4.88 | 0.59 ± 0.60 | 0.31 ± 0.46 | 0.2948 |
| RAGE | 0.00 | 12.25 | 0.41 ± 0.50 | 16.35 ± 3.58 | 0.0002 |
| NOX2 | 0.25 | 9.70 | 5.46 ± 0.80 | 11.68 ± 2.11 | <0.0001 |
| TNF-α | 0.50 | 13.10 | 4.03 ± 1.84 | 13.08 ± 2.14 | 0.0003 |
| e-NOS | 8.27 | 0.88 | 14.21 ± 1.46 | 4.44 ± 1.15 | 0.0002 |
| VEGFR-2 | 11.50 | 0.93 | 17.60 ± 2.94 | 3.82 ± 1.53 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlanga-Acosta, J.; Fernández-Mayola, M.; Mendoza-Marí, Y.; García-Ojalvo, A.; Martinez-Jimenez, I.; Rodriguez-Rodriguez, N.; Playford, R.J.; Reyes-Acosta, O.; Lopez-Marín, L.; Guillén-Nieto, G. Intralesional Infiltrations of Arteriosclerotic Tissue Cells-Free Filtrate Reproduce Vascular Pathology in Healthy Recipient Rats. Int. J. Mol. Sci. 2022, 23, 1511. https://doi.org/10.3390/ijms23031511
Berlanga-Acosta J, Fernández-Mayola M, Mendoza-Marí Y, García-Ojalvo A, Martinez-Jimenez I, Rodriguez-Rodriguez N, Playford RJ, Reyes-Acosta O, Lopez-Marín L, Guillén-Nieto G. Intralesional Infiltrations of Arteriosclerotic Tissue Cells-Free Filtrate Reproduce Vascular Pathology in Healthy Recipient Rats. International Journal of Molecular Sciences. 2022; 23(3):1511. https://doi.org/10.3390/ijms23031511
Chicago/Turabian StyleBerlanga-Acosta, Jorge, Maday Fernández-Mayola, Yssel Mendoza-Marí, Ariana García-Ojalvo, Indira Martinez-Jimenez, Nadia Rodriguez-Rodriguez, Raymond J. Playford, Osvaldo Reyes-Acosta, Laura Lopez-Marín, and Gerardo Guillén-Nieto. 2022. "Intralesional Infiltrations of Arteriosclerotic Tissue Cells-Free Filtrate Reproduce Vascular Pathology in Healthy Recipient Rats" International Journal of Molecular Sciences 23, no. 3: 1511. https://doi.org/10.3390/ijms23031511
APA StyleBerlanga-Acosta, J., Fernández-Mayola, M., Mendoza-Marí, Y., García-Ojalvo, A., Martinez-Jimenez, I., Rodriguez-Rodriguez, N., Playford, R. J., Reyes-Acosta, O., Lopez-Marín, L., & Guillén-Nieto, G. (2022). Intralesional Infiltrations of Arteriosclerotic Tissue Cells-Free Filtrate Reproduce Vascular Pathology in Healthy Recipient Rats. International Journal of Molecular Sciences, 23(3), 1511. https://doi.org/10.3390/ijms23031511






