Water-Soluble Products of Photooxidative Destruction of the Bisretinoid A2E Cause Proteins Modification in the Dark
Abstract
:1. Introduction
2. Results
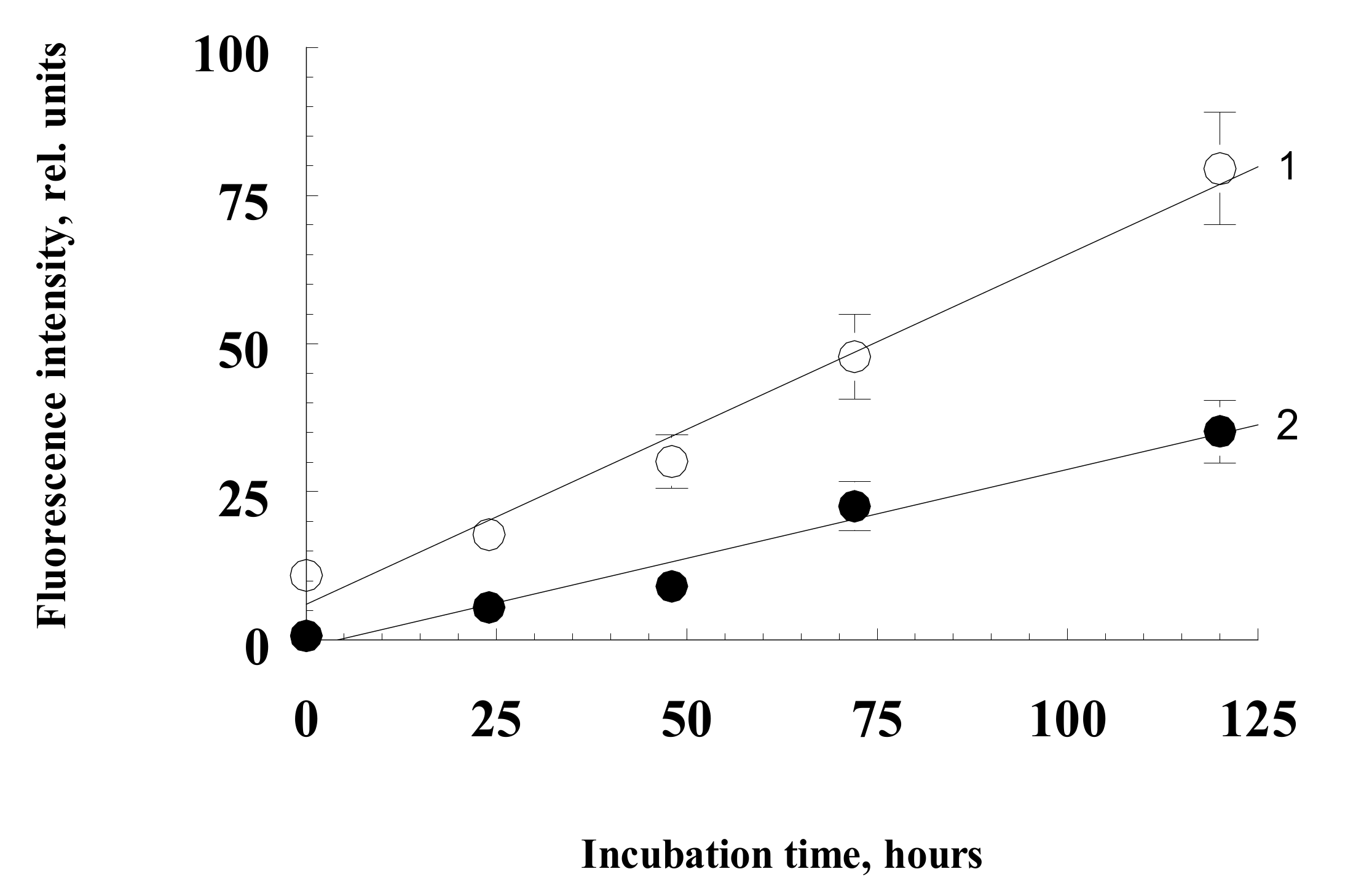
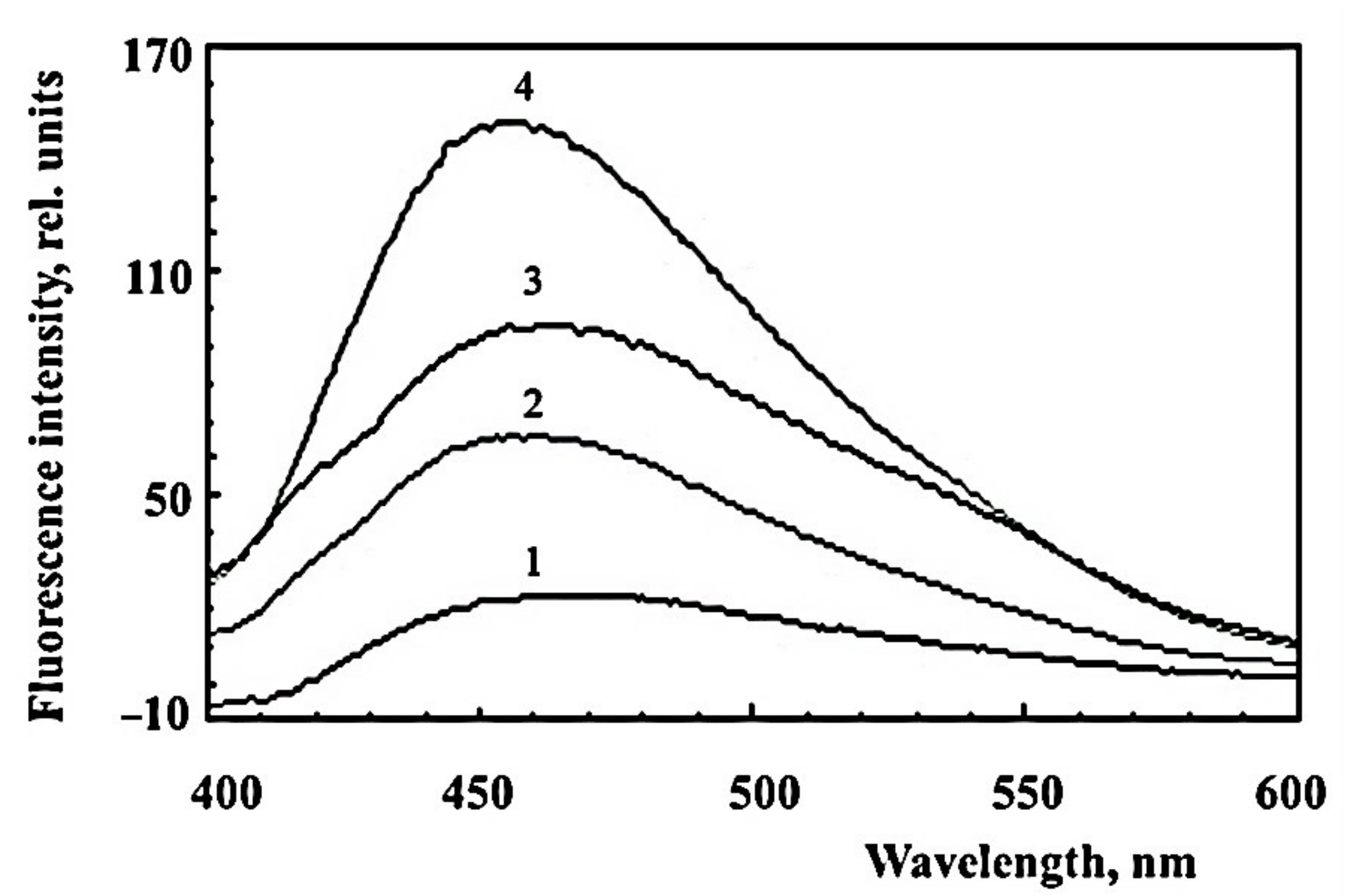
2.1. Formation of Water-Soluble Carbonyl Products during Photooxidation of Fluorophore A2E
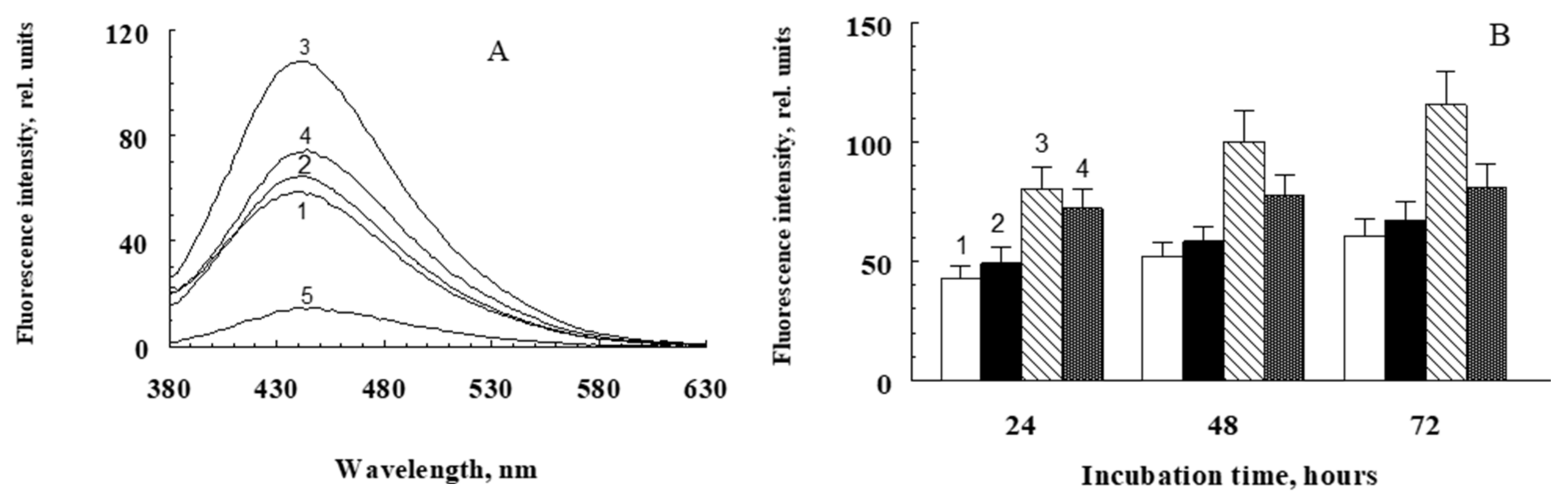
2.2. Modification of Proteins with Water-Soluble Fractions of Irradiated and Non-Irradiated Fluorophore A2E
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of A2E and Obtention of Water-Soluble Photooxidized Products
4.2.1. Solubilization of A2E in Methanol–Phosphate Buffer Mixture
4.2.2. Solubilization of A2E In Silica Gel Suspension
4.2.3. Solubilization of A2E in a Suspension of Polystyrene Latex Beads
4.3. Measurement of Fluorescence Spectra
4.4. Determination of TBA-Reactive Products
4.5. A2E Mass Spectrometry
4.6. Broadband CARS Microspectrometry
4.7. Measurement of Protein Modification-Induced Photooxidative Destruction Products of A2E
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Grune, T. Lipofuscin: Formation, effects and role of macro autophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dontsov, A.; Glickman, R.; Ostrovsky, M. Retinal pigment epithelium pigment granules stimulate the photo-oxidation of unsaturated fatty acid. Free Radic. Biol. Med. 1999, 26, 1436–1446. [Google Scholar] [CrossRef]
- Kennedy, C.; Rakoczy, P.; Constable, I. Lipofuscin of the retinal pigment epithelium: A review. Eye 1995, 9, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Sakai, N.; Decatur, J.; Nakanishi, K. Ocular age pigment A2E: An unprecedented pyridinium bisretinoid. J. Am. Chem. Soc. 1996, 118, 1559–1560. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Parish, C.; Hashimoto, M.; Liu, J.; Nakanishi, K.; Sparrow, J. Fluorescent pigments of the retinal pigment epithelium and age-related macular degeneration. Bioorg. Med. Chem. Lett. 2001, 11, 1533–1540. [Google Scholar] [CrossRef]
- Roberts, J.; Kukielczak, B.; Hu, D.; Miller, D.; Bilski, P.; Sik, R.; Motten, A.; Chignell, C. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem. Photobiol. 2002, 75, 184–190. [Google Scholar] [CrossRef]
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin is a photoinducible free radical generator. J. Photochem. Photobiol. B 1993, 19, 201–204. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef]
- Rozanowska, M.; Jarvis-Evans, J.; Korytowski, W.; Boulton, M.; Burke, J.; Sarna, T. Blue light-induced reactivity of retinal age pigment. J. Biol. Chem. 1995, 270, 18825–18830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutt, F.; Davies, S.; Kopitz, J.; Holz, F.; Boulton, M. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2303–2308. [Google Scholar]
- Sparrow, J.; Nakanishi, K.; Parish, C. The lipofuscin fluorophore A2E mediates blue light–induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 2007, 5, 32. [Google Scholar]
- Nakae, Y.; Stoward, P.J. The high correlation between counts and area fractions of lipofuscin granules, a biomarker of oxidative stress in muscular dystrophies. Histochem. Cell Biol. 2016, 146, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Bakall, B.; Radu, R.A.; Stanton, J.B.; Burke, J.M.; McKay, B.S.; Wadelius, C.; Mullins, R.F.; Stone, E.M.; Travis, G.H.; Marmorstein, A.D. Enhanced accumulation of A2E in individuals homozygous or heterozygous for mutations in BEST1 (VMD2). Exp. Eye Res. 2007, 85, 34–43. [Google Scholar] [CrossRef]
- Mata, N.L.; Weng, J.; Travis, G.H. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc. Natl. Acad. Sci. USA 2000, 97, 7154–7159. [Google Scholar] [CrossRef] [Green Version]
- Vasireddy, V.; Jablonski, M.M.; Khan, N.W.; Wang, X.F.; Sahu, P.; Sparrow, J.R.; Ayyagari, R. Elovl4 5-bp deletion knock-in mouse model for Stargardt-like macular degeneration demonstrates accumulation of ELOVL4 and lipofuscin. Exp. Eye Res. 2009, 89, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Poliakov, E.; Strunnikova, N.V.; Jiang, J.-k.; Martinez, B.; Parikh, T.; Lakkaraju, A.; Thomas, C.; Brooks, B.P.; Redmond, T.M. Multiple A2E treatments lead to melanization of rod outer segment–challenged ARPE-19 cells. Mol. Vis. 2014, 20, 285–300. [Google Scholar]
- Kim, H.J.; Montenegro, D.; Zhao, J.; Sparrow, J.R. Bisretinoids of the retina: Photo-oxidation, iron-catalyzed oxidation, and disease consequences. Antioxidants 2021, 10, 1382. [Google Scholar] [CrossRef]
- Zhang, D.; Mihai1, D.M.; Washington, I. Vitamin A cycle byproducts explain retinal damage and molecular changes thought to initiate retinal degeneration. Biol. Open 2021, 10, bio058600. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Wrona, M.; Rozanowska, M.; Zareba, M.; Lamb, L.; Roberts, J.; Simon, J.; Sarna, T. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem. Photobiol. 2003, 77, 253–258. [Google Scholar] [CrossRef]
- Ng, K.P.; Gugiu, B.G.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Rozanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteom. 2008, 7, 1397–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3663–3668. [Google Scholar] [CrossRef]
- Furso, J.; Zadlo, A.; Szewczyk, G.; Sarna, T.J. Photoreactivity of Bis-retinoid A2E Complexed with a Model Protein in Selected Model Systems. Cell Biochem. Biophys. 2020, 78, 415–427. [Google Scholar] [CrossRef]
- Zhou, J.; Ueda, K.; Zhao, J.; Sparrow, J.R. Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl adduct deposition. J. Biol. Chem. 2015, 290, 27215–27227. [Google Scholar] [CrossRef] [Green Version]
- Thao, M.T.; Renfus, D.J.; Dillon, J.; Gaillard, E.R. A2E mediated photochemical modification to fibronectin and its implications to age related changes in Bruch’s membrane. Photochem. Photobiol. 2014, 90, 329–334. [Google Scholar] [CrossRef]
- Wiktor, A.; Sarna, M.; Wnuk, D.; Sarna, T. Lipofuscin-mediated photodynamic stress induces adverse changes in nanomechanical properties of retinal pigment epithelium cells. Sci. Rep. 2018, 8, 17929. [Google Scholar] [CrossRef] [Green Version]
- Tarau, I.-S.; Berlin, A.; Curcio, C.A.; Ach, T. The Cytoskeleton of the Retinal Pigment Epithelium: From Normal Aging to Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3578. [Google Scholar] [CrossRef] [Green Version]
- Glenn, J.V.; Beattie, J.R.; Barrett, L.; Frizzell, N.; Thorpe, S.R.; Boulton, M.E.; McGarvey, J.J.; Stitt, A.W. Confocal Raman microscopy can quantify advanced glycation end products (AGE) modifications Bruch’s membrane leading to accurate, nondestructive prediction of ocular aging. FASEB J. 2007, 21, 3542–3552. [Google Scholar] [CrossRef]
- Beattie, J.R.; Pawlak, A.M.; Boulton, M.E.; Zhang, J.; Monnier, V.M.; McGarvey, J.J.; Stitt, A.W. Multiplex analysis of age-related protein and lipid modifications in human Bruch’s membrane. FASEB J. 2010, 24, 4816–4824. [Google Scholar] [PubMed]
- Murdaugh, L.S.; Dillon, J.; Gaillard, E.R. Modifications to the basement membrane protein laminin using glycolaldehyde and A2E: A model for aging in Bruch’s membrane. Exp. Eye Res. 2009, 89, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.M.; Glenn, J.V.; Beattie, J.R.; McGarvey, J.J.; Stitt, A.W. Advanced glycation as a basis for understanding retinal aging and noninvasive risk prediction. Ann. N. Y. Acad. Sci. 2008, 1126, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Walker, G.B.; Kurji, K.; Fang, E.; Law, G.; Prasad, S.S.; Kojic, L.; Cao, S.; White, V.; Cui, J.Z.; et al. Parainflammation associated with advanced glycation endproduct stimulation of RPE In Vitro: Implications for age-related degenerative diseases of the eye. Cytokine 2013, 62, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Prasad, C.; Imrhan, V.; Marutta, F.; Juma, S.; Vijayagupal, P. Lifestyle and advanced glycation end-products (AGEs) burden: Its relevance to healthy aging. Aging Dis. 2014, 5, 212–217. [Google Scholar] [CrossRef]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic targeting of advanced glycation end-products in age-related diseases. BBA Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Bierhaus, A.; Haring, H.U.; Nawroth, P.P.; Lehmann, R. Chemistry and pathobiology of advanced glycation end products. Contrib. Nephrol. 2001, 131, 1–9. [Google Scholar]
- Lin, J.-A.; Wu, C.-H.; Lu, C.-C.; Hsia, S.-M.; Yen, G.-C. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: An emerging biological factor in cancer onset and progression. Mol. Nutr. Food Res. 2016, 60, 1850–1864. [Google Scholar] [CrossRef]
- Yakovleva, M.A.; Sakina, N.L.; Kononikhin, A.S.; Feldman, T.B.; Nikolaev, E.N.; Dontsov, A.E.; Ostrovsky, M.A. Detection and study of the products of photooxidation of N-retinylidene-N-retinylethanolamine, the fluorophore of lipofuscin granules from retinal pigment epithelium of human donor eyes. Dokl. Biochem. Biophys. 2006, 409, 223–225. [Google Scholar] [CrossRef]
- Dontsov, A.E.; Sakina, N.L.; Golubkov, A.M.; Ostrovsky, M.A. Light-induced release of A2E photooxidation toxic products from lipofuscin granules of human retinal pigment epithelium. Dokl. Biochem. Biophys. 2009, 425, 98–101. [Google Scholar] [CrossRef]
- Wang, Z.; Keller, L.M.M.; Dillon, J.; Gaillard, E.R. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem. Photobiol. 2006, 82, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aybush, A.V.; Gulin, A.A.; Vasin, A.A.; Dontsov, A.E.; Nadtochenko, V.A.; Ostrovsky, M.A. Multimodal approach to reveal the effect of light irradiation on chemical composition of lipofuscin granules of human RPE tissues. J. Phys. Conf. Ser. 2020, 1695, 012063. [Google Scholar] [CrossRef]
- Jang, Y.P.; Matsuda, H.H.; Itagaki, Y.Y.; Nakanishi, K.K.; Sparrow, J.R. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J. Biol. Chem. 2005, 280, 39732–39739. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yanase, E.; Feng, X.; Siegel, M.M.; Sparrow, J.R. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 7275–7280. [Google Scholar] [CrossRef] [Green Version]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Fan, Y.; Xu, X. The effects of aminoguanidine on retinopathy in STZ-induced diabetic rats. Bioorg. Med. Chem. Lett. 2012, 22, 4386–4390. [Google Scholar] [CrossRef]
- Courderot-Masuyer, C.; Dalloz, F.; Maupoil, V.; Rochette, L. Antioxidant properties of aminoguanidine. Fundam. Clin. Pharmacol. 1999, 13, 535–540. [Google Scholar] [CrossRef]
- Pasten, C.; Lozano, M.; Rocco, J.; Carrión, F.; Alvarado, C.; Liberona, J.; Michea, L.; Irarrázabal, C.E. Aminoguanidine Prevents the Oxidative Stress, Inhibiting Elements of Inflammation, Endothelial Activation, Mesenchymal Markers, and Confers a Renoprotective Effect in Renal Ischemia and Reperfusion Injury. Antioxidants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine to prevent the formation of advanced glycation end products. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Adler, L., IV; Boyer, N.P.; Anderson, D.M.; Spraggins, J.M.; Schey, K.L.; Hanneken, A.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Determination of N-retinylidene-N-retinylethanolamine (A2E) levels in central and peripheral areas of human retinal pigment epithelium. Photochem. Photobiol. Sci. 2015, 14, 1983–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-Related Macular Degeneration: Role of Oxidative Stress and Blood Vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jang, Y.; Kim, S.; Sparrow, J. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2006, 103, 16182–16187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Leitner, W.; Staples, M.; Anderson, D. Complement activation and inflammatory processes in drusen formation and age-related macular degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Despriet, D.D.; van Duijn, C.M.; Oostra, B.A.; Uitterlinden, A.G.; Hofman, A.; Wright, A.F.; Jacoline, B.; Bakker, A.; de Jong, P.T.; Vingerling, J.R.; et al. Complement component C3 and risk of age-related macular degeneration. Ophthalmology 2009, 116, 474–480. [Google Scholar] [CrossRef]
- Hollyfield, J.G. Age-Related Macular Degeneration: The Molecular Link between Oxidative Damage, Tissue-Specific Inflammation and Outer Retinal Disease: The Proctor Lecture. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1276–1281. [Google Scholar] [CrossRef] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [Green Version]
- Morgan, B.P.; Harris, C.L. Complement Regulatory Proteins; Academic Press: San Diego, CA, USA, 1999. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [Green Version]
- Hageman, G.S.; Anderson, D.H.; Johnson, L.V.; Hancox, L.S.; Taiber, A.J.; Hardisty, L.I.; Hageman, J.L.; Stockman, H.A.; Borchardt, J.D.; Gehrs, K.M.; et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 7227–7232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollyfield, J.G.; Kuttner-Kondo, L. Animal models for age-related macular degeneration. In Animal Models for Retinal Diseases; Pang, I.H., Clark, A., Eds.; Neuromethods; Springer: Berlin, Germany, 2010; Volume 46, pp. 81–98. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G.; Crabb, J.W.; Salomon, R.G. Proteomic approaches to understanding age-related macular degeneration. Adv. Exp. Med. Biol. 2003, 533, 83–89. [Google Scholar] [PubMed]
- Parish, C.A.; Hashimoto, M.; Nakanishi, K.; Dillon, J.; Sparrow, J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 1998, 95, 14609–14613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dontsov, A.; Yakovleva, M.; Trofimova, N.; Sakina, N.; Gulin, A.; Aybush, A.; Gostev, F.; Vasin, A.; Feldman, T.; Ostrovsky, M. Water-Soluble Products of Photooxidative Destruction of the Bisretinoid A2E Cause Proteins Modification in the Dark. Int. J. Mol. Sci. 2022, 23, 1534. https://doi.org/10.3390/ijms23031534
Dontsov A, Yakovleva M, Trofimova N, Sakina N, Gulin A, Aybush A, Gostev F, Vasin A, Feldman T, Ostrovsky M. Water-Soluble Products of Photooxidative Destruction of the Bisretinoid A2E Cause Proteins Modification in the Dark. International Journal of Molecular Sciences. 2022; 23(3):1534. https://doi.org/10.3390/ijms23031534
Chicago/Turabian StyleDontsov, Alexander, Marina Yakovleva, Natalia Trofimova, Natalia Sakina, Alexander Gulin, Arseny Aybush, Fedor Gostev, Alexander Vasin, Tatiana Feldman, and Mikhail Ostrovsky. 2022. "Water-Soluble Products of Photooxidative Destruction of the Bisretinoid A2E Cause Proteins Modification in the Dark" International Journal of Molecular Sciences 23, no. 3: 1534. https://doi.org/10.3390/ijms23031534
APA StyleDontsov, A., Yakovleva, M., Trofimova, N., Sakina, N., Gulin, A., Aybush, A., Gostev, F., Vasin, A., Feldman, T., & Ostrovsky, M. (2022). Water-Soluble Products of Photooxidative Destruction of the Bisretinoid A2E Cause Proteins Modification in the Dark. International Journal of Molecular Sciences, 23(3), 1534. https://doi.org/10.3390/ijms23031534







