A Review of the Advantages, Disadvantages and Limitations of Chemotaxis Assays for Campylobacter spp.
Abstract
:1. Introduction
2. Agar Plug-Based Assays
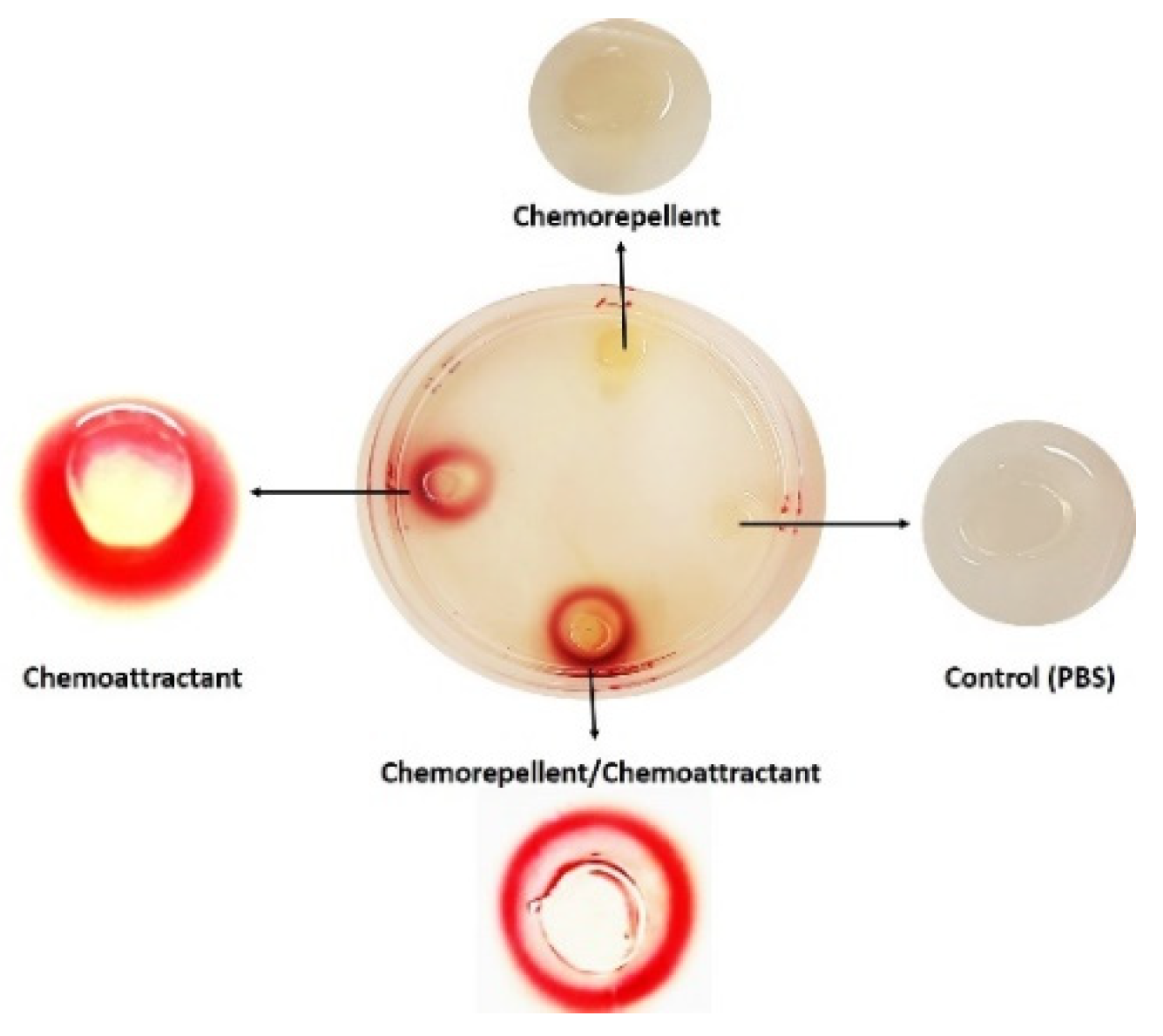
2.1. Hard Plug Agar Assay (HAP)
2.2. Tube-Based Chemotaxis Assays
2.3. Nutrient-Depletion Assay
3. Capillary Assays
4. Slide-Based Chemotaxis Assay
5. Comparison of t-HAP, Nutrient-Depletion and μ-Slide Assays
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korolik, V. The role of chemotaxis during Campylobacter jejuni colonisation and pathogenesis. Curr. Opin. Microbiol. 2018, 47, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, C.M.; Nachmkin, I.; Blaser, M.J. Campylobacter, 3rd ed.; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Szymanski, C.M.; King, M.; Haardt, M.; Armstrong, G.D. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect. Immun. 1995, 63, 4295–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Miller, J.F. Campylobacter jejuni Colonization of Mice with Limited Enteric Flora. Infect. Immun. 2006, 74, 5261–5271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.; Burr, D.H.; Guerry, P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol. Microbiol. 1997, 23, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Takata, T.; Fujimoto, S.; Amako, K. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect. Immun. 1992, 60, 3596–3600. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.P.D.; Hereld, D. Chemotaxis: Methods and Protocols; Humana Press: New York, NY, USA, 2009; Volume 571. [Google Scholar]
- King, R.M.; Korolik, V. Characterization of ligand–receptor interactions: Chemotaxis, biofilm, cell culture assays, and animal model methodologies. In Campylobacter jejuni: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 149–161. [Google Scholar]
- Jin, T.; Hereld, D. Chemotaxis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Mazumder, R.; Phelps, T.J.; Krieg, N.R.; Benoit, R. Determining chemotactic responses by two subsurface microaerophiles using a simplified capillary assay method. J. Microbiol. Methods 1999, 37, 255–263. [Google Scholar] [CrossRef]
- Sandhu, R. The Transducer-like Proteins of Campylobacter jejuni. Ph.D. Thesis, University of Leicester, Leicester, UK, 2011. [Google Scholar]
- Li, Z.; Lou, H.; Ojcius, D.; Sun, A.; Sun, D.; Zhao, J.; Lin, X.; Yan, J. Methyl-accepting chemotaxis proteins 3 and 4 are responsible for Campylobacter jejuni chemotaxis and jejuna colonization in mice in response to sodium deoxycholate. J. Med. Microbiol. 2014, 63, 343–354. [Google Scholar] [CrossRef]
- Vegge, C.S.; Brøndsted, L.; Li, Y.-P.; Bang, D.D.; Ingmer, H. Energy Taxis Drives Campylobacter jejuni toward the Most Favorable Conditions for Growth. Appl. Environ. Microbiol. 2009, 75, 5308–5314. [Google Scholar] [CrossRef] [Green Version]
- Hartley-Tassell, L.E.; Shewell, L.K.; Day, C.J.; Wilson, J.C.; Sandhu, R.; Ketley, J.M.; Korolik, V. Identification and characterization of the aspartate chemosensory receptor of Campylobacter jejuni. Mol. Microbiol. 2010, 75, 710–730. [Google Scholar] [CrossRef]
- Kakuda, T.; DiRita, V.J. Cj1496c Encodes a Campylobacter jejuni Glycoprotein That Influences Invasion of Human Epithelial Cells and Colonization of the Chick Gastrointestinal Tract. Infect. Immun. 2006, 74, 4715–4723. [Google Scholar] [CrossRef] [Green Version]
- Hugdahl, M.B.; Beery, J.T.; Doyle, M.P. Chemotactic behavior of Campylobacter jejuni. Infect. Immun. 1988, 56, 1560–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, B.; Miller, W.G.; Bates, A.H.; Mandrell, R.E. Autoinducer-2 Production in Campylobacter jejuni Contributes to Chicken Colonization. Appl. Environ. Microbiol. 2009, 75, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, J. Chemotaxis in Bacteria. Science 1966, 153, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Tso, W.-W.; Adler, J. Negative Chemotaxis in Escherichia coli. J. Bacteriol. 1974, 118, 560–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Go, A.C.; Ward, M.J.; Ottemann, K.M. The chemical-in-plug bacterial chemotaxis assay is prone to false positive responses. BMC Res. Notes 2010, 3, 77. [Google Scholar] [CrossRef] [Green Version]
- Sampedro, I.; Parales, R.E.; Krell, T.; Hill, J.E. Pseudomonas chemotaxis. FEMS Microbiol. Rev. 2014, 39, 12081. [Google Scholar]
- Gao, B.; Vorwerk, H.; Huber, C.; Lara-Tejero, M.; Mohr, J.; Goodman, A.L.; Eisenreich, W.; Galán, J.E.; Hofreuter, D. Metabolic and fitness determinants for in vitro growth and intestinal colonization of the bacterial pathogen Campylobacter jejuni. PLoS Biol. 2017, 15, e2001390. [Google Scholar] [CrossRef] [PubMed]
- Guccione, E.; Leon-Kempis, M.D.R.; Pearson, B.M.; Hitchin, E.; Mulholland, F.; Van Diemen, P.M.; Stevens, M.P.; Kelly, D.J. Amino acid-dependent growth of Campylobacter jejuni: Key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol. Microbiol. 2008, 69, 77–93. [Google Scholar] [CrossRef]
- Lübke, A.-L.; Minatelli, S.; Riedel, T.; Lugert, R.; Schober, I.; Spröer, C.; Overmann, J.; Groß, U.; Zautner, A.E.; Bohne, W. The transducer-like protein Tlp12 of Campylobacter jejuni is involved in glutamate and pyruvate chemotaxis. BMC Microbiol. 2018, 18, 111. [Google Scholar] [CrossRef]
- Zautner, A.E.; Tareen, A.M.; Groß, U.; Lugert, R. Chemotaxis in Campylobacter jejuni. Eur. J. Microbiol. Immunol. 2012, 2, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Elgamoudi, B.A.; Ketley, J.M.; Korolik, V. New approach to distinguishing chemoattractants, chemorepellents and catabolised chemoeffectors for Campylobacter jejuni. J. Microbiol. Methods 2018, 146, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; van Vliet, A.H. Signal balancing by the CetABC and CetZ chemoreceptors controls energy taxis in Campylobacter jejuni. PLoS ONE 2013, 8, e54390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, M.; Bhavsar, S.; Kapadnis, B. Effect of temperature on growth and chemotactic behaviour of Campylobacter jejuni. Lett. Appl. Microbiol. 2006, 43, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.M.; Dasti, J.I.; Zautner, A.E.; Groß, U.; Lugert, R. Campylobacter jejuni proteins Cj0952c and Cj0951c affect chemotactic behaviour towards formic acid and are important for invasion of host cells. Microbiology 2010, 156, 3123–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Wang, N.; Ren, F.; Tang, H.; Jiao, X.; Huang, J. cj0371: A Novel Virulence-Associated Gene of Campylobacter jejuni. Front. Microbiol. 2016, 7, 1094. [Google Scholar] [CrossRef] [PubMed]
- Hazeleger, W.C.; Wouters, J.A.; Rombouts, F.M.; Abee, T. Physiological Activity of Campylobacter jejuni Far below the Minimal Growth Temperature. Appl. Environ. Microbiol. 1998, 64, 3917–3922. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Machuca, M.A.; Rahman, M.M.; Koç, C.; Norton, R.S.; Smith, B.J.; Roujeinikova, A. Structure–Activity Relationship Study Reveals the Molecular Basis for Specific Sensing of Hydrophobic Amino Acids by the Campylobacter jejuni Chemoreceptor Tlp3. Biomolecules 2020, 10, 744. [Google Scholar] [CrossRef]
- Kanungpean, D.; Kakuda, T.; Takai, S. False Positive Responses of Campylobacter jejuni when Using the Chemical-In-Plug Chemotaxis Assay. J. Vet. Med. Sci. 2011, 73, 389–391. [Google Scholar] [CrossRef] [Green Version]
- Day, C.; King, R.M.; Shewell, L.K.; Tram, G.; Najnin, T.; Hartley-Tassell, L.E.; Wilson, J.C.; Fleetwood, A.D.; Zhulin, I.B.; Korolik, V. A direct-sensing galactose chemoreceptor recently evolved in invasive strains of Campylobacter jejuni. Nat. Commun. 2016, 7, 13206. [Google Scholar] [CrossRef]
- Rahman, H.; King, R.M.; Shewell, L.K.; Semchenko, E.A.; Hartley-Tassell, L.E.; Wilson, J.C.; Day, C.J.; Korolik, V. Character-isation of a multi-ligand binding chemoreceptor CcmL (Tlp3) of Campylobacter jejuni. PLoS Pathog. 2014, 10, e1003822. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, R.; Nothaft, H.; Garber, J.; Kin, L.X.; Stahl, M.; Flint, A.; van Vliet, A.; Stintzi, A.; Szymanski, C.M. L-fucose influences chemotaxis and biofilm formation in Campylobacter jejuni. Mol. Microbiol. 2016, 101, 575–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekhar, K.; Gangaiah, D.; Pina-Mimbela, R.; Kassem, I.; Jeon, B.H.; Rajashekara, G. Transducer like proteins of Campylobacter jejuni 81-176: Role in chemotaxis and colonization of the chicken gastrointestinal tract. Front. Cell. Infect. Microbiol. 2015, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgamoudi, B.; Ketley, J.M. Determination of the Chemotactic Behavior of Campylobacter jejuni by using the μ-Slide Chemotaxis. In User Protocols-Ibidi, 1st ed.; ibidi: Fitchburg, WI, USA, 2016; Volume 1. [Google Scholar]
- Elgamoudi, B.A.; Andrianova, E.P.; Shewell, L.K.; Day, C.J.; King, R.M.; Rahman, H.; Hartley-Tassell, L.E.; Zhulin, I.B.; Korolik, V. The Campylobacter jejuni chemoreceptor Tlp10 has a bimodal ligand-binding domain and specificity for multiple classes of chemoeffectors. Sci. Signal. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Reuter, M.; Salt, L.J.; Cross, K.L.; Betts, R.P.; van Vliet, A.H.M. Chicken Juice Enhances Surface Attachment and Biofilm Formation of Campylobacter jejuni. Appl. Environ. Microbiol. 2014, 80, 7053–7060. [Google Scholar] [CrossRef] [Green Version]
- Korolik, V.; Ottemann, K.M. Two Spatial Chemotaxis Assays: The Nutrient-Depleted Chemotaxis Assay and the Aga-rose-Plug-Bridge Assay. In Bacterial Chemosensing; Springer: Berlin/Heidelberg, Germany, 2018; pp. 23–31. [Google Scholar]
- Adler, J. A Method for Measuring Chemotaxis and Use of the Method to Determine Optimum Conditions for Chemotaxis by Escherichia coli. J. Gen. Microbiol. 1973, 74, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Bainer, R.; Park, H.; Cluzel, P. A high-throughput capillary assay for bacterial chemotaxis. J. Microbiol. Methods 2003, 55, 315–319. [Google Scholar] [CrossRef]
- Gordillo, F.; Chãvez, F.P.; Jerez, C.A. Motility and chemotaxis of Pseudomonas sp. B4 towards polychlorobiphenyls and chlorobenzoates. FEMS Microbiol. Ecol. 2007, 60, 322–328. [Google Scholar] [CrossRef]
- Moulton, R.C.; Montie, T.C. Chemotaxis by Pseudomonas aeruginosa. J. Bacteriol. 1979, 137, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Tumewu, S.A.; Matsui, H.; Yamamoto, M.; Noutoshi, Y.; Toyoda, K.; Ichinose, Y. Identification of chemoreceptor proteins for amino acids involved in host plant infection in Pseudomonas syringae pv. tabaci 6605. Microbiol. Res. 2021, 253, 126869. [Google Scholar] [CrossRef]
- Law, A.M.J.; Aitken, M.D. Continuous-Flow Capillary Assay for Measuring Bacterial Chemotaxis. Appl. Environ. Microbiol. 2005, 71, 3137–3143. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.S.; Elgamoudi, B.A.; Jen, F.E.-C.; Day, C.J.; Sweeney, E.G.; Pryce, M.L.; Guillemin, K.; Haselhorst, T.; Korolik, V.; Ottemann, K.M. The dCache Chemoreceptor TlpA of Helicobacter pylori Binds Multiple Attractant and Antagonistic Ligands via Distinct Sites. mBio 2021, 12, 01819–01821. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, K.; Srivastava, V.; Hwang, S.; Jeon, B.; Ryu, S.; Rajashekara, G. Transducer-Like Protein in Campylobacter jejuni With a Role in Mediating Chemotaxis to Iron and Phosphate. Front. Microbiol. 2018, 9, 2674. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Sweeney, E.G.; Sigal, M.; Zhang, H.C.; Remington, S.J.; Cantrell, M.A.; Kuo, C.J.; Guillemin, K.; Amieva, M.R. Chemodetection and Destruction of Host Urea Allows Helicobacter pylori to Locate the Epithelium. Cell Host Microbe 2015, 18, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocking, B. The use of attractants and repellents in vector control. Bull. World Health Organ. 1963, 29, 121–126. [Google Scholar]
- Karim, Q.N.; Logan, R.P.; Puels, J.; Karnholz, A.; Worku, M.L. Measurement of motility of Helicobacter pylori, Campylobacter jejuni, and Escherichia coli by real time computer tracking using the Hobson BacTracker. J. Clin. Pathol. 1998, 51, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Grognot, M.; Taute, K.M. A multiscale 3D chemotaxis assay reveals bacterial navigation mechanisms. Commun. Biol. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Staropoli, J.F.; Alon, U. Computerized Analysis of Chemotaxis at Different Stages of Bacterial Growth. Biophys. J. 2000, 78, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.S.; Alam, M. An agarose-in-plug bridge method to study chemotaxis in the Archaeon Halobacterium salinarum. FEMS Microbiol. Lett. 1997, 156, 265–269. [Google Scholar] [CrossRef]
- Boyeldieu, A.; Chaouche, A.A.; Méjean, V.; Jourlin-Castelli, C. Combining two optimized and affordable methods to assign chemoreceptors to a specific signal. Anal. Biochem. 2021, 620, 114139. [Google Scholar] [CrossRef]
- Parales, R.E.; Ditty, J.L.; Harwood, C.S. Toluene-Degrading Bacteria Are Chemotactic towards the Environmental Pollutants Benzene, Toluene, and Trichloroethylene. Appl. Environ. Microbiol. 2000, 66, 4098–4104. [Google Scholar] [CrossRef] [Green Version]

| Method | Detection Time | Molar Concentration | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Agar-based assays | |||||
| Hard-plug agar assay (HAP assay) | 3 h | 10–100 mM | -Easy to prepare. -Gives quantitative data. -Requires minimal equipment. -Strains can be compared directly. | -Chemorepellent taxis are difficult to observe. -False positive results are possible. | [16] |
| Modified hard-plug agar assay (t-HAP assay) | 10 min to 3 h | 10–100 mM | -Easy to prepare. -Gives quantitative data. -Requires minimal equipment. -Strains can be compared directly. -Differentiations between catabolised and non-catabolised ligands are possible | -Chemorepellent taxis are difficult to observe. | [26] |
| Nutrient-depletion assay | 3–6 h | 2–10 mM | -Gives quantitative data. -Easy to prepare. -Requires minimal equipment. -Strains can be compared directly. - chemorepellents taxis can be quantitated. -Gradients are created by diffusion, not metabolism. | -Sensitive to any motions around the assays. -One strain and conditions can be monitored per assay. -Visual observation is difficult. | [34,35] |
| Tube-based assay | 75 h | 1 M | -Easy to prepare. -Requires minimal equipment. -Strains can be compared directly. | -Not suitable for studying chemorepellents. -Semi-quantitative. | [36] |
| Capillary assay | |||||
| Capillary assay | 1 h | 10–100 mM | -Gives quantitative data. -Requires minimal equipment. -Gradients are created by diffusion, not metabolism. | -Not suitable for studying chemorepellents. -One strain and condition can be monitored per assay. | [37] |
| Chemotaxis chamber | |||||
| μ-slide chemotaxis chamber | 3 h | 5–10 mM | -Ideal to study the behaviour of a single cell. -Chemoresponses can be measured for a group of cells or a single cell. Clear visualisation of cell migration. -Gives quantitative data. | -One strain and condition can be monitored per assay. -Tracking system is relatively expensive. | [38,39] |
| Ligands | t-HAP | Nutrient-Depletion Assay | μ-Slide Assays | |||
|---|---|---|---|---|---|---|
| 11168-O WT | Δtlp10 | 11168-O WT | Δtlp10 | 11168-O WT | Δtlp10 | |
| fucose | 6.7 ± 0.61 | 5.3 ± 0.02 | 5.8 ± 0.63 | 3.86 ± 0.43 | 4.96 ± 0.39 | 3.61 ± 0.08 |
| isoleucine | 6.6 ± 0.34 | 5.9 ± 0.18 | 5.7 ± 0.4 | 4 ± 0.61 | 6.61 ± 0.33 | 5.52 ± 0.17 |
| aspartate | 6.38 ± 0.4 | 5.33 ± 0.22 | 5.61 ± 0.93 | 3.32 ± 0.18 | 5.71± 0.4 | 4.21 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgamoudi, B.A.; Korolik, V. A Review of the Advantages, Disadvantages and Limitations of Chemotaxis Assays for Campylobacter spp. Int. J. Mol. Sci. 2022, 23, 1576. https://doi.org/10.3390/ijms23031576
Elgamoudi BA, Korolik V. A Review of the Advantages, Disadvantages and Limitations of Chemotaxis Assays for Campylobacter spp. International Journal of Molecular Sciences. 2022; 23(3):1576. https://doi.org/10.3390/ijms23031576
Chicago/Turabian StyleElgamoudi, Bassam A., and Victoria Korolik. 2022. "A Review of the Advantages, Disadvantages and Limitations of Chemotaxis Assays for Campylobacter spp." International Journal of Molecular Sciences 23, no. 3: 1576. https://doi.org/10.3390/ijms23031576
APA StyleElgamoudi, B. A., & Korolik, V. (2022). A Review of the Advantages, Disadvantages and Limitations of Chemotaxis Assays for Campylobacter spp. International Journal of Molecular Sciences, 23(3), 1576. https://doi.org/10.3390/ijms23031576







