Mechanisms of Pannexin 1 (PANX1) Channel Mechanosensitivity and Its Pathological Roles
Abstract
1. An Introduction to Pannexins
2. Mechanisms of PANX1 Activation
- [K+]e: It has been found that the effect of [K+]e on PANX1 is dose-dependent. A [K+]e larger than 10 mM induces PANX1 channel opening and a [K+]e of 130 mM activates the PANX1 channel over a wide range of voltages [18]. The mechanism by which high [K+]e induces the opening of PANX1 channels remains unknown. PANX1 current is still activated even when voltage is clamped [18]. Instead, it is proposed that high K+-induced activation of PANX1 requires a direct interaction of K+ with its first EL [19]. Mutations of amino acids (R75A, S82A, L94A) in the ELs of PANX1 change these responses [20]. However, recent results question the ability of high [K+]e to activate PANX1; it has been found that the activation of PANX1 by K+ is not observed in all cell types [15,21].
- [Ca2+]i: PANX1 channels can be stimulated by Gαq-containing G protein-coupled receptors (GPCRs), including P2Y purinergic receptors [22] and ion channels [23,24,25]. This kind of channel activation is mediated by an increase in [Ca2+]i. For example, fluid shear stress exerted by flowing blood induces the activation of Piezo1, which increases ATP release and NO production in endothelial cells. These effects are mediated in part by PANX channels activated by [Ca2+]i [23]. In addition, the alveolar epithelium in the lung comprises alveolar epithelial type I (ATI) and surfactant secreting type II (ATII) cells. In ATI cells, when mechanical tension is imposed upon the membrane, it triggers the activation of Piezo1 channels in the caveolae. The resulting Ca2+ influx leads to the opening of PANX1, which induces ATP release and stimulates the secretion of surfactants from ATII cells [24]. Recently, it has been proposed that a Piezo1–PANX1 complex mediates the stretch-induced ATP release in cholangiocytes. The Piezo1 channel senses the membrane stretch and increases [Ca2+]i, which activates PANX1 and releases ATP [25]. The mechanism of [Ca2+]i-induced PANX1 activation has been revealed by a recent study. It proposes that the increase in [Ca2+]i induces the activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII), which phosphorylates the amino acid residue S394 of PANX1, resulting in its opening and ATP release [26].
- Src family kinase (SFK)-mediated phosphorylation: In addition to being activated by multiple metabotropic receptors, PANX1 channels can also be opened by ionotropic receptors and chemokine receptors, including N-methyl-D-aspartate receptor (NMDAR) and tumor necrosis factor alpha (TNFα) [27,28,29], which is mediated by the SFK phosphorylation of PANX1 channels. In hippocampal neurons, anoxia induces the opening of PANX1 channels via NMDARs [30,31]. This anoxia-induced PANX1 current is reduced by D-APV (an NMDAR antagonist) as well as by PP2 (an SFK inhibitor). However, MK-801 (an NMDAR pore blocker) does not block PANX1 current, suggesting that ion permeation via NMDARs is not required for this mechanism of activation of PANX1 channels. Instead, NMDARs activate the PANX1 channel metabotropically via SFKs [31,32]. A possible Src phosphorylation site on PANX1 channels is located at Tyr308. Activation of NMDARs increases the phosphorylation of Tyr308 in the presence of PP2 or a PANX1 mutant, which cannot be phosphorylated at Tyr308; the NMDAR-dependent increase at Tyr308 is abolished in cells [32]. SFKs are also involved in the activation of PANX1 channels induced by TNFα in human umbilical vein endothelial cells (HUVECs). TNFα-induced PANX1 opening requires the activation of type-1 TNF receptors and downstream signaling pathways through SFKs [29]. Further, the application of TNFα increases the phosphorylation of Tyr198 in PANX1 channels, and this increase is inhibited by PP2 [29], suggesting that the Tyr198 of PANX1 is a target of SFK phosphorylation in response to TNFα stimulation.
- Caspase cleavage: At basal conditions, the C-terminus of PANX1 interacts with the channel pore and prevents channel activation. During apoptosis, after caspase 3/7 is activated, it cleaves the C-terminus of PANX1, resulting in constitutive activation of the channel [33]. Even without the occurrence of apoptosis, the direct application of constitutively activated caspase 3/7 also potentiates PANX1 channels, suggesting that the cleavage directly regulates channel opening without the involvement of other apoptotic mediators [34]. In addition, the isolated CT tail of PANX1 blocks the channel pore [34]. Caspase 11 also activates the PANX1 channel by cleavage, which is involved in lipopolysaccharide (LPS)-induced pyroptosis in bone marrow-derived macrophages (BMDMs) [35].
- Mechanical stimulation: PANX1 channels can also be stimulated by a wide range of mechanical stresses. In Xenopus oocytes injected with the cDNA of human PANX1, the PANX1 channel is activated mechanically by suction applied to a patch pipette. The increased channel activity occurs over a wide range of holding potentials when stressed mechanically [36]. The authors also showed that the activation of PANX1 can release ATP [36]. Subsequently, this mechanical sensitivity of PANX1 has been identified in other cells, including erythrocytes, lung epithelium and neurons [37,38,39,40]. Further, PANX1 is activated when cancer cells are subjected to deformation as they travel along the microvasculature, which contributes to cancer metastasis [41]. Moreover, focused ultrasound (FUS) can stimulate ER-localized mechanosensitive PANX1 and result in the release of Ca2+ from the ER in invasive cancer cells [42].
3. Gating Mechanisms of PANX1 Channel Mechanosensitivity
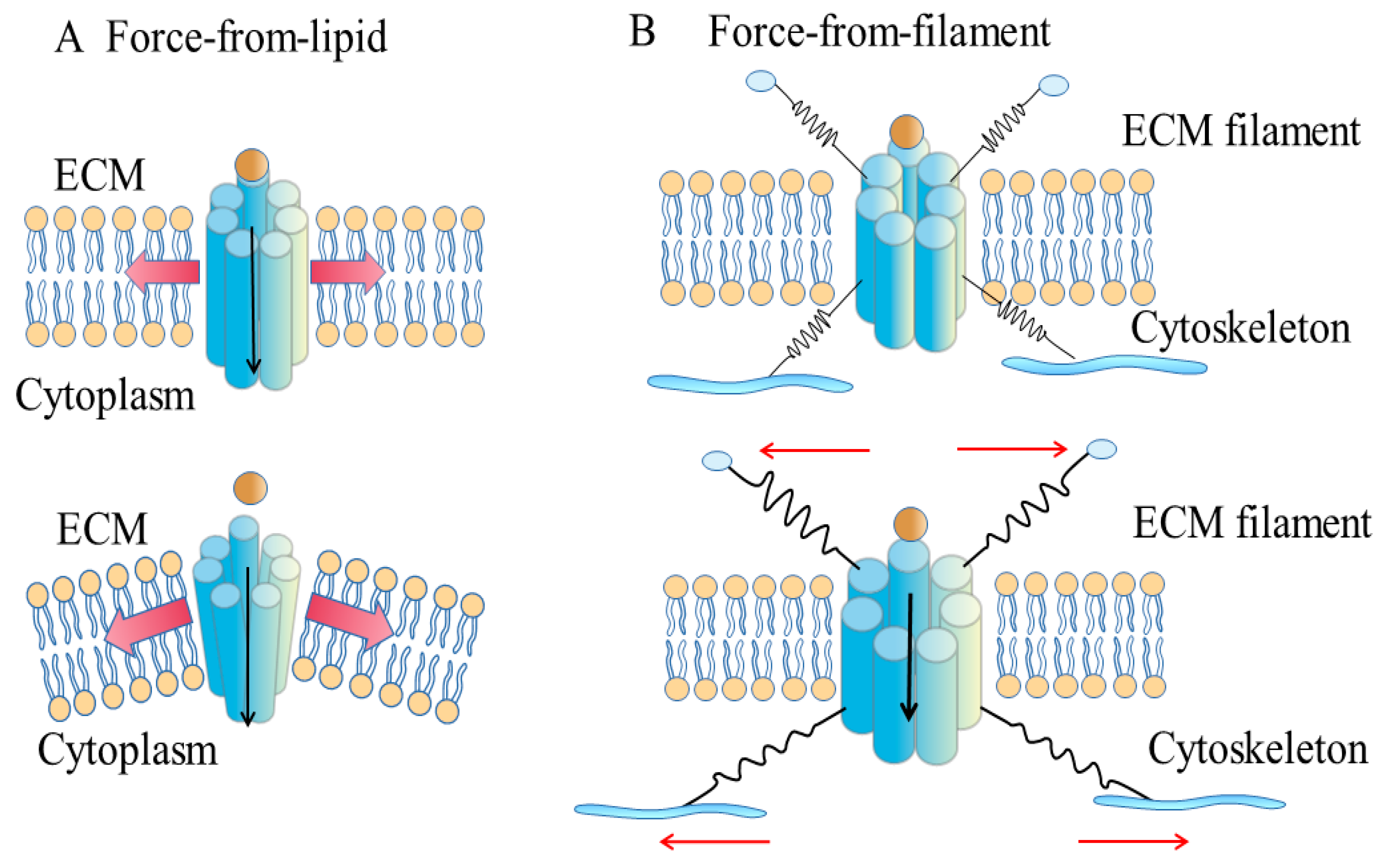
3.1. Force-from-Lipids Model
3.2. Force-from-Filaments Model
3.3. Hybrid Model
4. Mechanosensitive PANX1 in Physiological Processes
4.1. Airway Defense
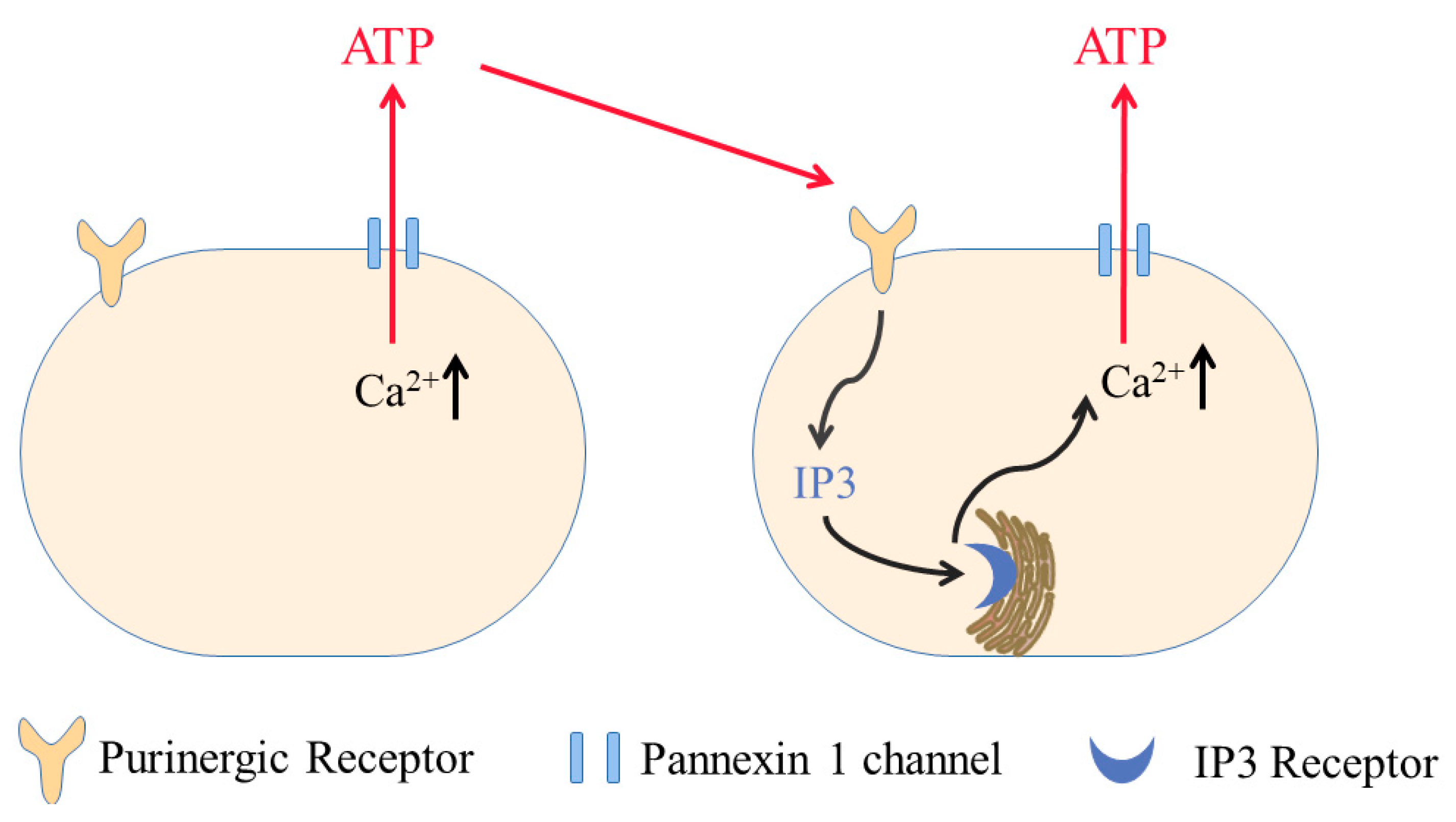
4.2. Ca2+ Wave Signaling
5. Mechanosensitive PANX1 in Diseases
5.1. Glaucoma
5.2. Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Panchin, Y.; Kelmanson, I.; Matz, M.; Lukyanov, K.; Usman, N.; Lukyanov, S. A ubiquitous family of putative gap junction molecules. Curr. Biol. 2000, 10, R473–R474. [Google Scholar] [CrossRef]
- Baranova, A.; Ivanov, D.; Petrash, N.; Pestova, A.; Skoblov, M.; Kelmanson, I.; Shagin, D.; Nazarenko, S.; Geraymovych, E.; Litvin, O.; et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 2004, 83, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Bhalla, R.; Gong, X.Q.; Cowan, K.N.; Celetti, S.J.; Cowan, B.J.; Bai, D.; Shao, Q.; Laird, D.W. Pannexin 1 and Pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 2007, 120, 3772–3783. [Google Scholar] [CrossRef] [PubMed]
- Le Vasseur, M.; Lelowski, J.; Bechberger, J.F.; Sin, W.C.; Naus, C.C. Pannexin 2 protein expression is not restricted to the CNS. Front. Cell Neurosci. 2014, 8, 392. [Google Scholar] [CrossRef]
- Ishikawa, M.; Iwamoto, T.; Nakamura, T.; Doyle, A.; Fukumoto, S.; Yamada, Y. Pannexin 3 functions as an ER Ca2+ channel, hemichannel, and gap junction to promote osteoblast differentiation. J. Cell Biol. 2011, 193, 1257–1274. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nakamura, T.; Ishikawa, M.; Yoshizaki, K.; Sugimoto, A.; Ida-Yonemochi, H.; Ohshima, H.; Saito, M.; Yamada, Y.; Fukumoto, S. Pannexin 3 regulates proliferation and differentiation of odontoblasts via its hemichannel activities. PLoS ONE 2017, 12, e0177557. [Google Scholar] [CrossRef]
- Vogt, A.; Hormuzdi, S.G.; Monyer, H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res. Mol. Brain Res. 2005, 141, 113–120. [Google Scholar] [CrossRef]
- Deng, Z.; He, Z.; Maksaev, G.; Bitter, R.M.; Rau, M.; Fitzpatrick, J.A.J.; Yuan, P. Cryo-EM structures of the ATP release channel pannexin 1. Nat. Struct. Mol. Biol. 2020, 27, 373–381. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, B.; Zheng, X.; Li, N.; Xu, L.; Xie, Y.; Song, F.; Bhat, E.A.; Chen, Y.; Gao, N.; et al. Cryo-EM structures of human pannexin 1 channel. Cell Res. 2020, 30, 449–451. [Google Scholar] [CrossRef]
- Michalski, K.; Syrjanen, J.L.; Henze, E.; Kumpf, J.; Furukawa, H.; Kawate, T. The cryo-EM structure of Pannexin 1 reveals unique motifs for ion selection and inhibition. eLife 2020, 9, e54670. [Google Scholar] [CrossRef]
- Mou, L.; Ke, M.; Song, M.; Shan, Y.; Xiao, Q.; Liu, Q.; Li, J.; Sun, K.; Pu, L.; Guo, L.; et al. Structural basis for gating mechanism of Pannexin 1 channel. Cell Res. 2020, 30, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Dong, L.; Zhang, J.; Yu, X.; Wang, L.; Zhu, S. Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res. 2020, 30, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Orozco, I.J.; Du, J.; Lu, W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 2020, 584, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Gassmann, O.; Pranskevich, J.N.; Boassa, D.; Smock, A.; Wang, J.; Dahl, G.; Steinem, C.; Sosinsky, G.E. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J. Biol. Chem. 2010, 285, 24420–24431. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ambrosi, C.; Qiu, F.; Jackson, D.G.; Sosinsky, G.; Dahl, G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci. Signal. 2014, 7, ra69. [Google Scholar] [CrossRef]
- Dahl, G. The Pannexin1 membrane channel: Distinct conformations and functions. FEBS Lett. 2018, 592, 3201–3209. [Google Scholar] [CrossRef]
- Sabirov, R.Z.; Okada, Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys. J. 2004, 87, 1672–1685. [Google Scholar] [CrossRef]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Jackson, D.G.; Wang, J.; Keane, R.W.; Scemes, E.; Dahl, G. ATP and potassium ions: A deadly combination for astrocytes. Sci. Rep. 2014, 4, 4576. [Google Scholar] [CrossRef]
- Wang, J.; Jackson, D.G.; Dahl, G. Cationic control of Panx1 channel function. Am. J. Physiol. Cell Physiol. 2018, 315, C279–C289. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Jin, X.; Medina, C.B.; Leonhardt, S.A.; Kiessling, V.; Bennett, B.C.; Shu, S.; Tamm, L.K.; Yeager, M.; Ravichandran, K.; et al. A quantized mechanism for activation of pannexin channels. Nat. Commun. 2017, 8, 14324. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Diem, K.; Fauler, M.; Fois, G.; Hellmann, A.; Winokurow, N.; Schumacher, S.; Kranz, C.; Frick, M. Mechanical stretch activates piezo1 in caveolae of alveolar type I cells to trigger ATP release and paracrine stimulation of surfactant secretion from alveolar type II cells. FASEB J. 2020, 34, 12785–12804. [Google Scholar] [CrossRef] [PubMed]
- Desplat, A.; Penalba, V.; Gros, E.; Parpaite, T.; Coste, B.; Delmas, P. Piezo1-Pannexin1 complex couples force detection to ATP secretion in cholangiocytes. J. Gen. Physiol. 2021, 153, e202112871. [Google Scholar] [CrossRef]
- Lopez, X.; Palacios-Prado, N.; Guiza, J.; Escamilla, R.; Fernandez, P.; Vega, J.L.; Rojas, M.; Marquez-Miranda, V.; Chamorro, E.; Cárdenas, A.M.; et al. A physiologic rise in cytoplasmic calcium ion signal increases pannexin1 channel activity via a C-terminus phosphorylation by CaMKII. Proc. Natl. Acad Sci. USA 2021, 118, e2108967118. [Google Scholar] [CrossRef]
- Iglesias, R.; Locovei, S.; Roque, A.; Alberto, A.P.; Dahl, G.; Spray, D.C.; Scemes, E. P2X7 receptor-pannexin1 complex: Pharmacology and signaling. Am. J. Physiol. Cell Physiol. 2008, 295, C752–C760. [Google Scholar] [CrossRef]
- Thompson, R.J.; Jackson, M.F.; Olah, M.E.; Rungta, R.L.; Hines, D.J.; Beazely, M.A.; MacDonald, J.F.; MacVicar, B.A. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 2008, 322, 1555–1559. [Google Scholar] [CrossRef]
- Lohman, A.W.; Leskov, I.L.; Butcher, J.T.; Johnstone, S.R.; Stokes, T.A.; Begandt, D.; DeLalio, L.; Best, A.K.; Penuela, S.; Leitinger, N.; et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat. Commun. 2015, 6, 7965. [Google Scholar] [CrossRef]
- Thompson, R.J.; Zhou, N.; MacVicar, B.A. Ischemia opens neuronal gap junction hemichannels. Science 2006, 312, 924–927. [Google Scholar] [CrossRef]
- Weilinger, N.L.; Tang, P.L.; Thompson, R.J. Anoxia-induced NMDA receptor activation opens pannexin channels via Src family kinases. J. Neurosci. 2012, 32, 12579–12588. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Sandilos, J.K.; Chiu, Y.H.; Chekeni, F.B.; Armstrong, A.J.; Walk, S.F.; Ravichandran, K.S.; Bayliss, D.A. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 2012, 287, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Y.; Munoz-Planillo, R.; Liu, Q.; Nunez, G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in erythrocytes: Function without a gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef]
- Seminario-Vidal, L.; Okada, S.F.; Sesma, J.I.; Kreda, S.M.; van Heusden, C.A.; Zhu, Y.; Jones, L.C.; O’Neal, W.K.; Penuela, S.; Laird, D.W.; et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 2011, 286, 26277–26286. [Google Scholar] [CrossRef]
- Xia, J.; Lim, J.C.; Lu, W.; Beckel, J.M.; Macarak, E.J.; Laties, A.M.; Mitchell, C.H. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J. Physiol. 2012, 590, 2285–2304. [Google Scholar] [CrossRef]
- Richter, K.; Kiefer, K.P.; Grzesik, B.A.; Clauss, W.G.; Fronius, M. Hydrostatic pressure activates ATP-sensitive K+ channels in lung epithelium by ATP release through pannexin and connexin hemichannels. FASEB J. 2014, 28, 45–55. [Google Scholar] [CrossRef]
- Furlow, P.W.; Zhang, S.; Soong, T.D.; Halberg, N.; Goodarzi, H.; Mangrum, C.; Wu, Y.G.; Elemento, O.; Tavazoie, S.F. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat. Cell Biol. 2015, 17, 943–952. [Google Scholar] [CrossRef]
- Lee, N.S.; Yoon, C.W.; Wang, Q.; Moon, S.; Koo, K.M.; Jung, H.; Chen, R.; Jiang, L.; Lu, G.; Fernandez, A.; et al. Focused ultrasound stimulates ER localized mechanosensitive PANNEXIN-1 to mediate intracellular calcium release in invasive cancer cells. Front. Cell Dev. Biol. 2020, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B.; Adler, J.; Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 1990, 348, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Markin, V.S.; Martinac, B. Mechanosensitive ion channels as reporters of bilayer expansion. A theoretical model. Biophys. J. 1991, 60, 1120–1127. [Google Scholar] [CrossRef]
- Martinac, B.; Rohde, P.R.; Battle, A.R.; Petrov, E.; Pal, P.; Foo, A.F.; Vásquez, V.; Huynh, T.; Kloda, A. Studying mechanosensitive ion channels using liposomes. Methods Mol. Biol. 2010, 606, 31–53. [Google Scholar] [CrossRef]
- Martinac, B.; Bavi, N.; Ridone, P.; Nikolaev, Y.A.; Martinac, A.D.; Nakayama, Y.; Rohde, P.R.; Bavi, O. Tuning ion channel mechanosensitivity by asymmetry of the transbilayer pressure profile. Biophys. Rev. 2018, 10, 1377–1384. [Google Scholar] [CrossRef]
- Cantor, R.S. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 1999, 76, 2625–2639. [Google Scholar] [CrossRef]
- Cox, C.D.; Bavi, N.; Martinac, B. Biophysical principles of ion-channel-mediated mechanosensory transduction. Cell Rep. 2019, 29, 1–12. [Google Scholar] [CrossRef]
- Perozo, E.; Kloda, A.; Cortes, D.M.; Martinac, B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002, 9, 696–703. [Google Scholar] [CrossRef]
- Gullingsrud, J.; Schulten, K. Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys. J. 2004, 86, 3496–3509. [Google Scholar] [CrossRef]
- Ridone, P.; Grage, S.L.; Patkunarajah, A.; Battle, A.R.; Ulrich, A.S.; Martinac, B. Force-from-lipids gating of mechanosensitive channels modulated by PUFAs. J. Mech. Behav. Biomed. Mater. 2018, 79, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Waldrop, S.L.; Khimji, A.K.; Kilic, G. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am. J. Physiol. Cell Physiol. 2012, 303, C1034–C1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, Z.; Varghese, Z.; Guo, Y.; Moorhead, J.F.; Unwin, R.J.; Ruan, X.Z. Nonesterified free fatty acids enhance the inflammatory response in renal tubules by inducing extracellular ATP release. Am. J. Physiol. Renal Physiol. 2020, 319, F292–F303. [Google Scholar] [CrossRef] [PubMed]
- Tam, T.H.; Chan, K.L.; Boroumand, P.; Liu, Z.; Brozinick, J.T.; Bui, H.H.; Roth, K.; Wakefield, C.B.; Penuela, S.; Bilan, P.J.; et al. Nucleotides released from palmitate-activated murine macrophages attract neutrophils. J. Biol. Chem. 2020, 295, 4902–4911. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Li, Y.E.; Fink, L.N.; Brozinick, J.T.; Nikolayev, A.; Kuo, M.S.; Bilan, P.J.; Klip, A. Nucleotides released from palmitate-challenged muscle cells through pannexin-3 attract monocytes. Diabetes 2014, 63, 3815–3826. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, P.; Manosalva, C.; Quiroga, J.; Belmar, I.; Alvarez, K.; Diaz, G.; Taubert, A.; Hermosilla, C.; Carretta, M.D.; Burgos, R.A.; et al. Oleic and linoleic acids induce the release of neutrophil extracellular traps via pannexin 1-dependent ATP release and P2X1 receptor activation. Front. Vet. Sci. 2020, 7, 260. [Google Scholar] [CrossRef]
- Samuels, S.E.; Lipitz, J.B.; Wang, J.; Dahl, G.; Muller, K.J. Arachidonic acid closes innexin/pannexin channels and thereby inhibits microglia cell movement to a nerve injury. Dev. Neurobiol. 2013, 73, 621–631. [Google Scholar] [CrossRef]
- Bhalla-Gehi, R.; Penuela, S.; Churko, J.M.; Shao, Q.; Laird, D.W. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J. Biol. Chem. 2010, 285, 9147–9160. [Google Scholar] [CrossRef]
- Wicki-Stordeur, L.E.; Swayne, L.A. Panx1 regulates neural stem and progenitor cell behaviours associated with cytoskeletal dynamics and interacts with multiple cytoskeletal elements. Cell. Commun. Signal. 2013, 11, 62. [Google Scholar] [CrossRef]
- Xiang, X.; Langlois, S.; St-Pierre, M.E.; Blinder, A.; Charron, P.; Graber, T.E.; Fowler, S.L.; Baird, S.D.; Bennett, S.A.L.; Alain, T.; et al. Identification of pannexin 1-regulated genes, interactome, and pathways in rhabdomyosarcoma and its tumor inhibitory interaction with AHNAK. Oncogene 2021, 40, 1868–1883. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Qu, H.L.; Dai, Y.J.; Wang, Q.; Ling, Z.M.; Su, W.F.; Zhao, Y.-Y.; Shen, W.-X. Pannexin 1, a large-pore membrane channel, contributes to hypotonicity-induced ATP release in Schwann cells. Neural Regen. Res. 2021, 16, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wicki-Stordeur, L.E.; Sanchez-Arias, J.C.; Liu, M.; Weaver, M.S.; Choi, C.S.W.; Swayne, L.A. Probenecid disrupts a novel Pannexin 1-collapsin response mediator protein 2 interaction and increases microtubule stability. Front. Cell Neurosci. 2018, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.A.; Lai, C.P.; Naus, C.C.; Morgan, J.R. Pannexin1 drives multicellular aggregate compaction via a signaling cascade that remodels the actin cytoskeleton. J. Biol. Chem. 2012, 287, 8407–8416. [Google Scholar] [CrossRef] [PubMed]
- Saez, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Dumenil, A.M.; Saez, J.C. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors. Sci. Signal. 2017, 10, eaah7107. [Google Scholar] [CrossRef]
- Flores-Munoz, C.; Maripillan, J.; Vasquez-Navarrete, J.; Novoa-Molina, J.; Ceriani, R.; Sanchez, H.A.; Abbott, A.; Weinstein-Oppenheimer, C.; Brown, D.; Cárdenas, A.; et al. Restraint of human skin fibroblast motility, migration, and cell surface actin dynamics, by Pannexin 1 and P2X7 receptor signaling. Int. J. Mol. Sci. 2021, 22, 1069. [Google Scholar] [CrossRef]
- Hamill, O.P. Twenty odd years of stretch-sensitive channels. Pflugers Arch. 2006, 453, 333–351. [Google Scholar] [CrossRef]
- Teng, J.; Loukin, S.; Anishkin, A.; Kung, C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2015, 467, 27–37. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, L.E.; Kittelmann, M.; Li, J.; Petkovic, M.; Cheng, T.; Jin, P.; Guo, Z.; Göpfert, M.C.; Jan, L.; et al. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 2015, 162, 1391–1403. [Google Scholar] [CrossRef]
- Jin, P.; Bulkley, D.; Guo, Y.; Zhang, W.; Guo, Z.; Huynh, W.; Wu, S.; Meltzer, S.; Cheng, T.; Jan, L.; et al. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 2017, 547, 118–122. [Google Scholar] [CrossRef]
- Li Fraine, S.; Patel, A.; Duprat, F.; Sharif-Naeini, R. Dynamic regulation of TREK1 gating by polycystin 2 via a filamin A-mediated cytoskeletal mechanism. Sci. Rep. 2017, 7, 17403. [Google Scholar] [CrossRef]
- Ransford, G.A.; Fregien, N.; Qiu, F.; Dahl, G.; Conner, G.E.; Salathe, M. Pannexin 1 contributes to ATP release in airway epithelia. Am. J. Respir. Cell Mol. Biol. 2009, 41, 525–534. [Google Scholar] [CrossRef] [PubMed]
- D’hondt, C.; Himpens, B.; Bultynck, G. Mechanical stimulation-induced calcium wave propagation in cell monolayers: The example of bovine corneal endothelial cells. J. Vis. Exp. 2013, 77, e50443. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, D.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Reigada, D.; Lu, W.; Zhang, M.; Mitchell, C.H. Elevated pressure triggers a physiological release of ATP from the retina: Possible role for pannexin hemichannels. Neuroscience 2008, 157, 396–404. [Google Scholar] [CrossRef][Green Version]
- Beckel, J.M.; Argall, A.J.; Lim, J.C.; Xia, J.; Lu, W.; Coffey, E.E.; Macarak, E.J.; Shahidullah, M.; Delamere, N.; Zode, G.S.; et al. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: A mechanism for purinergic involvement in chronic strain. Glia 2014, 62, 1486–1501. [Google Scholar] [CrossRef]
- Weitz, A.C.; Lee, N.S.; Yoon, C.W.; Bonyad, A.; Goo, K.S.; Kim, S.; Moon, S.; Jung, H.; Zhou, Q.; Chow, R.H.; et al. Functional assay of cancer cell invasion potential based on mechanotransduction of focused ultrasound. Front. Oncol. 2017, 7, 161. [Google Scholar] [CrossRef]
- Dalecki, D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 2004, 6, 229–248. [Google Scholar] [CrossRef]
- Gaitan-Penas, H.; Gradogna, A.; Laparra-Cuervo, L.; Solsona, C.; Fernandez-Duenas, V.; Barrallo-Gimeno, A.; Ciruela, F.; Lakadamyali, M.; Pusch, M.; Estévez, R. Investigation of LRRC8-mediated volume-regulated anion currents in Xenopus oocytes. Biophys. J. 2016, 111, 1429–1443. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Xiao, Z.; He, X.; Weng, R.; Zhao, X.; Sun, T. Mechanisms of Pannexin 1 (PANX1) Channel Mechanosensitivity and Its Pathological Roles. Int. J. Mol. Sci. 2022, 23, 1523. https://doi.org/10.3390/ijms23031523
Yang K, Xiao Z, He X, Weng R, Zhao X, Sun T. Mechanisms of Pannexin 1 (PANX1) Channel Mechanosensitivity and Its Pathological Roles. International Journal of Molecular Sciences. 2022; 23(3):1523. https://doi.org/10.3390/ijms23031523
Chicago/Turabian StyleYang, Kai, Zhupeng Xiao, Xueai He, Ruotong Weng, Xinyue Zhao, and Taolei Sun. 2022. "Mechanisms of Pannexin 1 (PANX1) Channel Mechanosensitivity and Its Pathological Roles" International Journal of Molecular Sciences 23, no. 3: 1523. https://doi.org/10.3390/ijms23031523
APA StyleYang, K., Xiao, Z., He, X., Weng, R., Zhao, X., & Sun, T. (2022). Mechanisms of Pannexin 1 (PANX1) Channel Mechanosensitivity and Its Pathological Roles. International Journal of Molecular Sciences, 23(3), 1523. https://doi.org/10.3390/ijms23031523




