Polymodal Control of TMEM16x Channels and Scramblases
Abstract
1. Introduction to TMEM16x Physiology
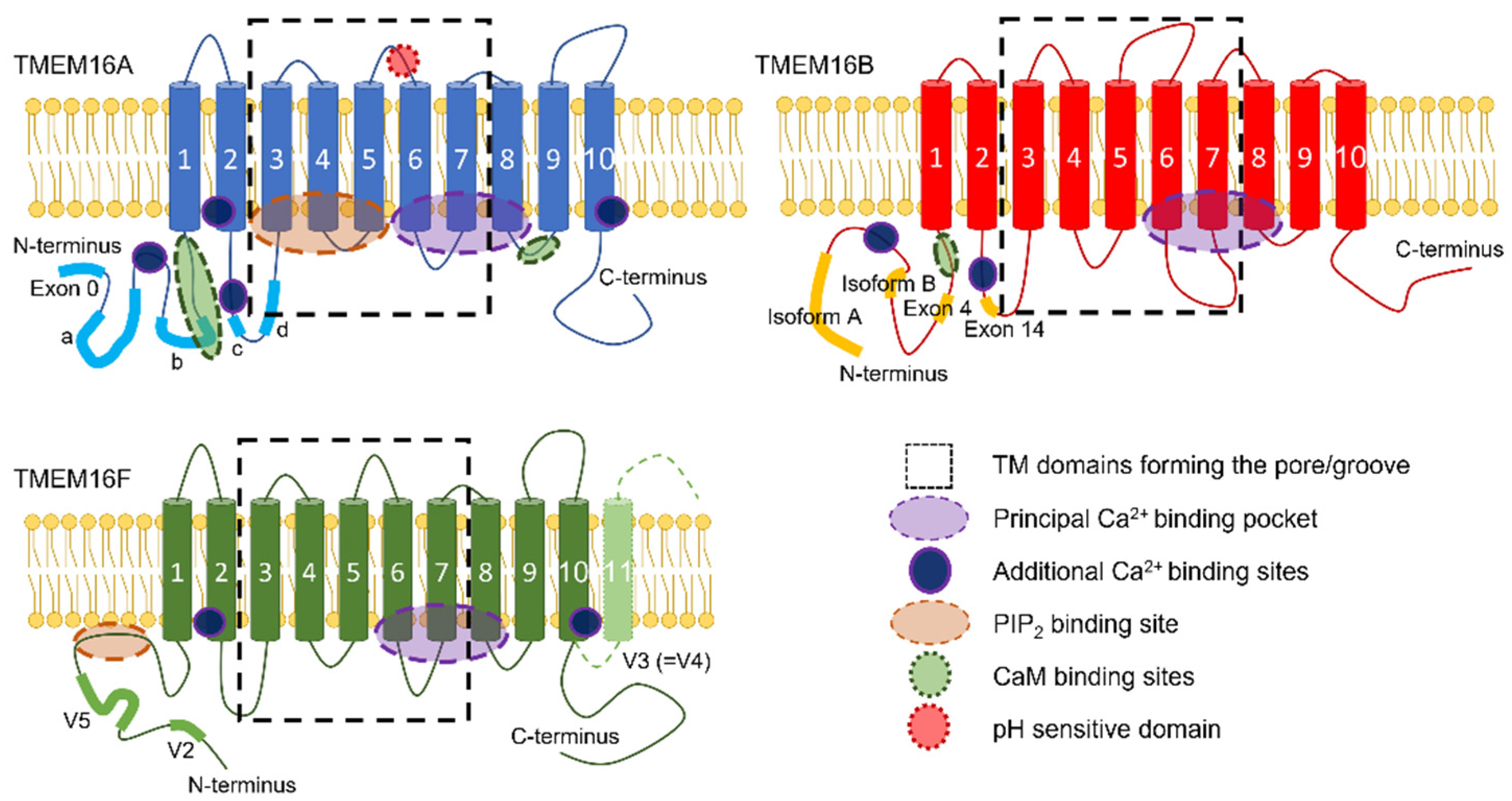
2. TMEM16x Splice Variants
3. The Factors Controlling Ion and Lipid Transport in TMEM16x Proteins
Biophysics of Ion and Lipid Transport
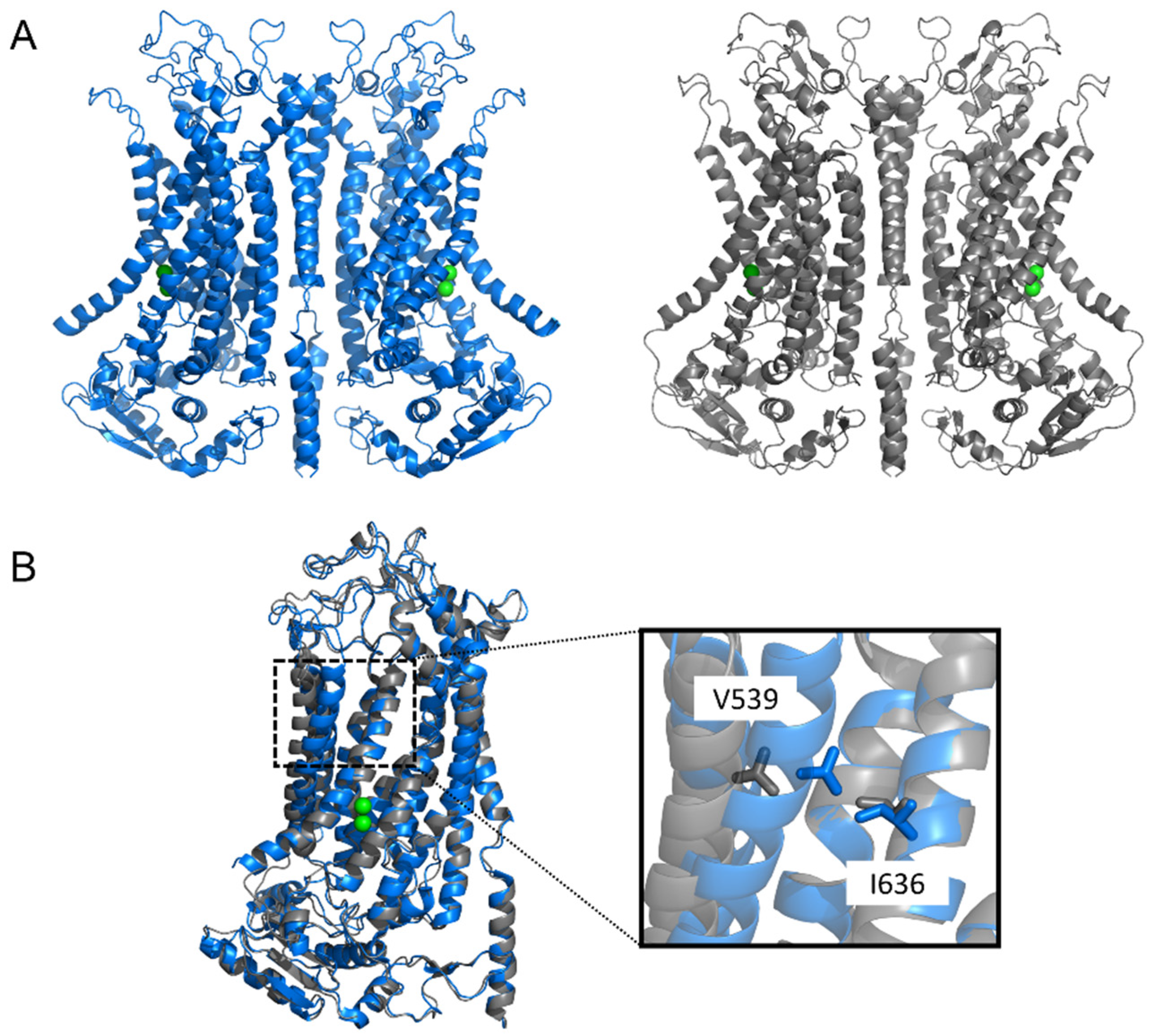
4. Overview of the Structure–Function Relationship in TMEM16x Proteins
4.1. Gating Mechanisms
4.2. Ion and Lipid Conduction Pathways
5. Mechanisms of Modulation of TMEM16x Proteins’ Function
5.1. Ca2+ and Other Divalent Cations
5.2. Calmodulin
5.3. Intra- and Extracellular pH
5.4. Intra- and Extracellular ATP
5.5. Hypoxia and Reactive Oxygen Species (ROS)
5.6. Heat
5.7. Lipids
5.7.1. PIP2
5.7.2. Other Lipids
6. Other Regulators of TMEM16x Activity
6.1. Ca2+-Activated Chloride Channel Regulators 1 and 2 (CLCA1 and 2)
6.2. KCNE1
7. Additional Regulatory Mechanisms of TMEM16x Proteins
8. A Role for TMEM16F in the Pathology of SARS-CoV-2
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression Cloning of TMEM16A as a Calcium-Activated Chloride Channel Subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Bushell, S.R.; Pike, A.C.W.; Falzone, M.E.; Rorsman, N.J.G.; Ta, C.M.; Corey, R.A.; Newport, T.D.; Christianson, J.C.; Scofano, L.F.; Shintre, C.A.; et al. The Structural Basis of Lipid Scrambling and Inactivation in the Endoplasmic Reticulum Scramblase TMEM16K. Nat. Commun. 2019, 10, 3956. [Google Scholar] [CrossRef] [PubMed]
- Di Zanni, E.; Gradogna, A.; Scholz-Starke, J.; Boccaccio, A. Gain of Function of TMEM16E/ANO5 Scrambling Activity Caused by a Mutation Associated with Gnathodiaphyseal dysplasia. Cell. Mol. Life Sci. 2018, 75, 1657–1670. [Google Scholar] [CrossRef]
- Guo, J.; Wang, D.; Dong, Y.; Gao, X.; Tong, H.; Liu, W.; Zhang, L.; Sun, M. ANO7: Insights into Topology, Function, and Potential Applications as a Biomarker and Immunotherapy Target. Tissue Cell 2021, 72, 101546. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Lee, J.; Lee, B.; Kim, H.-R.; Jung, J.; Lee, M.-O.; Oh, U. Anoctamin 9/TMEM16J Is a Cation Channel Activated by CAMP/PKA Signal. Cell Calcium. 2018, 71, 75–85. [Google Scholar] [CrossRef]
- Reichhart, N.; Schöberl, S.; Keckeis, S.; Alfaar, A.S.; Roubeix, C.; Cordes, M.; Crespo-Garcia, S.; Haeckel, A.; Kociok, N.; Föckler, R.; et al. Anoctamin-4 Is a Bona Fide Ca2+-Dependent Non-Selective Cation Channel. Sci. Rep. 2019, 9, 2257. [Google Scholar] [CrossRef]
- Scudieri, P.; Caci, E.; Venturini, A.; Sondo, E.; Pianigiani, G.; Marchetti, C.; Ravazzolo, R.; Pagani, F.; Galietta, L.J.V. Ion Channel and Lipid Scramblase Activity Associated with Expression of TMEM16F/ANO6 Isoforms. J. Physiol. 2015, 593, 3829–3848. [Google Scholar] [CrossRef]
- Yu, K.; Whitlock, J.M.; Lee, K.; Ortlund, E.A.; Yuan Cui, Y.; Hartzell, H.C. Identification of a Lipid Scrambling Domain in ANO6/TMEM16F. eLife 2015, 4, e06901. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Ostertag, E.M.; Nuwal, T.; Huang, B.; Jan, Y.-N.; Basbaum, A.I.; Jan, L.Y. TMEM16C Facilitates Na+-Activated K+ Currents in Rat Sensory Neurons and Regulates Pain Processing. Nat. Neurosci. 2013, 16, 1284–1290. [Google Scholar] [CrossRef]
- Jha, A.; Chung, W.Y.; Vachel, L.; Maleth, J.; Lake, S.; Zhang, G.; Ahuja, M.; Muallem, S. Anoctamin 8 Tethers Endoplasmic Reticulum and Plasma Membrane for Assembly of Ca2+ Signaling Complexes at the ER/PM Compartment. EMBO J. 2019, 38, e101452. [Google Scholar] [CrossRef] [PubMed]
- Galindo, B.E.; Vacquier, V.D. Phylogeny of the TMEM16 Protein Family: Some Members Are Overexpressed in Cancer. Int. J. Mol. Med. 2005, 16, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Brockmann, M.; Stöhr, H.; Weber, B.H.; Strauss, O. Evolution and Functional Divergence of the Anoctamin Family of Membrane Proteins. BMC Evol. Biol. 2010, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Pelz, T.; Drose, D.R.; Fleck, D.; Henkel, B.; Ackels, T.; Spehr, M.; Neuhaus, E.M. An Ancestral TMEM16 Homolog from Dictyostelium Discoideum Forms a Scramblase. PLoS ONE 2018, 13, e0191219. [Google Scholar] [CrossRef]
- Ferrera, L.; Zegarra-Moran, O.; Galietta, L.J.V. Ca2+-Activated Cl− Channels. Compr. Physiol. 2011, 1, 2155–2174. [Google Scholar] [CrossRef]
- Hartzell, H.C.; Yu, K.; Xiao, Q.; Chien, L.-T.; Qu, Z. Anoctamin/TMEM16 Family Members Are Ca2+-Activated Cl− Channels. J. Physiol. 2009, 587, 2127–2139. [Google Scholar] [CrossRef]
- Huang, F.; Wong, X.; Jan, L.Y. International Union of Basic and Clinical Pharmacology. LXXXV: Calcium-Activated Chloride Channels. Pharmacol. Rev. 2012, 64, 1–15. [Google Scholar] [CrossRef]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.-S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.-M.; et al. TMEM16A Confers Receptor-Activated Calcium-Dependent Chloride Conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Adomaviciene, A.; Smith, K.J.; Garnett, H.; Tammaro, P. Putative Pore-Loops of TMEM16/Anoctamin Channels Affect Channel Density in Cell Membranes. J. Physiol. 2013, 591, 3487–3505. [Google Scholar] [CrossRef]
- Betto, G.; Cherian, O.L.; Pifferi, S.; Cenedese, V.; Boccaccio, A.; Menini, A. Interactions between Permeation and Gating in the TMEM16B/Anoctamin2 Calcium-Activated Chloride Channel. J. Gen. Physiol. 2014, 143, 703–718. [Google Scholar] [CrossRef]
- Cruz-Rangel, S.; Jesús-Pérez, J.J.D.; Contreras-Vite, J.A.; Pérez-Cornejo, P.; Hartzell, H.C.; Arreola, J. Gating Modes of Calcium-Activated Chloride Channels TMEM16A and TMEM16B. J. Physiol. 2015, 593, 5283–5298. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, S. Permeation Mechanisms in the TMEM16B Calcium-Activated Chloride Channels. PLoS ONE 2017, 12, e0169572. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, S.; Dibattista, M.; Menini, A. TMEM16B Induces Chloride Currents Activated by Calcium in Mammalian Cells. Pflüg. Arch. Eur. J. Physiol. 2009, 458, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Scudieri, P.; Sondo, E.; Ferrera, L.; Galietta, L.J.V. The Anoctamin Family: TMEM16A and TMEM16B as Calcium-Activated Chloride Channels. Exp. Physiol. 2012, 97, 177–183. [Google Scholar] [CrossRef]
- Cherian, O.L.; Menini, A.; Boccaccio, A. Multiple Effects of Anthracene-9-Carboxylic Acid on the TMEM16B/Anoctamin2 Calcium-Activated Chloride Channel. Biochim. Biophys. Acta BBA Biomembr. 2015, 1848, 1005–1013. [Google Scholar] [CrossRef]
- Ta, C.M.; Adomaviciene, A.; Rorsman, N.J.G.; Garnett, H.; Tammaro, P. Mechanism of Allosteric Activation of TMEM16A/ANO1 Channels by a Commonly Used Chloride Channel Blocker. Br. J. Pharmacol. 2016, 173, 511–528. [Google Scholar] [CrossRef]
- Bradley, E.; Fedigan, S.; Webb, T.; Hollywood, M.A.; Thornbury, K.D.; McHale, N.G.; Sergeant, G.P. Pharmacological Characterization of TMEM16A Currents. Channels 2014, 8, 308–320. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Huang, D.; Qi, J.; Xu, J.; Gao, H.; Du, X.; Gamper, N.; Zhang, H. Characterization of the Effects of Cl− Channel Modulators on TMEM16A and Bestrophin-1 Ca2+ Activated Cl− Channels. Pflugers Arch. 2015, 467, 1417–1430. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H.K.; Park, J.; Jeon, D.; Jo, S.; Jo, M.; Namkung, W. Ani9, A Novel Potent Small-Molecule ANO1 Inhibitor with Negligible Effect on ANO2. PLoS ONE 2016, 11, e0155771. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs That Inhibit TMEM16 Proteins Block SARS-CoV-2 Spike-Induced Syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Miner, K.; Labitzke, K.; Liu, B.; Wang, P.; Henckels, K.; Gaida, K.; Elliott, R.; Chen, J.J.; Liu, L.; Leith, A.; et al. Drug Repurposing: The Anthelmintics Niclosamide and Nitazoxanide Are Potent TMEM16A Antagonists That Fully Bronchodilate Airways. Front. Pharmacol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Uliyakina, I.; Kongsuphol, P.; Warth, R.; Mirza, M.; Martins, J.R.; Kunzelmann, K. Expression and Function of Epithelial Anoctamins. J. Biol. Chem. 2010, 285, 7838–7845. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Anoctamins (TMEM16 Proteins): Functions and Involvement in Neurologic Disease. Neurology 2017, 89, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Yang, H.; Jan, L.Y. Ca2+-Activated Cl− Channels at a Glance. J. Cell Sci. 2012, 125, 1367–1371. [Google Scholar] [CrossRef]
- Crottès, D.; Jan, L.Y. The Multifaceted Role of TMEM16A in Cancer. Cell Calcium. 2019, 82, 102050. [Google Scholar] [CrossRef]
- Duran, C.; Hartzell, H.C. Physiological Roles and Diseases of Tmem16/Anoctamin Proteins: Are They All Chloride Channels? Acta Pharmacol. Sin. 2011, 32, 685–692. [Google Scholar] [CrossRef]
- Duran, C.; Qu, Z.; Osunkoya, A.O.; Cui, Y.; Hartzell, H.C. ANOs 3–7 in the Anoctamin/Tmem16 Cl− Channel Family Are Intracellular Proteins. Am. J. Physiol. Cell Physiol. 2012, 302, C482–C493. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Tian, Y.; Martins, J.R.; Faria, D.; Kongsuphol, P.; Ousingsawat, J.; Thevenod, F.; Roussa, E.; Rock, J.; Schreiber, R. Anoctamins. Pflüg. Arch. Eur. J. Physiol. 2011, 462, 195–208. [Google Scholar] [CrossRef]
- Oh, U.; Jung, J. Cellular Functions of TMEM16/Anoctamin. Pflüg. Arch. Eur. J. Physiol. 2016, 468, 443–453. [Google Scholar] [CrossRef]
- Pedemonte, N.; Galietta, L.J.V. Structure and Function of TMEM16 Proteins (Anoctamins). Physiol. Rev. 2014, 94, 419–459. [Google Scholar] [CrossRef] [PubMed]
- Picollo, A.; Malvezzi, M.; Accardi, A. TMEM16 Proteins: Unknown Structure and Confusing Functions. J. Mol. Biol. 2015, 427, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Salzer, I.; Boehm, S. Calcium-Activated Chloride Channels: Potential Targets for Antinociceptive Therapy. Int. J. Biochem. Cell Biol. 2019, 111, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.M.; Hartzell, H.C. Anoctamins/TMEM16 Proteins: Chloride Channels Flirting with Lipids and Extracellular Vesicles. Annu. Rev. Physiol. 2017, 79, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. TMEM16A, A Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Dutta, A.K.; Khimji, A.; Kresge, C.; Bugde, A.; Dougherty, M.; Esser, V.; Ueno, Y.; Glaser, S.S.; Alpini, G.; Rockey, D.C.; et al. Identification and Functional Characterization of TMEM16A, a Ca2+-Activated Cl− Channel Activated by Extracellular Nucleotides, in Biliary Epithelium. J. Biol. Chem. 2011, 286, 766–776. [Google Scholar] [CrossRef]
- Huang, W.C.; Xiao, S.; Huang, F.; Harfe, B.D.; Jan, Y.N.; Jan, L.Y. Calcium-Activated Chloride Channels (CaCCs) Regulate Action Potential and Synaptic Response in Hippocampal Neurons. Neuron 2012, 74, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.J.; Forrest, A.S.; Jepps, T.A.; Valencik, M.L.; Wiwchar, M.; Singer, C.A.; Sones, W.R.; Greenwood, I.A.; Leblanc, N. Expression Profile and Protein Translation of TMEM16A in Murine Smooth Muscle. Am. J. Physiol. Cell Physiol. 2010, 299, C948–C959. [Google Scholar] [CrossRef]
- Heinze, C.; Seniuk, A.; Sokolov, M.V.; Huebner, A.K.; Klementowicz, A.E.; Szijártó, I.A.; Schleifenbaum, J.; Vitzthum, H.; Gollasch, M.; Ehmke, H.; et al. Disruption of Vascular Ca2+-Activated Chloride Currents Lowers Blood Pressure. J. Clin. Investig. 2014, 124, 675–686. [Google Scholar] [CrossRef]
- Manoury, B.; Tamuleviciute, A.; Tammaro, P. TMEM16A/Anoctamin 1 Protein Mediates Calcium-Activated Chloride Currents in Pulmonary Arterial Smooth Muscle Cells. J. Physiol. 2010, 588, 2305–2314. [Google Scholar] [CrossRef]
- Thomas-Gatewood, C.; Neeb, Z.P.; Bulley, S.; Adebiyi, A.; Bannister, J.P.; Leo, M.D.; Jaggar, J.H. TMEM16A Channels Generate Ca2+-Activated Cl− Currents in Cerebral Artery Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1819–H1827. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Huai, R.; Qu, Z. Overexpression of ANO1/TMEM16A, an Arterial Ca2+-Activated Cl− Channel, Contributes to Spontaneous Hypertension. J. Mol. Cell. Cardiol. 2015, 82, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Dauner, K.; Möbus, C.; Frings, S.; Möhrlen, F. Targeted Expression of Anoctamin Calcium-Activated Chloride Channels in Rod Photoreceptor Terminals of the Rodent Retina. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3126–3136. [Google Scholar] [CrossRef] [PubMed]
- Hengl, T.; Kaneko, H.; Dauner, K.; Vocke, K.; Frings, S.; Möhrlen, F. Molecular Components of Signal Amplification in Olfactory Sensory Cilia. Proc. Natl. Acad. Sci. USA 2010, 107, 6052–6057. [Google Scholar] [CrossRef] [PubMed]
- Pietra, G.; Dibattista, M.; Menini, A.; Reisert, J.; Boccaccio, A. The Ca2+-Activated Cl− Channel TMEM16B Regulates Action Potential Firing and Axonal Targeting in Olfactory Sensory Neurons. J. Gen. Physiol. 2016, 148, 293–311. [Google Scholar] [CrossRef]
- Stephan, A.B.; Shum, E.Y.; Hirsh, S.; Cygnar, K.D.; Reisert, J.; Zhao, H. ANO2 Is the Cilial Calcium-Activated Chloride Channel That May Mediate Olfactory Amplification. Proc. Natl. Acad. Sci. USA 2009, 106, 11776–11781. [Google Scholar] [CrossRef]
- Stöhr, H.; Heisig, J.B.; Benz, P.M.; Schöberl, S.; Milenkovic, V.M.; Strauss, O.; Aartsen, W.M.; Wijnholds, J.; Weber, B.H.F.; Schulz, H.L. TMEM16B, a Novel Protein with Calcium-Dependent Chloride Channel Activity, Associates with a Presynaptic Protein Complex in Photoreceptor Terminals. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 6809–6818. [Google Scholar] [CrossRef]
- Ayoglu, B.; Mitsios, N.; Kockum, I.; Khademi, M.; Zandian, A.; Sjöberg, R.; Forsström, B.; Bredenberg, J.; Bomfim, I.L.; Holmgren, E.; et al. Anoctamin 2 Identified as an Autoimmune Target in Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 2188–2193. [Google Scholar] [CrossRef]
- Ha, G.E.; Lee, J.; Kwak, H.; Song, K.; Kwon, J.; Jung, S.-Y.; Hong, J.; Chang, G.-E.; Hwang, E.M.; Shin, H.-S.; et al. The Ca2+-Activated Chloride Channel Anoctamin-2 Mediates Spike-Frequency Adaptation and Regulates Sensory Transmission in Thalamocortical Neurons. Nat. Commun. 2016, 7, 13791. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Xiao, S.; Tien, J.; Le, S.; Le, T.; Jan, L.Y.; Yang, H. Inferior Olivary TMEM16B Mediates Cerebellar Motor Learning. Neuron 2017, 95, 1103–1111.e4. [Google Scholar] [CrossRef]
- Suzuki, J.; Fujii, T.; Imao, T.; Ishihara, K.; Kuba, H.; Nagata, S. Calcium-Dependent Phospholipid Scramblase Activity of TMEM16 Protein Family Members. J. Biol. Chem. 2013, 288, 13305–13316. [Google Scholar] [CrossRef]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-Dependent Phospholipid Scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, N.; Milenkovic, V.M.; Wetzel, C.H.; Strauß, O. Prediction of Functional Consequences of Missense Mutations in ANO4 Gene. Int. J. Mol. Sci. 2021, 22, 2732. [Google Scholar] [CrossRef] [PubMed]
- Maniero, C.; Scudieri, P.; Haris Shaikh, L.; Zhao, W.; Gurnell, M.; Galietta, L.J.V.; Brown, M.J. ANO4 (Anoctamin 4) Is a Novel Marker of Zona Glomerulosa That Regulates Stimulated Aldosterone Secretion. Hypertension 2019, 74, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, A.; Sanna, S.; Uda, M.; Deiana, B.; Usala, G.; Busonero, F.; Maschio, A.; Scally, M.; Patriciu, N.; Chen, W.-M.; et al. Genome-Wide Association Scan for Five Major Dimensions of Personality. Mol. Psychiatry 2010, 15, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Athanasiu, L.; Mattingsdal, M.; Kähler, A.K.; Brown, A.; Gustafsson, O.; Agartz, I.; Giegling, I.; Muglia, P.; Cichon, S.; Rietschel, M.; et al. Gene Variants Associated with Schizophrenia in a Norwegian Genome-Wide Study Are Replicated in a Large European Cohort. J. Psychiatr. Res. 2010, 44, 748–753. [Google Scholar] [CrossRef]
- Sherva, R.; Tripodis, Y.; Bennett, D.A.; Chibnik, L.B.; Crane, P.K.; de Jager, P.L.; Farrer, L.A.; Saykin, A.J.; Shulman, J.M.; Naj, A.; et al. Genome-Wide Association Study of the Rate of Cognitive Decline in Alzheimer’s Disease. Alzheimers Dement. 2014, 10, 45–52. [Google Scholar] [CrossRef]
- Webb, B.T.; Guo, A.-Y.; Maher, B.S.; Zhao, Z.; van den Oord, E.J.; Kendler, K.S.; Riley, B.P.; Gillespie, N.A.; Prescott, C.A.; Middeldorp, C.M.; et al. Meta-Analyses of Genome-Wide Linkage Scans of Anxiety-Related Phenotypes. Eur. J. Hum. Genet. 2012, 20, 1078–1084. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Yu, K.; Cui, Y.Y.; Hartzell, H.C. Anoctamin 5/TMEM16E Facilitates Muscle Precursor Cell Fusion. J. Gen. Physiol. 2018, 150, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Di Zanni, E.; Gradogna, A.; Picco, C.; Scholz-Starke, J.; Boccaccio, A. TMEM16E/ANO5 Mutations Related to Bone Dysplasia or Muscular Dystrophy Cause Opposite Effects on Lipid Scrambling. Hum. Mutat. 2020, 41, 1157–1170. [Google Scholar] [CrossRef]
- van Kruchten, R.; Mattheij, N.J.A.; Saunders, C.; Feijge, M.A.H.; Swieringa, F.; Wolfs, J.L.N.; Collins, P.W.; Heemskerk, J.W.M.; Bevers, E.M. Both TMEM16F-Dependent and TMEM16F-Independent Pathways Contribute to Phosphatidylserine Exposure in Platelet Apoptosis and Platelet Activation. Blood 2013, 121, 1850–1857. [Google Scholar] [CrossRef]
- Fujii, T.; Sakata, A.; Nishimura, S.; Eto, K.; Nagata, S. TMEM16F Is Required for Phosphatidylserine Exposure and Microparticle Release in Activated Mouse Platelets. Proc. Natl. Acad. Sci. USA 2015, 112, 12800–12805. [Google Scholar] [CrossRef] [PubMed]
- Foltz, S.J.; Cui, Y.Y.; Choo, H.J.; Hartzell, H.C. ANO5 Ensures Trafficking of Annexins in Wounded Myofibers. J. Cell Biol. 2021, 220, 7059. [Google Scholar] [CrossRef] [PubMed]
- Stamelou, M.; Charlesworth, G.; Cordivari, C.; Schneider, S.A.; Kägi, G.; Sheerin, U.-M.; Rubio-Agusti, I.; Batla, A.; Houlden, H.; Wood, N.W.; et al. The Phenotypic Spectrum of DYT24 Due to ANO3 Mutations. Mov. Disord. 2014, 29, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, E.; Rantapero, T.; Zhang, Q.; Taimen, P.; Laitinen, V.; Kallajoki, M.; Jambulingam, D.; Ettala, O.; Knaapila, J.; Boström, P.J.; et al. ANO7 Is Associated with Aggressive Prostate Cancer. Int. J. Cancer 2018, 143, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Schreiber, R.; Kunzelmann, K. Anoctamins Are a Family of Ca2+-Activated Cl− Channels. J. Cell Sci. 2012, 125, 4991–4998. [Google Scholar] [CrossRef]
- Blanco, M.A.; Alečković, M.; Hua, Y.; Li, T.; Wei, Y.; Xu, Z.; Cristea, I.M.; Kang, Y. Identification of Staphylococcal Nuclease Domain-Containing 1 (SND1) as a Metadherin-Interacting Protein with Metastasis-Promoting Functions. J. Biol. Chem. 2011, 286, 19982–19992. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, E.; Takala, A.; Pursiheimo, J.-P.; Wahlström, G.; Schleutker, J. The Interactome of the Prostate-Specific Protein Anoctamin 7. Cancer Biomark. 2020, 28, 91–100. [Google Scholar] [CrossRef]
- Hammer, C.; Wanitchakool, P.; Sirianant, L.; Papiol, S.; Monnheimer, M.; Faria, D.; Ousingsawat, J.; Schramek, N.; Schmitt, C.; Margos, G.; et al. A Coding Variant of ANO10, Affecting Volume Regulation of Macrophages, Is Associated with Borrelia Seropositivity. Mol. Med. Camb. Mass 2015, 21, 26–37. [Google Scholar] [CrossRef]
- Petkovic, M.; Oses-Prieto, J.; Burlingame, A.; Jan, L.Y.; Jan, Y.N. TMEM16K Is an Interorganelle Regulator of Endosomal Sorting. Nat. Commun. 2020, 11, 3298. [Google Scholar] [CrossRef]
- Tsuji, T.; Cheng, J.; Tatematsu, T.; Ebata, A.; Kamikawa, H.; Fujita, A.; Gyobu, S.; Segawa, K.; Arai, H.; Taguchi, T.; et al. Predominant Localization of Phosphatidylserine at the Cytoplasmic Leaflet of the ER, and Its TMEM16K-Dependent Redistribution. Proc. Natl. Acad. Sci. USA 2019, 116, 13368–13373. [Google Scholar] [CrossRef]
- Schreiber, R.; Ousingsawat, J.; Kunzelmann, K. Targeting of Intracellular TMEM16 Proteins to the Plasma Membrane and Activation by Purinergic Signaling. Int. J. Mol. Sci. 2020, 21, 4065. [Google Scholar] [CrossRef]
- Katsurahara, K.; Shiozaki, A.; Kosuga, T.; Kudou, M.; Shoda, K.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; et al. ANO9 Regulated Cell Cycle in Human Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2020, 27, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Hawley, R.S. The Spindle-Associated Transmembrane Protein Axs Identifies a Membranous Structure Ensheathing the Meiotic Spindle. Nat. Cell Biol. 2003, 5, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; Cabrita, I.; Faria, D.; Schreiber, R.; Kunzelmann, K. Cellular Defects by Deletion of ANO10 Are Due to Deregulated Local Calcium Signaling. Cell. Signal. 2017, 30, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Balreira, A.; Boczonadi, V.; Barca, E.; Pyle, A.; Bansagi, B.; Appleton, M.; Graham, C.; Hargreaves, I.P.; Rasic, V.M.; Lochmüller, H.; et al. ANO10 Mutations Cause Ataxia and Coenzyme Q10 Deficiency. J. Neurol. 2014, 261, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, S.; Hoischen, A.; Meijer, R.P.P.; Gilissen, C.; Neveling, K.; Wieskamp, N.; de Brouwer, A.; Koenig, M.; Anheim, M.; Assoum, M.; et al. Targeted Next-Generation Sequencing of a 12.5 Mb Homozygous Region Reveals ANO10 Mutations in Patients with Autosomal-Recessive Cerebellar Ataxia. Am. J. Hum. Genet. 2010, 87, 813–819. [Google Scholar] [CrossRef]
- Jun, I.; Park, H.S.; Piao, H.; Han, J.W.; An, M.J.; Yun, B.G.; Zhang, X.; Cha, Y.H.; Shin, Y.K.; Yook, J.I.; et al. ANO9/TMEM16J Promotes Tumourigenesis via EGFR and Is a Novel Therapeutic Target for Pancreatic Cancer. Br. J. Cancer 2017, 117, 1798–1809. [Google Scholar] [CrossRef]
- Katsurahara, K.; Shiozaki, A.; Kosuga, T.; Shimizu, H.; Kudou, M.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; et al. ANO9 Regulates PD-L2 Expression and Binding Ability to PD-1 in Gastric Cancer. Cancer Sci. 2021, 112, 1026–1037. [Google Scholar] [CrossRef]
- Ferrera, L.; Caputo, A.; Ubby, I.; Bussani, E.; Zegarra-Moran, O.; Ravazzolo, R.; Pagani, F.; Galietta, L.J.V. Regulation of TMEM16A Chloride Channel Properties by Alternative Splicing. J. Biol. Chem. 2009, 284, 33360–33368. [Google Scholar] [CrossRef]
- Han, J.; Kim, H.; Seo, D.-G.; Lee, G.; Jeung, E.-B.; Yu, F.H. Multiple Transcripts of Anoctamin Genes Expressed in the Mouse Submandibular Salivary Gland. J. Periodontal Implant Sci. 2015, 45, 69–75. [Google Scholar] [CrossRef]
- Huanosta-Gutiérrez, A.; Espino-Saldaña, A.E.; Reyes, J.P.; Pétriz, A.; Miledi, R.; Martínez-Torres, A. TMEM16A Alternative Splicing Isoforms in Xenopus Tropicalis: Distribution and Functional Properties. Biochem. Biophys. Res. Commun. 2014, 446, 1096–1101. [Google Scholar] [CrossRef]
- Ponissery Saidu, S.; Stephan, A.B.; Talaga, A.K.; Zhao, H.; Reisert, J. Channel Properties of the Splicing Isoforms of the Olfactory Calcium-Activated Chloride Channel Anoctamin 2. J. Gen. Physiol. 2013, 141, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Suzuki, J.; Nagata, S. Constitutive Exposure of Phosphatidylserine on Viable Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19246–19251. [Google Scholar] [CrossRef] [PubMed]
- Strege, P.R.; Bernard, C.E.; Mazzone, A.; Linden, D.R.; Beyder, A.; Gibbons, S.J.; Farrugia, G. A Novel Exon in the Human Ca2+-Activated Cl− Channel Ano1 Imparts Greater Sensitivity to Intracellular Ca2+. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 309, G743–G749. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, L.; Lau, Y.S.; Gao, Y.; Moore, S.A.; Han, R. A Novel ANO5 Splicing Variant in a LGMD2L Patient Leads to Production of a Truncated Aggregation-Prone Ano5 Peptide. J. Pathol. Clin. Res. 2018, 4, 135–145. [Google Scholar] [CrossRef]
- O’Driscoll, K.E.; Pipe, R.A.; Britton, F.C. Increased Complexity of Tmem16a/Anoctamin 1 Transcript Alternative Splicing. BMC Mol. Biol. 2011, 12, 35. [Google Scholar] [CrossRef]
- Mazzone, A.; Gibbons, S.J.; Bernard, C.E.; Nowsheen, S.; Middha, S.; Almada, L.L.; Ordog, T.; Kendrick, M.L.; Lombardo, K.R.; Shen, K.R.; et al. Identification and Characterization of a Novel Promoter for the Human ANO1 Gene Regulated by the Transcription Factor Signal Transducer and Activator of Transcription 6 (STAT6). FASEB J. 2015, 29, 152–163. [Google Scholar] [CrossRef]
- Mazzone, A.; Bernard, C.E.; Strege, P.R.; Beyder, A.; Galietta, L.J.V.; Pasricha, P.J.; Rae, J.L.; Parkman, H.P.; Linden, D.R.; Szurszewski, J.H.; et al. Altered Expression of Ano1 Variants in Human Diabetic Gastroparesis. J. Biol. Chem. 2011, 286, 13393–13403. [Google Scholar] [CrossRef]
- Sondo, E.; Scudieri, P.; Tomati, V.; Caci, E.; Mazzone, A.; Farrugia, G.; Ravazzolo, R.; Galietta, L.J.V. Non-Canonical Translation Start Sites in the TMEM16A Chloride Channel. Biochim. Biophys. Acta BBA Biomembr. 2014, 1838, 89–97. [Google Scholar] [CrossRef]
- Hawn, M.B.; Akin, E.; Hartzell, H.C.; Greenwood, I.A.; Leblanc, N. Molecular Mechanisms of Activation and Regulation of ANO1-Encoded Ca2+-Activated Cl− Channels. Channels 2021, 197, 5411. [Google Scholar] [CrossRef]
- Xiao, Q.; Yu, K.; Perez-Cornejo, P.; Cui, Y.; Arreola, J.; Hartzell, H.C. Voltage- and Calcium-Dependent Gating of TMEM16A/Ano1 Chloride Channels Are Physically Coupled by the First Intracellular Loop. Proc. Natl. Acad. Sci. USA 2011, 108, 8891–8896. [Google Scholar] [CrossRef]
- Armstrong, C.M. Interaction of Tetraethylammonium Ion Derivatives with the Potassium Channels of Giant Axons. J. Gen. Physiol. 1971, 58, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, I.A.; Large, W.A. Modulation of the Decay of Ca2+-Activated Cl− Currents in Rabbit Portal Vein Smooth Muscle Cells by External Anions. J. Physiol. 1999, 516 Pt 2, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Yellen, G. Single Channel Seeks Permeant Ion for Brief but Intimate Relationship. J. Gen. Physiol. 1997, 110, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Jung, S.-R.; Kim, K.-W.; Yeon, J.-H.; Park, C.-G.; Nam, J.H.; Hille, B.; Suh, B.-C. Allosteric Modulation of Alternatively Spliced Ca2+-Activated Cl− Channels TMEM16A by PI(4,5)P2 and CaMKII. Proc. Natl. Acad. Sci. USA 2020, 20, 117. [Google Scholar] [CrossRef]
- Dang, S.; Feng, S.; Tien, J.; Peters, C.J.; Bulkley, D.; Lolicato, M.; Zhao, J.; Zuberbühler, K.; Ye, W.; Qi, L.; et al. Cryo-EM Structures of the TMEM16A Calcium-Activated Chloride Channel. Nature 2017, 552, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.M.; Rheinberger, J.; Paulino, C.; Dutzler, R. Gating the Pore of the Calcium-Activated Chloride Channel TMEM16A. Nat. Commun. 2021, 12, 785. [Google Scholar] [CrossRef]
- Paulino, C.; Kalienkova, V.; Lam, A.K.M.; Neldner, Y.; Dutzler, R. Activation Mechanism of the Calcium-Activated Chloride Channel TMEM16A Revealed by Cryo-EM. Nature 2017, 552, 421–425. [Google Scholar] [CrossRef]
- Paulino, C.; Neldner, Y.; Lam, A.K.; Kalienkova, V.; Brunner, J.D.; Schenck, S.; Dutzler, R. Structural Basis for Anion Conduction in the Calcium-Activated Chloride Channel TMEM16A. eLife 2017, 6, e26232. [Google Scholar] [CrossRef]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-Ray Structure of a Calcium-Activated TMEM16 Lipid Scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef]
- Falzone, M.E.; Rheinberger, J.; Lee, B.-C.; Peyear, T.; Sasset, L.; Raczkowski, A.M.; Eng, E.T.; Di Lorenzo, A.; Andersen, O.S.; Nimigean, C.M.; et al. Structural Basis of Ca2+-Dependent Activation and Lipid Transport by a TMEM16 Scramblase. eLife 2019, 8, e43229. [Google Scholar] [CrossRef]
- Kalienkova, V.; Clerico Mosina, V.; Bryner, L.; Oostergetel, G.T.; Dutzler, R.; Paulino, C. Stepwise Activation Mechanism of the Scramblase NhTMEM16 Revealed by Cryo-EM. eLife 2019, 8, e44364. [Google Scholar] [CrossRef] [PubMed]
- Khelashvili, G.; Falzone, M.E.; Cheng, X.; Lee, B.-C.; Accardi, A.; Weinstein, H. Dynamic Modulation of the Lipid Translocation Groove Generates a Conductive Ion Channel in Ca2+-Bound nhTMEM16. Nat. Commun. 2019, 10, 4972. [Google Scholar] [CrossRef] [PubMed]
- Alvadia, C.; Lim, N.K.; Clerico Mosina, V.; Oostergetel, G.T.; Dutzler, R.; Paulino, C. Cryo-EM Structures and Functional Characterization of the Murine Lipid Scramblase TMEM16F. eLife 2019, 8, e44365. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dang, S.; Han, T.W.; Ye, W.; Jin, P.; Cheng, T.; Li, J.; Jan, Y.N.; Jan, L.Y.; Cheng, Y. Cryo-EM Studies of TMEM16F Calcium-Activated Ion Channel Suggest Features Important for Lipid Scrambling. Cell Rep. 2019, 28, 567–579.e4. [Google Scholar] [CrossRef] [PubMed]
- Bethel, N.P.; Grabe, M. Atomistic Insight into Lipid Translocation by a TMEM16 Scramblase. Proc. Natl. Acad. Sci. USA 2016, 113, 14049–14054. [Google Scholar] [CrossRef]
- Stansfeld, P.J.; Goose, J.E.; Caffrey, M.; Carpenter, E.P.; Parker, J.L.; Newstead, S.; Sansom, M.S.P. MemProtMD: Automated Insertion of Membrane Protein Structures into Explicit Lipid Membranes. Structure 2015, 23, 1350–1361. [Google Scholar] [CrossRef]
- Jeng, G.; Aggarwal, M.; Yu, W.-P.; Chen, T.-Y. Independent Activation of Distinct Pores in Dimeric TMEM16A Channels. J. Gen. Physiol. 2016, 148, 393–404. [Google Scholar] [CrossRef]
- Lim, N.K.; Lam, A.K.M.; Dutzler, R. Independent Activation of Ion Conduction Pores in the Double-Barreled Calcium-Activated Chloride Channel TMEM16A. J. Gen. Physiol. 2016, 148, 375–392. [Google Scholar] [CrossRef]
- Gyobu, S.; Miyata, H.; Ikawa, M.; Yamazaki, D.; Takeshima, H.; Suzuki, J.; Nagata, S. A Role of TMEM16E Carrying a Scrambling Domain in Sperm Motility. Mol. Cell. Biol. 2016, 36, 645–659. [Google Scholar] [CrossRef]
- Gyobu, S.; Ishihara, K.; Suzuki, J.; Segawa, K.; Nagata, S. Characterization of the Scrambling Domain of the TMEM16 Family. Proc. Natl. Acad. Sci. USA 2017, 114, 6274–6279. [Google Scholar] [CrossRef]
- Peters, C.J.; Gilchrist, J.M.; Tien, J.; Bethel, N.P.; Qi, L.; Chen, T.; Wang, L.; Jan, Y.N.; Grabe, M.; Jan, L.Y. The Sixth Transmembrane Segment Is a Major Gating Component of the TMEM16A Calcium-Activated Chloride Channel. Neuron 2018, 97, 1063–1077.e4. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, R.L.; Pipatpolkai, T.; Agostinelli, E.; Russell, A.J.; Stansfeld, P.J.; Tammaro, P. An Outer-Pore Gate Modulates the Pharmacology of the TMEM16A Channel. Proc. Natl. Acad. Sci. USA 2021, 118, 2118. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Dutzler, R. Calcium-Dependent Electrostatic Control of Anion Access to the Pore of the Calcium-Activated Chloride Channel TMEM16A. eLife 2018, 7, e39122. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, S.; Ren, S.; Chen, Y.; Yuan, H.; Chai, R.; Yu, H.; Zhang, H.; Zhan, Y.; An, H. Two Ca2+-Binding Sites Cooperatively Couple Together in TMEM16A Channel. J. Membr. Biol. 2016, 249, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, J.; Tak, M.H.; Wee, J.; Lee, B.; Jang, Y.; Chun, H.; Yang, D.-J.; Yang, Y.D.; Park, S.H.; et al. Two Helices in the Third Intracellular Loop Determine Anoctamin 1 (TMEM16A) Activation by Calcium. Pflugers Arch. 2015, 467, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.-L.; Yuan, H.-B.; Cao, T.-G.; Su, J.-G.; Chen, Y.-F.; Liu, H.; Yu, H.; Zhang, H.-L.; Zhan, Y.; An, H.-L.; et al. Molecular Simulation Assisted Identification of Ca2+ Binding Residues in TMEM16A. J. Comput. Aided Mol. Des. 2015, 29, 1035–1043. [Google Scholar] [CrossRef]
- Tien, J.; Peters, C.J.; Wong, X.M.; Cheng, T.; Jan, Y.N.; Jan, L.Y.; Yang, H. A Comprehensive Search for Calcium Binding Sites Critical for TMEM16A Calcium-Activated Chloride Channel Activity. eLife 2014, 3, e02772. [Google Scholar] [CrossRef]
- Yang, H.; Kim, A.; David, T.; Palmer, D.; Jin, T.; Tien, J.; Huang, F.; Cheng, T.; Coughlin, S.R.; Jan, Y.N.; et al. TMEM16F Forms a Ca2+-Activated Cation Channel Required for Lipid Scrambling in Platelets during Blood Coagulation. Cell 2012, 151, 111–122. [Google Scholar] [CrossRef]
- Yu, K.; Duran, C.; Qu, Z.; Cui, Y.-Y.; Hartzell, H.C. Explaining Calcium-Dependent Gating of Anoctamin-1 Chloride Channels Requires a Revised Topology. Circ. Res. 2012, 110, 990–999. [Google Scholar] [CrossRef]
- Le, S.C.; Yang, H. An Additional Ca2+ Binding Site Allosterically Controls TMEM16A Activation. Cell Rep. 2020, 33, 108570. [Google Scholar] [CrossRef]
- Tak, M.H.; Jang, Y.; Son, W.S.; Yang, Y.D.; Oh, U. EF-Hand like Region in the N-Terminus of Anoctamin 1 Modulates Channel Activity by Ca2+ and Voltage. Exp. Neurobiol. 2019, 28, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.W.; Hwang, G.E.; Kim, W.K.; Nam, J.H. Ca2+ Sensitivity of Anoctamin 6/TMEM16F Is Regulated by the Putative Ca2+-Binding Reservoir at the N-Terminal Domain. Mol. Cells 2021, 44, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Cenedese, V.; Betto, G.; Celsi, F.; Cherian, O.L.; Pifferi, S.; Menini, A. The Voltage Dependence of the TMEM16B/Anoctamin2 Calcium-Activated Chloride Channel Is Modified by Mutations in the First Putative Intracellular Loop. J. Gen. Physiol. 2012, 139, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Cui, Y. Acidic Amino Acids in the First Intracellular Loop Contribute to Voltage- and Calcium- Dependent Gating of Anoctamin1/TMEM16A. PLoS ONE 2014, 9, e99376. [Google Scholar] [CrossRef]
- Lee, B.-C.; Khelashvili, G.; Falzone, M.; Menon, A.K.; Weinstein, H.; Accardi, A. Gating Mechanism of the Extracellular Entry to the Lipid Pathway in a TMEM16 Scramblase. Nat. Commun. 2018, 9, 3251. [Google Scholar] [CrossRef]
- Lam, A.K.M.; Dutzler, R. Mechanism of Pore Opening in the Calcium-Activated Chloride Channel TMEM16A. Nat. Commun. 2021, 12, 786. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, J. Specific PIP2 Binding Promotes Calcium Activation of TMEM16A Chloride Channels. Commun. Biol. 2021, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, K.; Hartzell, H.C.; Tajkhorshid, E. Lipids and Ions Traverse the Membrane by the Same Physical Pathway in the NhTMEM16 Scramblase. eLife 2017, 6, e28671. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Chalat, M.; Janjusevic, R.; Picollo, A.; Terashima, H.; Menon, A.K.; Accardi, A. Ca2+-Dependent Phospholipid Scrambling by a Reconstituted TMEM16 Ion Channel. Nat. Commun. 2013, 4, 2367. [Google Scholar] [CrossRef]
- Qu, Z.; Hartzell, H.C. Anion Permeation in Ca2+-Activated Cl− Channels. J. Gen. Physiol. 2000, 116, 825–844. [Google Scholar] [CrossRef]
- Ni, Y.-L.; Kuan, A.-S.; Chen, T.-Y. Activation and Inhibition of TMEM16A Calcium-Activated Chloride Channels. PLoS ONE 2014, 9, e86734. [Google Scholar] [CrossRef] [PubMed]
- Grubb, S.; Poulsen, K.A.; Juul, C.A.; Kyed, T.; Klausen, T.K.; Larsen, E.H.; Hoffmann, E.K. TMEM16F (Anoctamin 6), an Anion Channel of Delayed Ca2+ Activation. J. Gen. Physiol. 2013, 141, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.; Chen, L.S.; Yu, W.-P.; Chen, T.-Y. Comparison of Ion Transport Determinants between a TMEM16 Chloride Channel and Phospholipid Scramblase. J. Gen. Physiol. 2019, 151, 518–531. [Google Scholar] [CrossRef]
- Shimizu, T.; Iehara, T.; Sato, K.; Fujii, T.; Sakai, H.; Okada, Y. TMEM16F Is a Component of a Ca2+-Activated Cl− Channel but Not a Volume-Sensitive Outwardly Rectifying Cl− Channel. Am. J. Physiol. Cell Physiol. 2013, 304, C748–C759. [Google Scholar] [CrossRef] [PubMed]
- Stabilini, S.; Menini, A.; Pifferi, S. Anion and Cation Permeability of the Mouse TMEM16F Calcium-Activated Channel. Int. J. Mol. Sci. 2021, 22, 8578. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Yu, H.; Tien, J.; Jan, Y.N.; Li, M.; Jan, L.Y. Four Basic Residues Critical for the Ion Selectivity and Pore Blocker Sensitivity of TMEM16A Calcium-Activated Chloride Channels. Proc. Natl. Acad. Sci. USA 2015, 112, 3547–3552. [Google Scholar] [CrossRef] [PubMed]
- Aoun, J.; Hayashi, M.; Sheikh, I.A.; Sarkar, P.; Saha, T.; Ghosh, P.; Bhowmick, R.; Ghosh, D.; Chatterjee, T.; Chakrabarti, P.; et al. Anoctamin 6 Contributes to Cl− Secretion in Accessory Cholera Enterotoxin (Ace)-Stimulated Diarrhea: An Essential Role for Phosphatidylinositol 4,5-Bisphosphate (PIP2) Signaling in Cholera. J. Biol. Chem. 2016, 291, 26816–26836. [Google Scholar] [CrossRef]
- Henkel, B.; Drose, D.R.; Ackels, T.; Oberland, S.; Spehr, M.; Neuhaus, E.M. Co-Expression of Anoctamins in Cilia of Olfactory Sensory Neurons. Chem. Senses 2015, 40, 73–87. [Google Scholar] [CrossRef]
- Kim, H.J.; Jun, I.; Yoon, J.S.; Jung, J.; Kim, Y.K.; Kim, W.K.; Kim, B.J.; Song, J.; Kim, S.J.; Nam, J.H.; et al. Selective Serotonin Reuptake Inhibitors Facilitate ANO6 (TMEM16F) Current Activation and Phosphatidylserine Exposure. Pflugers Arch. 2015, 467, 2243–2256. [Google Scholar] [CrossRef]
- Muratori, C.; Pakhomov, A.G.; Gianulis, E.; Meads, J.; Casciola, M.; Mollica, P.A.; Pakhomova, O.N. Activation of the Phospholipid Scramblase TMEM16F by Nanosecond Pulsed Electric Fields (NsPEF) Facilitates Its Diverse Cytophysiological Effects. J. Biol. Chem. 2017, 292, 19381–19391. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Kmit, A.; Romao, A.M.; Jantarajit, W.; Schreiber, R.; Kunzelmann, K. Anoctamin 6 Mediates Effects Essential for Innate Immunity Downstream of P2X 7 Receptors in Macrophages. Nat. Commun. 2015, 6, 6245. [Google Scholar] [CrossRef] [PubMed]
- Simões, F.; Ousingsawat, J.; Wanitchakool, P.; Fonseca, A.; Cabrita, I.; Benedetto, R.; Schreiber, R.; Kunzelmann, K. CFTR Supports Cell Death through ROS-Dependent Activation of TMEM16F (Anoctamin 6). Pflüg. Arch. Eur. J. Physiol. 2018, 470, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Mahaut-Smith, M.P. A Major Interspecies Difference in the Ionic Selectivity of Megakaryocyte Ca2+-Activated Channels Sensitive to the TMEM16F Inhibitor CaCCinh-A01. Platelets 2019, 30, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Bricogne, C.; Fine, M.; Pereira, P.M.; Sung, J.; Tijani, M.; Wang, Y.; Henriques, R.; Collins, M.K.; Hilgemann, D.W. TMEM16F Activation by Ca2+ Triggers Plasma Membrane Expansion and Directs PD-1 Trafficking. Sci. Rep. 2019, 9, 619. [Google Scholar] [CrossRef]
- Pomorski, T.; Menon, A.K. Lipid Flippases and Their Biological Functions. Cell. Mol. Life Sci. CMLS 2006, 63, 2908–2921. [Google Scholar] [CrossRef]
- Falzone, M.E.; Malvezzi, M.; Lee, B.-C.; Accardi, A. Known Structures and Unknown Mechanisms of TMEM16 Scramblases and Channels. J. Gen. Physiol. 2018, 150, 933–947. [Google Scholar] [CrossRef]
- Kalienkova, V.; Clerico Mosina, V.; Paulino, C. The Groovy TMEM16 Family: Molecular Mechanisms of Lipid Scrambling and Ion Conduction. J. Mol. Biol. 2021, 433, 166941. [Google Scholar] [CrossRef]
- Whitlock, J.M.; Hartzell, H.C. A Pore Idea: The Ion Conduction Pathway of TMEM16/ANO Proteins Is Composed Partly of Lipid. Pflüg. Arch. Eur. J. Physiol. 2016, 468, 455–473. [Google Scholar] [CrossRef]
- Kostritskii, A.Y.; Machtens, J.-P. Molecular Mechanisms of Ion Conduction and Ion Selectivity in TMEM16 Lipid Scramblases. Nat. Commun. 2021, 12, 2826. [Google Scholar] [CrossRef]
- Hartzell, C.; Putzier, I.; Arreola, J. Calcium-Activated Chloride Channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Angermann, J.E.; Sanguinetti, A.R.; Kenyon, J.L.; Leblanc, N.; Greenwood, I.A. Mechanism of the Inhibition of Ca2+-Activated Cl− Currents by Phosphorylation in Pulmonary Arterial Smooth Muscle Cells. J. Gen. Physiol. 2006, 128, 73–87. [Google Scholar] [CrossRef]
- Arreola, J.; Melvin, J.E.; Begenisich, T. Activation of Calcium-Dependent Chloride Channels in Rat Parotid Acinar Cells. J. Gen. Physiol. 1996, 108, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kuruma, A.; Hartzell, H.C. Bimodal Control of a Ca2+-Activated Cl− Channel by Different Ca2+ Signals. J. Gen. Physiol. 2000, 115, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Han, T.W.; Nassar, L.M.; Zubia, M.; Jan, Y.N.; Jan, L.Y. Phosphatidylinositol-(4,5)-Bisphosphate Regulates Calcium Gating of Small-Conductance Cation Channel TMEM16F. Proc. Natl. Acad. Sci. USA 2018, 115, E1667–E1674. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Vite, J.A.; Cruz-Rangel, S.; De Jesús-Pérez, J.J.; Figueroa, I.A.A.; Rodríguez-Menchaca, A.A.; Pérez-Cornejo, P.; Hartzell, H.C.; Arreola, J. Revealing the Activation Pathway for TMEM16A Chloride Channels from Macroscopic Currents and Kinetic Models. Pflüg. Arch. Eur. J. Physiol. 2016, 468, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- De Jesús-Pérez, J.J.; Cruz-Rangel, S.; Espino-Saldaña, Á.E.; Martínez-Torres, A.; Qu, Z.; Hartzell, H.C.; Corral-Fernandez, N.E.; Pérez-Cornejo, P.; Arreola, J. Phosphatidylinositol 4,5-Bisphosphate, Cholesterol, and Fatty Acids Modulate the Calcium-Activated Chloride Channel TMEM16A (ANO1). Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2018, 1863, 299–312. [Google Scholar] [CrossRef]
- Le, S.C.; Jia, Z.; Chen, J.; Yang, H. Molecular Basis of PIP2-Dependent Regulation of the Ca2+-Activated Chloride Channel TMEM16A. Nat. Commun. 2019, 10, 3769. [Google Scholar] [CrossRef]
- Tembo, M.; Carlson, A.E. PIP2 and Ca2+ Are Both Required to Open TMEM16a Channels in Xenopus Laevis Oocytes. Biophys. J. 2018, 114, 610a. [Google Scholar] [CrossRef][Green Version]
- Lin, H.; Roh, J.; Woo, J.H.; Kim, S.J.; Nam, J.H. TMEM16F/ANO6, a Ca2+-Activated Anion Channel, Is Negatively Regulated by the Actin Cytoskeleton and Intracellular MgATP. Biochem. Biophys. Res. Commun. 2018, 503, 2348–2354. [Google Scholar] [CrossRef]
- Ye, W.; Han, T.W.; He, M.; Jan, Y.N.; Jan, L.Y. Dynamic Change of Electrostatic Field in TMEM16F Permeation Pathway Shifts Its Ion Selectivity. eLife 2019, 8, e45187. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, C.; Chen, Y.; Jia, M.; Geng, J.; Zhang, H.; Zhan, Y.; Boland, L.M.; An, H. Divalent Cations Modulate TMEM16A Calcium-Activated Chloride Channels by a Common Mechanism. J. Membr. Biol. 2013, 246, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.; Kwon, H.C.; Chen, T.-Y. Divalent Cation Modulation of Ion Permeation in TMEM16 Proteins. Int. J. Mol. Sci. 2021, 22, 2209. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.M.; Chen, L.S.; Jeng, G.; Yu, W.-P.; Chen, T.-Y. Cobalt Ion Interaction with TMEM16A Calcium-Activated Chloride Channel: Inhibition and Potentiation. PLoS ONE 2020, 15, e0231812. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhu, J.; Qu, Z.; Cui, Y.-Y.; Hartzell, H.C. Activation of the Ano1 (TMEM16A) Chloride Channel by Calcium Is Not Mediated by Calmodulin. J. Gen. Physiol. 2014, 143, 253–267. [Google Scholar] [CrossRef]
- Vocke, K.; Dauner, K.; Hahn, A.; Ulbrich, A.; Broecker, J.; Keller, S.; Frings, S.; Möhrlen, F. Calmodulin-Dependent Activation and Inactivation of Anoctamin Calcium-Gated Chloride Channels. J. Gen. Physiol. 2013, 142, 381–404. [Google Scholar] [CrossRef]
- Perez-Cornejo, P.; Gokhale, A.; Duran, C.; Cui, Y.; Xiao, Q.; Hartzell, H.C.; Faundez, V. Anoctamin 1 (Tmem16A) Ca2+-Activated Chloride Channel Stoichiometrically Interacts with an Ezrin–Radixin–Moesin Network. Proc. Natl. Acad. Sci. USA 2012, 109, 10376–10381. [Google Scholar] [CrossRef]
- Terashima, H.; Picollo, A.; Accardi, A. Purified TMEM16A Is Sufficient to Form Ca2+-Activated Cl− Channels. Proc. Natl. Acad. Sci. USA 2013, 110, 19354–19359. [Google Scholar] [CrossRef]
- Chao, S.H.; Suzuki, Y.; Zysk, J.R.; Cheung, W.Y. Activation of Calmodulin by Various Metal Cations as a Function of Ionic Radius. Mol. Pharmacol. 1984, 26, 75–82. [Google Scholar]
- Tian, Y.; Kongsuphol, P.; Hug, M.; Ousingsawat, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. Calmodulin-Dependent Activation of the Epithelial Calcium-Dependent Chloride Channel TMEM16A. FASEB J. 2011, 25, 1058–1068. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Zheng, L.-Y.; Zhang, Z.; Tang, Y.-B.; Wang, G.-L.; Du, Y.-H.; Lv, X.-F.; Liu, J.; Zhou, J.-G.; et al. Downregulation of TMEM16A Calcium-Activated Chloride Channel Contributes to Cerebrovascular Remodeling during Hypertension by Promoting Basilar Smooth Muscle Cell Proliferation. Circulation 2012, 125, 697–707. [Google Scholar] [CrossRef]
- Jung, J.; Nam, J.H.; Park, H.W.; Oh, U.; Yoon, J.-H.; Lee, M.G. Dynamic Modulation of ANO1/TMEM16A HCO3− Permeability by Ca2+/Calmodulin. Proc. Natl. Acad. Sci. USA 2012, 110, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, I.A.; Ledoux, J.; Leblanc, N. Differential Regulation of Ca2+-Activated Cl− Currents in Rabbit Arterial and Portal Vein Smooth Muscle Cells by Ca2+-Calmodulin-Dependent Kinase. J. Physiol. 2001, 534, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-X.; Lv, X.-F.; Yuan, F.; Li, X.-Y.; Ma, M.-M.; Liu, C.-Z.; Zhou, J.-G.; Wang, G.-L.; Guan, Y.-Y. Ca2+/Calmodulin-Dependent Protein Kinase II γ-Dependent Serine727 Phosphorylation Is Required for TMEM16A Ca2+-Activated Cl− Channel Regulation in Cerebrovascular Cells. Circ. J. Off. J. Jpn. Circ. Soc. 2018, 82, 903–913. [Google Scholar] [CrossRef]
- Wang, Y.X.; Kotlikoff, M.I. Inactivation of Calcium-Activated Chloride Channels in Smooth Muscle by Calcium/Calmodulin-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 14918–14923. [Google Scholar] [CrossRef]
- Ayon, R.J.; Hawn, M.B.; Aoun, J.; Wiwchar, M.; Forrest, A.S.; Cunningham, F.; Singer, C.A.; Valencik, M.L.; Greenwood, I.A.; Leblanc, N. Molecular Mechanism of TMEM16A Regulation: Role of CaMKII and PP1/PP2A. Am. J. Physiol.-Cell Physiol. 2019, 59, 418. [Google Scholar] [CrossRef]
- Wiwchar, M.; Ayon, R.; Greenwood, I.A.; Leblanc, N. Phosphorylation Alters the Pharmacology of Ca2+-Activated Cl− Channels in Rabbit Pulmonary Arterial Smooth Muscle Cells. Br. J. Pharmacol. 2009, 158, 1356–1365. [Google Scholar] [CrossRef]
- Sim, K.M.; Lee, Y.-S.; Kim, H.J.; Cho, C.-H.; Yi, G.-S.; Park, M.-J.; Hwang, E.M.; Park, J.-Y. Suppression of CaMKIIβ Inhibits ANO1-Mediated Glioblastoma Progression. Cells 2020, 9, 1079. [Google Scholar] [CrossRef]
- Chun, H.; Cho, H.; Choi, J.; Lee, J.; Kim, S.M.; Kim, H.; Oh, U. Protons Inhibit Anoctamin 1 by Competing with Calcium. Cell Calcium 2015, 58, 431–441. [Google Scholar] [CrossRef]
- Liang, P.; Yang, H. Molecular Underpinning of Intracellular PH Regulation on TMEM16F. J. Gen. Physiol. 2021, 153, 2704. [Google Scholar] [CrossRef]
- Han, Y.; Shewan, A.M.; Thorn, P. HCO3− Transport through Anoctamin/Transmembrane Protein ANO1/TMEM16A in Pancreatic Acinar Cells Regulates Luminal PH. J. Biol. Chem. 2016, 291, 20345–20352. [Google Scholar] [CrossRef]
- Lee, M.G.; Ohana, E.; Park, H.W.; Yang, D.; Muallem, S. Molecular Mechanism of Pancreatic and Salivary Gland Fluid and HCO3− Secretion. Physiol. Rev. 2012, 92, 39–74. [Google Scholar] [CrossRef] [PubMed]
- Segura-Covarrubias, G.; Aréchiga-Figueroa, I.A.; De Jesús-Pérez, J.J.; Sánchez-Solano, A.; Pérez-Cornejo, P.; Arreola, J. Voltage-Dependent Protonation of the Calcium Pocket Enable Activation of the Calcium-Activated Chloride Channel Anoctamin-1 (TMEM16A). Sci. Rep. 2020, 10, 6644. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated PH: A Perfect Storm for Cancer Progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Wanitchakool, P.; Wolf, L.; Koehl, G.E.; Sirianant, L.; Schreiber, R.; Kulkarni, S.; Duvvuri, U.; Kunzelmann, K. Role of Anoctamins in Cancer and Apoptosis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130096. [Google Scholar] [CrossRef]
- Cruz-Rangel, S.; Jesús-Pérez, J.J.D.; Aréchiga-Figueroa, I.A.; Rodríguez-Menchaca, A.A.; Pérez-Cornejo, P.; Hartzell, H.C.; Arreola, J. Extracellular Protons Enable Activation of the Calcium-Dependent Chloride Channel TMEM16A. J. Physiol. 2017, 595, 1515–1531. [Google Scholar] [CrossRef]
- Chiche, J.; Brahimi-Horn, M.C.; Pouysségur, J. Tumour Hypoxia Induces a Metabolic Shift Causing Acidosis: A Common Feature in Cancer. J. Cell. Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef]
- Chesler, M. Regulation and Modulation of PH in the Brain. Physiol. Rev. 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Mangel, S.C. Circadian Clock Regulation of PH in the Rabbit Retina. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 2897–2902. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Mangel, S.C. Retinal PH Reflects Retinal Energy Metabolism in the Day and Night. J. Neurophysiol. 2004, 91, 2404–2412. [Google Scholar] [CrossRef]
- Newman, E.A. Acid Efflux from Retinal Glial Cells Generated by Sodium Bicarbonate Cotransport. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 159–168. [Google Scholar] [CrossRef]
- Chatton, J.Y.; Spring, K.R. Acidic PH of the Lateral Intercellular Spaces of MDCK Cells Cultured on Permeable Supports. J. Membr. Biol. 1994, 140, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Montrose, M.H. Extracellular PH Regulation in Microdomains of Colonic Crypts: Effects of Short-Chain Fatty Acids. Proc. Natl. Acad. Sci. USA 1995, 92, 3303–3307. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Tanaka, S.; Kaunitz, J.D.; Montrose, M.H. Dynamic Regulation of Gastric Surface PH by Luminal PH. J. Clin. Investig. 1999, 103, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.; Chatton, J.Y.; Tran, P.H.; Bungay, P.M.; Spring, K.R. PH, Morphology, and Diffusion in Lateral Intercellular Spaces of Epithelial Cell Monolayers. Am. J. Physiol. 1994, 266, C73–C80. [Google Scholar] [CrossRef] [PubMed]
- Maouyo, D.; Chu, S.; Montrose, M.H. PH Heterogeneity at Intracellular and Extracellular Plasma Membrane Sites in HT29-C1 Cell Monolayers. Am. J. Physiol. Cell Physiol. 2000, 278, C973–C981. [Google Scholar] [CrossRef] [PubMed]
- Rechkemmer, G.; Wahl, M.; Kuschinsky, W.; von Engelhardt, W. PH-Microclimate at the Luminal Surface of the Intestinal Mucosa of Guinea Pig and Rat. Pflugers Arch. 1986, 407, 33–40. [Google Scholar] [CrossRef]
- Sandoval, M.; Burgos, J.; Sepúlveda, F.V.; Cid, L.P. Extracellular PH in Restricted Domains as a Gating Signal for Ion Channels Involved in Transepithelial Transport. Biol. Pharm. Bull. 2011, 34, 803–809. [Google Scholar] [CrossRef]
- Sondo, E.; Caci, E.; Galietta, L.J.V. The TMEM16A Chloride Channel as an Alternative Therapeutic Target in Cystic Fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 73–76. [Google Scholar] [CrossRef]
- Martins, J.R.; Faria, D.; Kongsuphol, P.; Reisch, B.; Schreiber, R.; Kunzelmann, K. Anoctamin 6 Is an Essential Component of the Outwardly Rectifying Chloride Channel. Proc. Natl. Acad. Sci. USA 2011, 108, 18168–18172. [Google Scholar] [CrossRef]
- Benedetto, R.; Ousingsawat, J.; Wanitchakool, P.; Zhang, Y.; Holtzman, M.J.; Amaral, M.; Rock, J.R.; Schreiber, R.; Kunzelmann, K. Epithelial Chloride Transport by CFTR Requires TMEM16A. Sci. Rep. 2017, 7, 12397. [Google Scholar] [CrossRef]
- Genovese, M.; Borrelli, A.; Venturini, A.; Guidone, D.; Caci, E.; Viscido, G.; Gambardella, G.; Bernardo, D.D.; Scudieri, P.; Galietta, L.J.V. TRPV4 and Purinergic Receptor Signalling Pathways Are Separately Linked in Airway Epithelia to CFTR and TMEM16A Chloride Channels. J. Physiol. 2019, 597, 5859–5878. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Faulhaber, J.; Srisawang, L.; Stortz, A.; Salomon, J.J.; Mall, M.A.; Frings, S.; Möhrlen, F. Cellular Distribution and Function of Ion Channels Involved in Transport Processes in Rat Tracheal Epithelium. Physiol. Rep. 2017, 5, e13290. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X.; et al. Calcium-Activated Chloride Channel TMEM16A Modulates Mucin Secretion and Airway Smooth Muscle Contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Tsuji, M.; Hara, K.; Arimura, K.; Yagi, O.; Tagaya, E.; Takeyama, K.; Tamaoki, J. Chloride Ion Transport and Overexpression of TMEM16A in a Guinea-Pig Asthma Model. Clin. Exp. Allergy 2017, 47, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Centeio, R.; Cabrita, I.; Benedetto, R.; Talbi, K.; Ousingsawat, J.; Schreiber, R.; Sullivan, J.K.; Kunzelmann, K. Pharmacological Inhibition and Activation of the Ca2+ Activated Cl− Channel TMEM16A. Int. J. Mol. Sci. 2020, 21, 2557. [Google Scholar] [CrossRef] [PubMed]
- Guarascio, D.M.; Gonzalez-Velandia, K.Y.; Hernandez-Clavijo, A.; Menini, A.; Pifferi, S. Functional Expression of TMEM16A in Taste Bud Cells. J. Physiol. 2021, 599, 3697–3714. [Google Scholar] [CrossRef] [PubMed]
- Henriques, T.; Agostinelli, E.; Hernandez-Clavijo, A.; Maurya, D.K.; Rock, J.R.; Harfe, B.D.; Menini, A.; Pifferi, S. TMEM16A Calcium-Activated Chloride Currents in Supporting Cells of the Mouse Olfactory Epithelium. J. Gen. Physiol. 2019, 151, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Suh, B.-C. Differential Regulation of Ca2+-Activated Cl− Channel TMEM16A Splice Variants by Membrane PI(4,5)P2. Int. J. Mol. Sci. 2021, 22, 4088. [Google Scholar] [CrossRef]
- Tembo, M.; Wozniak, K.L.; Bainbridge, R.E.; Carlson, A.E. Phosphatidylinositol 4,5-Bisphosphate (PIP2) and Ca2+ Are Both Required to Open the Cl− Channel TMEM16A. J. Biol. Chem. 2019, 294, 12556–12564. [Google Scholar] [CrossRef]
- Kloner, R.A.; King, K.S.; Harrington, M.G. No-Reflow Phenomenon in the Heart and Brain. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H550–H562. [Google Scholar] [CrossRef]
- Bulley, S.; Jaggar, J.H. Cl− Channels in Smooth Muscle Cells. Pflugers Arch. 2014, 466, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-M.; Lou, J.; Song, B.-L.; Gong, Y.-F.; Li, Y.-C.; Yu, C.-J.; Wang, Q.-S.; Ma, T.-X.; Ma, K.; Hartzell, H.C.; et al. Hypoxia Augments the Calcium-Activated Chloride Current Carried by Anoctamin-1 in Cardiac Vascular Endothelial Cells of Neonatal Mice. Br. J. Pharmacol. 2014, 171, 3680–3692. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xia, Y.; Paudel, O.; Yang, X.-R.; Sham, J.S.K. Chronic Hypoxia-Induced Upregulation of Ca2+-Activated Cl− Channel in Pulmonary Arterial Myocytes: A Mechanism Contributing to Enhanced Vasoreactivity. J. Physiol. 2012, 590, 3507–3521. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Nagaraj, C.; Zabini, D.; Nagy, B.M.; Lengyel, M.; Skofic Maurer, D.; Sharma, N.; Egemnazarov, B.; Kovacs, G.; Kwapiszewska, G.; et al. Targeting TMEM16A to Reverse Vasoconstriction and Remodelling in Idiopathic Pulmonary Arterial Hypertension. Eur. Respir. J. 2019, 53, 1800965. [Google Scholar] [CrossRef] [PubMed]
- Blount, A.; Zhang, S.; Chestnut, M.; Hixon, B.; Skinner, D.; Sorscher, E.J.; Woodworth, B.A. Transepithelial Ion Transport Is Suppressed in Hypoxic Sinonasal Epithelium. The Laryngoscope 2011, 121, 1929–1934. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Contribution of TMEM16F to Pyroptotic Cell Death. Cell Death Dis. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Schreiber, R.; Buchholz, B.; Kraus, A.; Schley, G.; Scholz, J.; Ousingsawat, J.; Kunzelmann, K. Lipid Peroxidation Drives Renal Cyst Growth In Vitro through Activation of TMEM16A. J. Am. Soc. Nephrol. 2019, 30, 228–242. [Google Scholar] [CrossRef]
- Schreiber, R.; Ousingsawat, J.; Wanitchakool, P.; Sirianant, L.; Benedetto, R.; Reiss, K.; Kunzelmann, K. Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 Ion Currents and Phospholipid Scrambling by Ca2+ and Plasma Membrane Lipid. J. Physiol. 2018, 596, 217–229. [Google Scholar] [CrossRef]
- Cho, H.; Oh, U. Anoctamin 1 Mediates Thermal Pain as a Heat Sensor. Curr. Neuropharmacol. 2013, 11, 641–651. [Google Scholar] [CrossRef]
- Cho, H.; Yang, Y.D.; Lee, J.; Lee, B.; Kim, T.; Jang, Y.; Back, S.K.; Na, H.S.; Harfe, B.D.; Wang, F.; et al. The Calcium-Activated Chloride Channel Anoctamin 1 Acts as a Heat Sensor in Nociceptive Neurons. Nat. Neurosci. 2012, 15, 1015–1021. [Google Scholar] [CrossRef]
- Lin, H.; Jun, I.; Woo, J.H.; Lee, M.G.; Kim, S.J.; Nam, J.H. Temperature-Dependent Increase in the Calcium Sensitivity and Acceleration of Activation of ANO6 Chloride Channel Variants. Sci. Rep. 2019, 9, 6706. [Google Scholar] [CrossRef] [PubMed]
- Brauchi, S.; Orio, P.; Latorre, R. Clues to Understanding Cold Sensation: Thermodynamics and Electrophysiological Analysis of the Cold Receptor TRPM8. Proc. Natl. Acad. Sci. USA 2004, 101, 15494–15499. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Brauchi, S.; Orta, G.; Zaelzer, C.; Vargas, G. ThermoTRP Channels as Modular Proteins with Allosteric Gating. Cell Calcium 2007, 42, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Voets, T. Quantifying and modeling the temperature-dependent gating of TRP channels. In Reviews of Physiology, Biochemistry and Pharmacology; Nilius, B., Amara, S.G., Gudermann, T., Jahn, R., Lill, R., Offermanns, S., Petersen, O.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 162, pp. 91–119. ISBN 978-3-642-29256-9. [Google Scholar]
- Clapham, D.E.; Miller, C. A Thermodynamic Framework for Understanding Temperature Sensing by Transient Receptor Potential (TRP) Channels. Proc. Natl. Acad. Sci. USA 2011, 108, 19492–19497. [Google Scholar] [CrossRef]
- Lee, B.; Cho, H.; Jung, J.; Yang, Y.D.; Yang, D.-J.; Oh, U. Anoctamin 1 Contributes to Inflammatory and Nerve-Injury Induced Hypersensitivity. Mol. Pain 2014, 10, 5. [Google Scholar] [CrossRef]
- Liu, B.; Linley, J.E.; Du, X.; Zhang, X.; Ooi, L.; Zhang, H.; Gamper, N. The Acute Nociceptive Signals Induced by Bradykinin in Rat Sensory Neurons Are Mediated by Inhibition of M-Type K+ Channels and Activation of Ca2+-Activated Cl– Channels. J. Clin. Investig. 2010, 120, 1240–1252. [Google Scholar] [CrossRef]
- Jang, W.; Kim, J.Y.; Cui, S.; Jo, J.; Lee, B.-C.; Lee, Y.; Kwon, K.-S.; Park, C.-S.; Kim, C. The Anoctamin Family Channel Subdued Mediates Thermal Nociception in Drosophila. J. Biol. Chem. 2015, 290, 2521–2528. [Google Scholar] [CrossRef]
- Watanabe, R.; Sakuragi, T.; Noji, H.; Nagata, S. Single-Molecule Analysis of Phospholipid Scrambling by TMEM16F. Proc. Natl. Acad. Sci. USA 2018, 115, 3066–3071. [Google Scholar] [CrossRef]
- Pritchard, H.A.T.; Leblanc, N.; Albert, A.P.; Greenwood, I.A. Inhibitory Role of Phosphatidylinositol 4,5-Bisphosphate on TMEM16A-Encoded Calcium-Activated Chloride Channels in Rat Pulmonary Artery. Br. J. Pharmacol. 2014, 171, 4311–4321. [Google Scholar] [CrossRef]
- Ta, C.M.; Acheson, K.E.; Rorsman, N.J.G.; Jongkind, R.C.; Tammaro, P. Contrasting Effects of Phosphatidylinositol 4,5-Bisphosphate on Cloned TMEM16A and TMEM16B Channels. Br. J. Pharmacol. 2017, 174, 2984–2999. [Google Scholar] [CrossRef]
- Yu, K.; Jiang, T.; Cui, Y.; Tajkhorshid, E.; Hartzell, H.C. A Network of Phosphatidylinositol 4,5-Bisphosphate Binding Sites Regulates Gating of the Ca2+-Activated Cl− Channel ANO1 (TMEM16A). Proc. Natl. Acad. Sci. USA 2019, 116, 19952–19962. [Google Scholar] [CrossRef] [PubMed]
- Arreola, J.; Hartzell, H.C. Wasted TMEM16A Channels Are Rescued by Phosphatidylinositol 4,5-Bisphosphate. Cell Calcium 2019, 84, 102103. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Menon, A.K.; Accardi, A. The nhTMEM16 Scramblase Is Also a Nonselective Ion Channel. Biophys. J. 2016, 111, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Sones, W.R.; Davis, A.J.; Leblanc, N.; Greenwood, I.A. Cholesterol Depletion Alters Amplitude and Pharmacology of Vascular Calcium-Activated Chloride Channels. Cardiovasc. Res. 2010, 87, 476–484. [Google Scholar] [CrossRef]
- Elinder, F.; Liin, S. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Li, R.; Zhang, A.; Niu, Z.; Cai, Z.; Duan, W.; Li, X.; Zhang, H. Increased TMEM16A Involved in Alveolar Fluid Clearance After Lipopolysaccharide Stimulation. Inflammation 2016, 39, 881–890. [Google Scholar] [CrossRef]
- Zhang, A.; Yan, X.; Li, H.; Gu, Z.; Zhang, P.; Zhang, H.; Li, Y.; Yu, H. TMEM16A Protein Attenuates Lipopolysaccharide-Mediated Inflammatory Response of Human Lung Epithelial Cell Line A549. Exp. Lung Res. 2014, 40, 237–250. [Google Scholar] [CrossRef]
- Sui, J.; Zhang, C.; Fang, X.; Wang, J.; Li, Y.; Wang, J.; Wang, L.; Dong, J.; Zhou, Z.; Li, C.; et al. Dual Role of Ca2+-Activated Cl− Channel Transmembrane Member 16A in Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction in Vitro. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.-L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between Plasma Levels of Lipopolysaccharide (LPS) and LPS-Binding Protein in Patients with Severe Sepsis and Septic Shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Sirianant, L.; Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Kunzelmann, K. Cellular Volume Regulation by Anoctamin 6: Ca2+, Phospholipase A2 and Osmosensing. Pflüg. Arch. Eur. J. Physiol. 2016, 468, 335–349. [Google Scholar] [CrossRef]
- Yoo, J.; Cui, Q. Curvature Generation and Pressure Profile Modulation in Membrane by Lysolipids: Insights from Coarse-Grained Simulations. Biophys. J. 2009, 97, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.L.; Desiderio, D.M.; Miller, D.D.; Tolley, B.; Tigyi, G.J. Direct Quantitative Analysis of Lysophosphatidic Acid Molecular Species by Stable Isotope Dilution Electrospray Ionization Liquid Chromatography–Mass Spectrometry. Anal. Biochem. 2001, 292, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Baker, D.; Virag, T.; Wada, A.; Yatomi, Y.; Kobayashi, T.; Igarashi, Y.; Tigyi, G. Multiple Mechanisms Linked to Platelet Activation Result in Lysophosphatidic Acid and Sphingosine 1-Phosphate Generation in Blood. J. Biol. Chem. 2002, 277, 21197–21206. [Google Scholar] [CrossRef]
- Bolen, A.L.; Naren, A.P.; Yarlagadda, S.; Beranova-Giorgianni, S.; Chen, L.; Norman, D.; Baker, D.L.; Rowland, M.M.; Best, M.D.; Sano, T.; et al. The Phospholipase A1 Activity of Lysophospholipase A-I Links Platelet Activation to LPA Production during Blood Coagulation. J. Lipid Res. 2011, 52, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Eichholtz, T.; Jalink, K.; Fahrenfort, I.; Moolenaar, W.H. The Bioactive Phospholipid Lysophosphatidic Acid Is Released from Activated Platelets. Biochem. J. 1993, 291, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Andrews, D.A.; Low, P.S. Lysophosphatidic Acid Opens a Ca2+ Channel in Human Erythrocytes. Blood 2000, 95, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.A.; Haining, E.J.; Geuss, E.; Beck, S.; Swieringa, F.; Wanitchakool, P.; Schuhmann, M.K.; Stegner, D.; Kunzelmann, K.; Kleinschnitz, C.; et al. TMEM16F-Mediated Platelet Membrane Phospholipid Scrambling Is Critical for Hemostasis and Thrombosis but Not Thromboinflammation in Mice—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2152–2157. [Google Scholar] [CrossRef]
- Chung, S.-M.; Bae, O.-N.; Lim, K.-M.; Noh, J.-Y.; Lee, M.-Y.; Jung, Y.-S.; Chung, J.-H. Lysophosphatidic Acid Induces Thrombogenic Activity Through Phosphatidylserine Exposure and Procoagulant Microvesicle Generation in Human Erythrocytes. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 414–421. [Google Scholar] [CrossRef]
- Liu, G.; Liu, G.; Chen, H.; Borst, O.; Gawaz, M.; Vortkamp, A.; Schreiber, R.; Kunzelmann, K.; Lang, F. Involvement of Ca2+ Activated Cl− Channel Ano6 in Platelet Activation and Apoptosis. Cell. Physiol. Biochem. 2015, 37, 1934–1944. [Google Scholar] [CrossRef]
- Öhlinger, T.; Müllner, E.W.; Fritz, M.; Sauer, T.; Werning, M.; Baron, D.M.; Salzer, U. Lysophosphatidic Acid-Induced pro-Thrombotic Phosphatidylserine Exposure and Ionophore-Induced Microvesiculation Is Mediated by the Scramblase TMEM16F in Erythrocytes. Blood Cells. Mol. Dis. 2020, 83, 102426. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, J.; Nagata, S. Functional Swapping between Transmembrane Proteins TMEM16A and TMEM16F. J. Biol. Chem. 2014, 289, 7438–7447. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Le, S.C.; Zhang, Y.; Liang, P.; Yang, H. Evidence That Polyphenols Do Not Inhibit the Phospholipid Scramblase TMEM16F. J. Biol. Chem. 2020, 295, 12537–12544. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dutta, A.; Kresge, C.; Bugde, A.; Feranchak, A.P. Bile Acids Stimulate Cholangiocyte Fluid Secretion by Activation of Transmembrane Member 16A Cl− Channels. Hepatology 2018, 68, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.A.; Awayda, M.S.; Bubien, J.K.; Ismailov, I.I.; Arrate, M.P.; Berdiev, B.K.; Benos, D.J.; Fuller, C.M. Cloning of an Epithelial Chloride Channel from Bovine Trachea. J. Biol. Chem. 1996, 271, 7244. [Google Scholar] [CrossRef]
- Yurtsever, Z.; Sala-Rabanal, M.; Randolph, D.T.; Scheaffer, S.M.; Roswit, W.T.; Alevy, Y.G.; Patel, A.C.; Heier, R.F.; Romero, A.G.; Nichols, C.G.; et al. Self-Cleavage of Human CLCA1 Protein by a Novel Internal Metalloprotease Domain Controls Calcium-Activated Chloride Channel Activation. J. Biol. Chem. 2012, 287, 42138–42149. [Google Scholar] [CrossRef]
- Patel, A.C.; Brett, T.J.; Holtzman, M.J. The Role of CLCA Proteins in Inflammatory Airway Disease. Annu. Rev. Physiol. 2009, 71, 425–449. [Google Scholar] [CrossRef]
- Alevy, Y.G.; Patel, A.C.; Romero, A.G.; Patel, D.A.; Tucker, J.; Roswit, W.T.; Miller, C.A.; Heier, R.F.; Byers, D.E.; Brett, T.J.; et al. IL-13–Induced Airway Mucus Production Is Attenuated by MAPK13 Inhibition. J. Clin. Investig. 2012, 122, 4555–4568. [Google Scholar] [CrossRef]
- Britton, F.C.; Ohya, S.; Horowitz, B.; Greenwood, I.A. Comparison of the Properties of CLCA1 Generated Currents and ICl(Ca) in Murine Portal Vein Smooth Muscle Cells. J. Physiol. 2002, 539, 107–117. [Google Scholar] [CrossRef]
- Elble, R.C.; Ji, G.; Nehrke, K.; DeBiasio, J.; Kingsley, P.D.; Kotlikoff, M.I.; Pauli, B.U. Molecular and Functional Characterization of a Murine Calcium-Activated Chloride Channel Expressed in Smooth Muscle. J. Biol. Chem. 2002, 277, 18586–18591. [Google Scholar] [CrossRef]
- Gandhi, R.; Elble, R.C.; Gruber, A.D.; Schreur, K.D.; Ji, H.-L.; Fuller, C.M.; Pauli, B.U. Molecular and Functional Characterization of a Calcium-Sensitive Chloride Channel from Mouse Lung. J. Biol. Chem. 1998, 273, 32096–32101. [Google Scholar] [CrossRef]
- Greenwood, I.A.; Miller, L.J.; Ohya, S.; Horowitz, B. The Large Conductance Potassium Channel β-Subunit Can Interact with and Modulate the Functional Properties of a Calcium-Activated Chloride Channel, CLCA1. J. Biol. Chem. 2002, 277, 22119–22122. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Yurtsever, Z.; Nichols, C.G.; Brett, T.J. Secreted CLCA1 Modulates TMEM16A to Activate Ca2+-Dependent Chloride Currents in Human Cells. eLife 2015, 4, e05875. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ramena, G.; Yin, Y.; Premkumar, L.; Elble, R.C. CLCA2 Is a Positive Regulator of Store-Operated Calcium Entry and TMEM16A. PLoS ONE 2018, 13, e0196512. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Koyama, R.; Maruyama, R.; Hirano, T.; Tamura, M.; Sugisaka, J.; Suzuki, H.; Idogawa, M.; Shinomura, Y.; Tokino, T. CLCA2, a Target of the P53 Family, Negatively Regulates Cancer Cell Migration and Invasion. Cancer Biol. Ther. 2012, 13, 1512–1521. [Google Scholar] [CrossRef]
- Shinmura, K.; Igarashi, H.; Kato, H.; Kawanishi, Y.; Inoue, Y.; Nakamura, S.; Ogawa, H.; Yamashita, T.; Kawase, A.; Funai, K.; et al. CLCA2 as a Novel Immunohistochemical Marker for Differential Diagnosis of Squamous Cell Carcinoma from Adenocarcinoma of the Lung. Dis. Markers 2014, 2014, 619273. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Shao, C.; Bill, A.; Kim, J.; Xiao, D.; Bertrand, C.A.; Seethala, R.S.; Sano, D.; Myers, J.N.; Ha, P.; et al. To “Grow” or “Go”: TMEM16A Expression as a Switch between Tumor Growth and Metastasis in SCCHN. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 4673–4688. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Yurtsever, Z.; Berry, K.N.; Nichols, C.G.; Brett, T.J. Modulation of TMEM16A Channel Activity by the von Willebrand Factor Type A (VWA) Domain of the Calcium-Activated Chloride Channel Regulator 1 (CLCA1). J. Biol. Chem. 2017, 292, 9164–9174. [Google Scholar] [CrossRef]
- Centeio, R.; Ousingsawat, J.; Schreiber, R.; Kunzelmann, K. CLCA1 Regulates Airway Mucus Production and Ion Secretion Through TMEM16A. Int. J. Mol. Sci. 2021, 22, 5133. [Google Scholar] [CrossRef]
- Barhanin, J.; Lesage, F.; Guillemare, E.; Fink, M.; Lazdunski, M.; Romey, G. K(V)LQT1 and LsK (MinK) Proteins Associate to Form the I(Ks) Cardiac Potassium Current. Nature 1996, 384, 78–80. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Curran, M.E.; Zou, A.; Shen, J.; Spector, P.S.; Atkinson, D.L.; Keating, M.T. Coassembly of K(V)LQT1 and MinK (IsK) Proteins to Form Cardiac I(Ks) Potassium Channel. Nature 1996, 384, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Ávalos Prado, P.; Häfner, S.; Comoglio, Y.; Wdziekonski, B.; Duranton, C.; Attali, B.; Barhanin, J.; Sandoz, G. KCNE1 Is an Auxiliary Subunit of Two Distinct Ion Channel Superfamilies. Cell 2021, 184, 534–544.e11. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.C.; Joiner, A.M.; Zhang, L.; Iñiguez-Lluhí, J.; Martens, J.R. SUMOylation Regulates Ciliary Localization of Olfactory Signaling Proteins. J. Cell Sci. 2015, 128, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L.; Emeson, R.B. Editing of Neurotransmitter Receptor and Ion Channel RNAs in the Nervous System. Curr. Top. Microbiol. Immunol. 2012, 353, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pöhlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784.e5. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- van den Eijnde, S.M.; van den Hoff, M.J.; Reutelingsperger, C.P.; van Heerde, W.L.; Henfling, M.E.; Vermeij-Keers, C.; Schutte, B.; Borgers, M.; Ramaekers, F.C. Transient Expression of Phosphatidylserine at Cell-Cell Contact Areas Is Required for Myotube Formation. J. Cell Sci. 2001, 114, 3631–3642. [Google Scholar] [CrossRef]
- Helming, L.; Gordon, S. Molecular Mediators of Macrophage Fusion. Trends Cell Biol. 2009, 19, 514–522. [Google Scholar] [CrossRef]
- Lyden, T.W.; Ng, A.K.; Rote, N.S. Modulation of Phosphatidylserine Epitope Expression by BeWo Cells during Forskolin Treatment. Placenta 1993, 14, 177–186. [Google Scholar] [CrossRef]
- Rival, C.M.; Xu, W.; Shankman, L.S.; Morioka, S.; Arandjelovic, S.; Lee, C.S.; Wheeler, K.M.; Smith, R.P.; Haney, L.B.; Isakson, B.E.; et al. Phosphatidylserine on Viable Sperm and Phagocytic Machinery in Oocytes Regulate Mammalian Fertilization. Nat. Commun. 2019, 10, 4456. [Google Scholar] [CrossRef]
- Verma, S.K.; Leikina, E.; Melikov, K.; Gebert, C.; Kram, V.; Young, M.F.; Uygur, B.; Chernomordik, L.V. Cell-Surface Phosphatidylserine Regulates Osteoclast Precursor Fusion. J. Biol. Chem. 2018, 293, 254–270. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Bonilla, H.; Jagannathan, P.; Shafer, R.W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e00109-21. [Google Scholar] [CrossRef] [PubMed]


| Modulator | TMEM16x | Effect |
|---|---|---|
| Ca2+, Sr2+, Ni2+, Ba2+ | CaCCs, A, B, F | Agonist |
| Co2+, Zn2+, Mg2+ | A | Antagonist |
| Mg2+, Gd3+ | A | Charge screening |
| Calmodulin | A, B | Modulation of ion channel properties (contrasting results) |
| CaMKII | CaCCs, A | Current inhibition |
| pHi | F | Inhibition of channel and scramblase activities |
| pHi | A | Current modulation |
| pHo | A | Current activation |
| ATP | A, F | Current activation/reduction of current rundown (contrasting effects) |
| Hypoxia | A | Current activation |
| ROS | A, F | Activation of scramblase and channel activities |
| Heat | A, F | Current activation; Modulation of scramblase activity |
| PIP2 | A, F | Activation of channel and scramblase activities |
| PIP2 | CaCCs, B | Current inhibition |
| Plasmalemmal lipids | afTMEM16, nhTMEM16 | Contrasting effects |
| Cholesterol | CaCCs, A | Current inhibition |
| Fatty acids | A | Current inhibition |
| LPS | A | Increase in current density |
| LPA | A, F | Activation of channel and scramblase activities |
| AA | F | Current inhibition |
| Bile acids (UDCA and TUDCA) | A | Current activation |
| CLACs | CaCCs, A | Increase in current density |
| KCNE1 | A | Increase in current density |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostinelli, E.; Tammaro, P. Polymodal Control of TMEM16x Channels and Scramblases. Int. J. Mol. Sci. 2022, 23, 1580. https://doi.org/10.3390/ijms23031580
Agostinelli E, Tammaro P. Polymodal Control of TMEM16x Channels and Scramblases. International Journal of Molecular Sciences. 2022; 23(3):1580. https://doi.org/10.3390/ijms23031580
Chicago/Turabian StyleAgostinelli, Emilio, and Paolo Tammaro. 2022. "Polymodal Control of TMEM16x Channels and Scramblases" International Journal of Molecular Sciences 23, no. 3: 1580. https://doi.org/10.3390/ijms23031580
APA StyleAgostinelli, E., & Tammaro, P. (2022). Polymodal Control of TMEM16x Channels and Scramblases. International Journal of Molecular Sciences, 23(3), 1580. https://doi.org/10.3390/ijms23031580






